Pathological vascular remodeling of the vessel wall refers to the structural and functional changes of the vessel wall that occur in response to injury that eventually leads to cardiovascular disease (CVD). The vessel wall is composed of two main types of cells, endothelial cells (EC) and vascular smooth muscle cells (VSMC), whose communication is crucial in both the development of the vasculature and the homeostasis of mature vessels. Changes in the dialogue between ECs and VSMCs are associated with various pathological states that triggers remodeling of the vascular wall. For many years, considerable efforts have been made to develop effective diagnoses and treatments for these pathologies by studying their mechanisms in both in vitro and in vivo models. Compared to animal models, in vitro models can provide great opportunities to obtain data in a more homogeneous, economical and massive way, providing an overview of the signaling pathways responsible for these pathologies. The implementation of three-dimensional in vitro co-culture models for the study of other pathologies has been postulated as a potentially applicable methodology, which determines the importance of its application in studies of cardiovascular diseases. In this article we present a method for culturing human endothelial cells and vascular smooth muscle cells, grown under non-adherent conditions, that generate three-dimensional spheroidal structures with greater physiological equivalence to in vivo conditions. This in vitro modeling could be used as a study tool to identify cellular and molecular mechanisms involved in the pathological processes underlying vascular remodeling.
El remodelado patológico de la pared vascular se refiere a los cambios estructurales y funcionales de la pared del vaso que ocurren en respuesta a una lesión que eventualmente conduce a una enfermedad cardiovascular (ECV). La pared de los vasos se compone de dos tipos principales de células, las células endoteliales (CEs) y las células del músculo liso vascular (CMLVs), cuya comunicación es crucial tanto en el desarrollo de la vasculatura como en la homeostasis de los vasos maduros. Cambios en el diálogo entre las CEs y las CMLVs se asocian a diversos estados patológicos que conllevan remodelado de la pared vascular. Durante muchos años se han realizado considerables esfuerzos dedicados al desarrollo de diagnósticos y tratamientos eficaces para estas patologías mediante el estudio de sus mecanismos tanto en modelos in vitro como in vivo. En comparación con los modelos animales, los modelos in vitro pueden brindar grandes oportunidades para obtener datos de manera más homogénea, económica y masiva, aportando una visión general de las rutas de señalización responsables de estas patologías. La implantación de modelos tridimensionales de co-cultivo in vitro para el estudio de otras patologías, se ha postulado como una metodología potencialmente aplicable, lo que determina la importancia de su aplicación en estudios de las enfermedades cardiovasculares. En este artículo exponemos un método de cultivo de células endoteliales y células de músculo liso vascular humanas, crecidas en condiciones de no adherencia, que generan estructuras tridimensionales esferoidales con una mayor equivalencia fisiológica a las condiciones in vivo. Este modelado in vitro podría utilizarse como herramienta de estudio para identificar mecanismos celulares y moleculares implicados en los procesos patológicos que subyacen al remodelado vascular.
Cardiovascular diseases (CVD) are the principal cause of death in developed countries, accounting for 17.3 million deaths per year and the likelihood is that this number will increase by 2030.1 This illustrates the importance of studying these pathologies, with the purpose of developing diagnostic and prognostic markers, together with new therapeutic approaches. Atherothrombosis is the main cause of CVD and can remain asymptomatic for many years, with ischaemic events often being its primary clinical manifestation. Vascular wall remodelling is key during atherothrombosis and involves multicellular and multifactorial action of both resident cells in the vessel (endothelial cells [EC], vascular smooth muscle cells [VSMC]), and circulating cells in the blood.2
The vascular wall is of a complex and multimodel nature, formed by different concentric layers of communicating cells. The intima or endothelial layer is in contact with the lumen of the vessel. The media is mainly composed of VSMC and elastin fibres responsible for the contactile identity of the vessel, and the adventitia is rich in elastin and collagen, responsible for maintaining structural integrity. Cell communication is established mainly by paracrine signalling and by direct physical contact, being bidirectional and controlling both the organotypic and physiological mechanisms of the vascular nature itself as well as the pathological mechanisms of vascular remodelling.3 In this sense, under physiological conditions the EC and VSMC constitutively secrete low concentrations of extracellular vesicles and vasoactive molecules (prostanoids, arachidonic acid metabolites or nitric oxide), favouring a contractile behaviour that maintains vascular tone. However, pathological stimuli such as mechanical stress (injury, change in blood flow) or chemical stress (increase in factors in the blood) lead to changes in the dialogue between EC-VSMC at both the paracrine level (changes in the levels of extracellular vesicles, changes in the synthesis and secretion of the extracellular matrix component) and at the structural level of physical contact between cells.4 Specifically, changes in the activation of the endothelium due to the increase in lipids in the blood lead to the recruitment of monocytes to the subendothelial space, which will differentiate into macrophages capable of ingesting oxidized low-density lipoproteins (ox-LDL). Likewise, the pro-inflammatory environment produced by the secretion of cytokines by inflammatory cells induces phenotypic changes in VSMC, which go from a contractile phenotype to a synthetic, proliferative and migratory one, triggering the formation of the so-called neointima and the development of atherosclerotic plaque.5
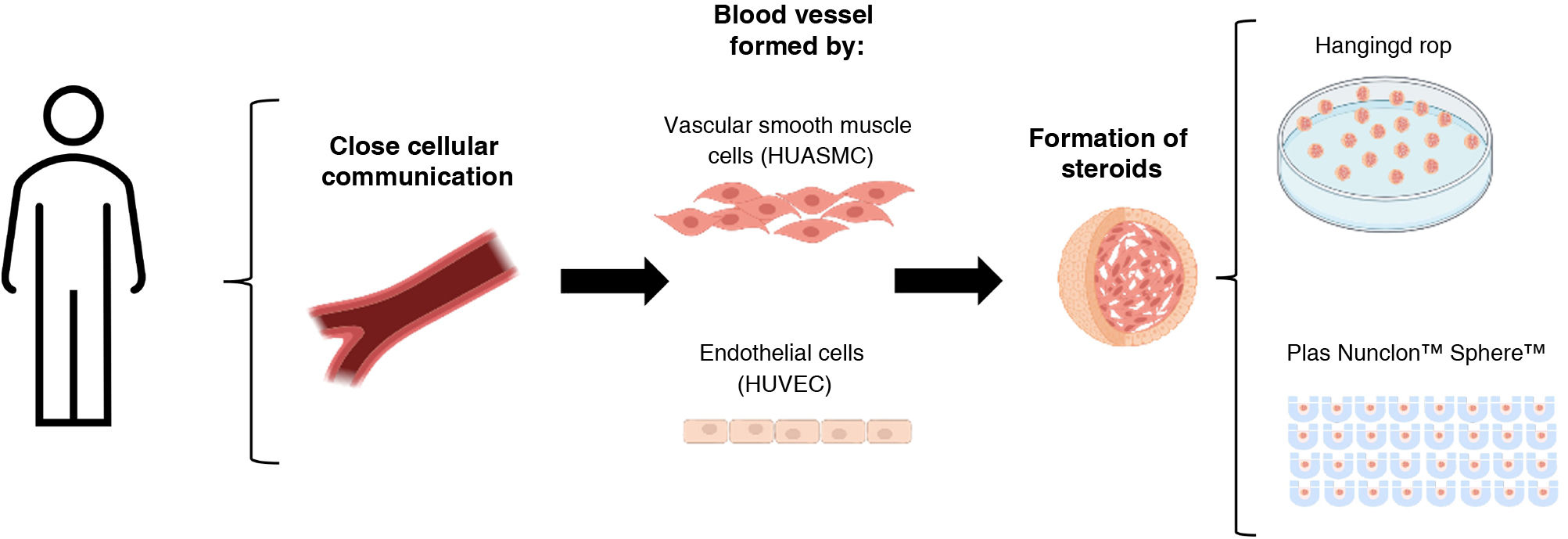
Preclinical models of vascular remodelling are an essential part of the study of pathophysiology and, consequently, for the development of new diagnostic and therapeutic targets. These types of studies provide important knowledge about the potential mechanisms involved in vascular damage, providing a systemic view.6 However, when massive screening studies are required, they are not the first option due to high costs and ethical considerations.7 In this regard, attempts have long been made to elucidate the functions of ECs and VSMCs as independent entities in vascular remodelling pathological processes using single cell cultures due to easier interpretation and analysis of results. Considering the complex and multimodal nature of the communication between EC and VSMC, attempts have been made to imitate and study in vitro different aspects of these interactions through the exploration of artificial co-culture configuration. One example that allows us to simultaneously and easily study both cell types are direct 2D co-cultures of EC and VSMC, or separated by a porous membrane (indirect co-cultures), such as transwells (static culture) or flow chambers (dynamic culture).8 Depending on its characteristics, such as permeability or pore size, this membrane allows for studies of communication, permeability, migration or paracrine regulation3 to be conducted. Despite its multiple advantages, these types of techniques have a limitation when it comes to simulating the complex interactions and structure that occur in vivo and cell-cell contact. The exploration of 3D co-culture techniques is novel in other fields such as oncological diseases and could be interesting for the study of cell-cell dialogue in vascular diseases. One of these tools is spheroids, three-dimensional cellular aggregates in the form of a sphere, which, when grown under different condiciones9,10 are being used as a tool for studies of cell communication and for mass diagnostic screening. Specifically, the three-dimensional or “vasculoids” system is a model based on the generation of suspended cell spheres that, depending on the vascular niche of origin of the EC or VSMC, can spontaneously form spheres with different structures and compositions.11
This study analyses the vasculoid co-culture model of endothelial cells and human vascular smooth muscle cells in vitro under different growth conditions (hanging drop and low-attachment plates).
MethodsCell cultureTwo cell types were used: human umbilical vein endothelial cells, HUVEC (P10961, Innoprot) and human umbilical artery smooth muscle cells, HUASMC (P10964, Innoprot) Both HUVEC and HUASMC were cultured with the reference commercial media recommended by the commercial company. Endothelial cells were cultured in Endothelial Cell Medium (ECM, P60104, Innoprot) supplemented with 20% foetal bovine serum (FBS, F7524, Sigma-Aldrich), streptomycin/penicillin solution (1:100), and endothelial growth factors (1:100). The smooth muscle cells were cultured in Smooth Muscle Cell Medium (SMCM, P60125, Innoprot), supplemented with 10% FBS, streptomycin/penicillin solution (1:100), and their respective growth factors (1:100). Both culture media promote an optimal and balanced nutritional environment for cell proliferation and growth following the instructions of the commercial company (Innoprot). Both cell types were grown at constant temperature (37 °C) and carbon dioxide values (5%). A 0.25% trypsin solution, 0.5 mM EDTA, 1 mM sodium pyruvate and 10 mM HEPES with a pH of 7.4 was used, diluted in DPBS 1:25 for HUVEC and 1:10 for HUASMC in order to separate adherent cells from the culture surface. They were incubated for 5 min in the incubator at 37 °C, and this reaction was neutralised with a neutralising solution or Trypsin Neutralisation Solution (TNS) containing HEPES buffer and 10% FBS as a trypsin inhibitor. After neutralisation, the cell suspension was centrifuged for 5 min at 1000 rpm according to the commercial company instructions.
Formation of HUVEC-HUASMC spheroidsTo make these three-dimensional spheroidal complexes, HUVEC and HUASMC were trypsinised and suspended in a mixture of both, in equal amounts (1500 cells of each type/spheroid). Two different culture approaches were used:
- (a)
(Hanging drop spheroid formation, for which 25 μl volume of cell suspension (3000 cells) in ECM medium supplemented with 5% FBS and .24% methylcellulose (Sigma, M7027) was placed in small drops on the sterile surface of a culture plate lid. The plate was then inverted 180° to generate the “hanging drop”, generating surface tension that favours spheroid formation.12
- (b)
Spheroid formation in low-attachment plates, for which 75 μl volume of cell suspension (3000 cells) was plated on Nunclon™ Sphera™ plates (174943, Scientific™) specified to prevent cell adhesion due to their low-attachment surface. In this case, the cell suspension medium is free of methylcellulose and is composed only of the ECM medium supplemented with 5% FBS.
In both cases, the development and generation of the spheres was visualised using an inverted Leica microscope (Model Dmi1) at different times. The area of the spheres was analysed over time using ImageJ software.
Immunofluorescence of spheroidsHUVEC-HUASMC spheres were carefully collected with a pipette and fixed in 4% paraformaldehyde in PBS for 20 min while moving. These spheres were then stained with hematoxylin (7211 Epredia) for 5 min to facilitate their subsequent visualisation and handling. 2% agarose in PBS was used as a mould to preserve the spheres. Briefly, agarose with the spheres was added to a 2 cm deep plastic cassette and allowed to solidify until a manageable structure was achieved. These agarose blocks with the spheres embedded inside were then processed in increasing concentrations of ethanol (70%, 95%, 100%) and embedded in paraffin blocks. The paraffin blocks were cut into 4-micron sections, until reaching the central sections of the spheres. For immunofluorescence, the sphere sections were deparaffinised and hydrated in decreasing concentrations of ethanol (100%, 95%, 70%). The samples were blocked with .05% TBST and 5% horse serum (Sigma) for 45 min at room temperature in a humid chamber. Subsequently, the samples were incubated overnight at 4 °C in a humid chamber with the corresponding antibodies, anti-alpha vascular smooth muscle actin-Cy3 (C6198, Sigma) 1:1000, and anti-CD31 (AF3628 R&D Systems) 1:100. An anti-goat Alexa 488 antibody (Invitrogen, 1:500) was used as a specific secondary and DAPI (Invitrogen 1:10000) for nuclear staining. Images were taken on a Zeiss Axioscope 5 fluorescence microscope, Axiocam 208 colour camera, with a 20x objective. Cell characterisation in the central part of the spheroid was analysed with Image J software.
For TUNNEL assays, the instructions required by the in situ cell death detection kit, TMR red (12156792910, Roche) were followed and the number of apoptotic cells out of the total number of cells was quantified with Image J software.
Statistical analysisData are presented as mean ± standard error of the mean (SEM) and the statistical significance of differences was assessed with Tukey's post hoc ANOVA test for multiple comparisons or Student's t-test. Significance was accepted at the level of P < .05. Data analysis was performed using GraphPad Prism 9.0a software (GraphPad, San Diego, CA).
Results- 1
Spheroid formation using the hanging drop technique and on low-attachment plates.
We proposed to optimise the method of developing vasculoids from HUVEC and HUASMC, cultured both in hanging drop and in another buoyancy condition, on specific low-attachment Nunclon Sphera plates (Fig. 1). We performed an analysis of their growth and structure, aimed at considering them as a tool applicable to the study of cell communication in vascular remodelling pathologies.
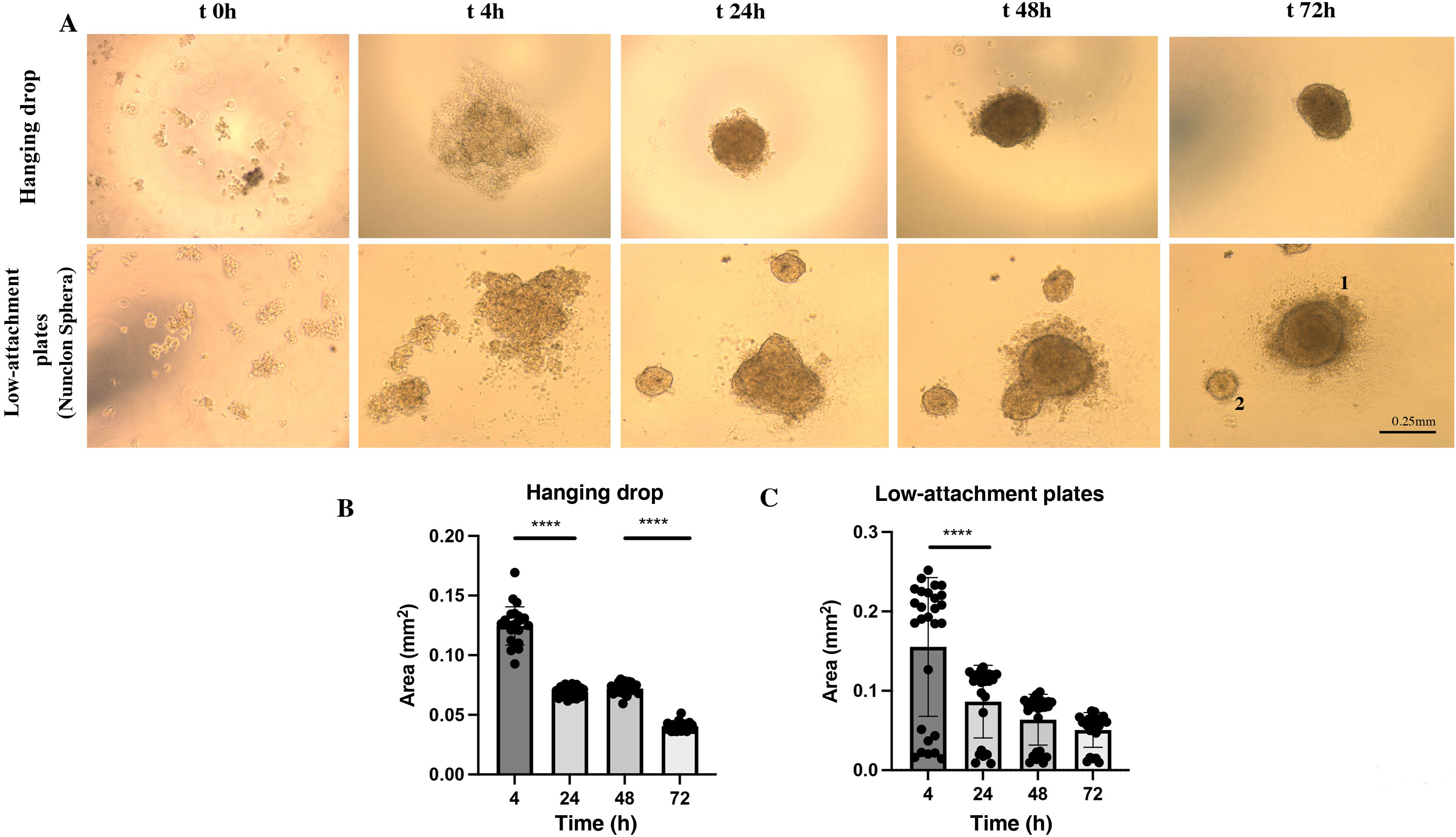
We observed that with the cell suspension of 25 μl of HUVEC-HUASMC in ECM medium supplemented with 5% FBS and .24% methylcellulose under conditions of inversion in a “hanging drop” at time 0 h, the cells appeared as single cells. After 4 h a single cell grouping with an unstable contour could be observed. At 24 h, this cell grouping became visibly clearer and more compact with a spheroidal morphology and its area decreased significantly (P < .0001) compared to 4 h (.069 mm2). Between 24 h and 48 h, the area remained the same (P = .6586) and the spheroidal structure was more opaque, unique and compact, with the presence of some cells on its periphery. At 72 h, a single sphere with a homogeneous contour was observed. However, a significant decrease in its area was observed (0.04 mm2) (P < .0001) (Fig. 2 A [upper panels] and B).
3D spheroid formation dynamics of HUVEC-HUASMC under hanging drop or low adhesion plate conditions (Nunclon Sphera). A) Representative images for a spheroid in bright field at a defined time kinetics (t0 seeding, 4 h, 24 h, 48 h, 72 h). Top images represent spheroid formation in hanging drop. Bottom images represent spheroid formation on Nunclon™ Sphera™ low adhesion plates. B). Quantification of spheroid area over time measured in mm2 in n = 24 spheroids in hanging drop, n = 22 on low adhesion plate. ****P < .0001. 1.2 indicative of various sphere sizes. Error bars .25 mm. One way-ANOVA, Turkey multiple comparison. Mean ± SEM. ****P < .0001.
In the case of the spheroid formation on a low-attachment plate by means of a 75 μl cell suspension of HUVEC-HUASMC in ECM medium supplemented with 5% methylcellulose-free FBS, it was observed that, at time 0 h, the cells appeared as individual cells. After 4 h, unstable cell grouping could be observed, which in many cases was not unique. After 24 h, these groupings were structured as spheres. Two groups of spheres were frequently observed, some larger (around .1 mm2) and others smaller (<.05 mm2), the areas of which were maintained for 48 h (P = .4295), with a tendency to decrease in a non-significant way (P = .8425) at 72 h (Fig. 2A [bottom panels] and C).
- 2
Phenotypic characterisation of spheroids in hanging drop and low-attachment plate.
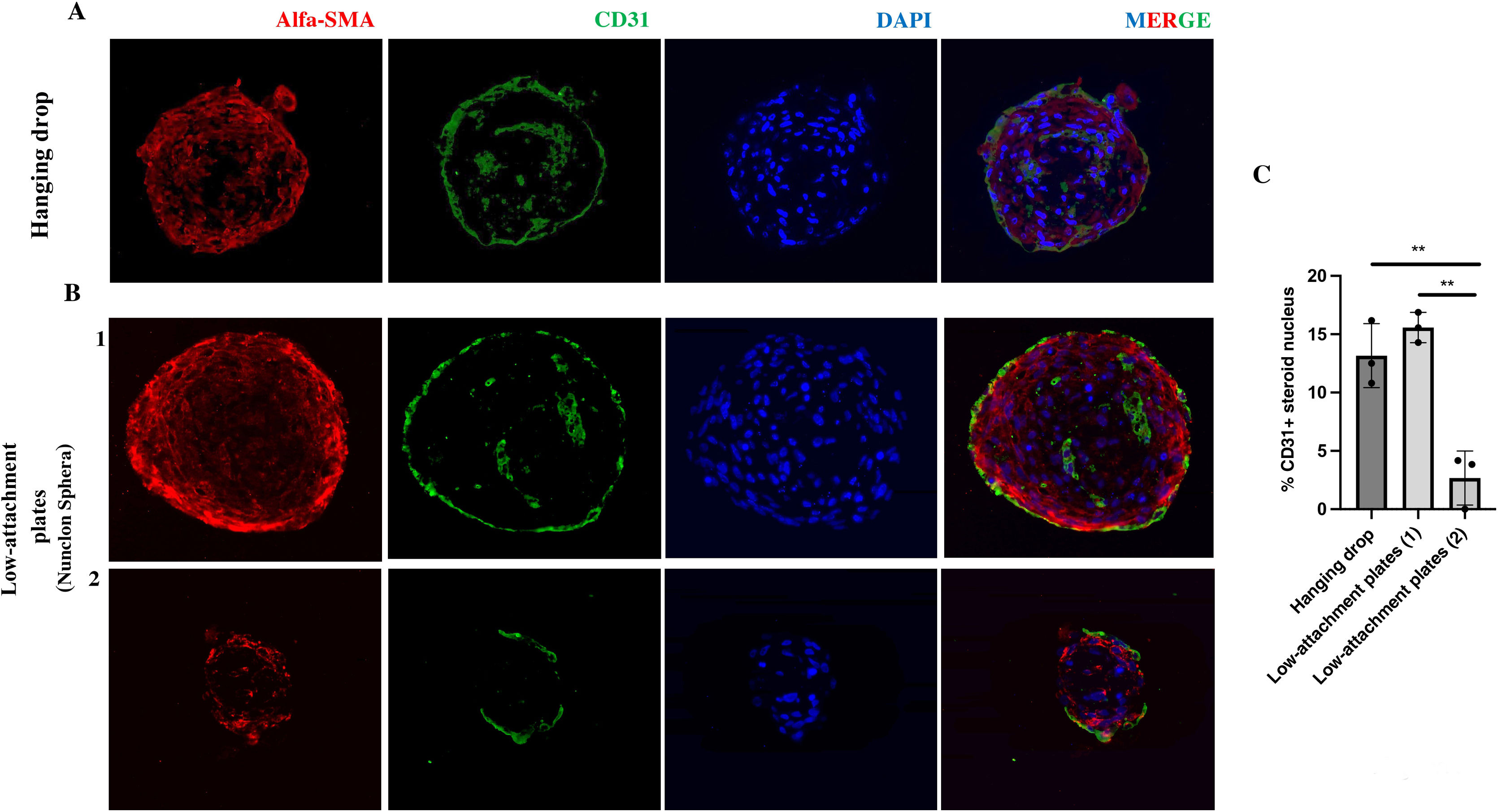
Both co-culture approaches allow the formation of three-dimensional structures. However, in the case of spheres developed by the hanging drop technique, there is greater homogeneity in their size and area over time. To characterise their nature, spheres developed in hanging drop and low-attachment plate were fixed and embedded in paraffin. Immunofluorescence of the CE marker CD31 (PECAM1) as well as the VSMC marker alpha-smooth muscle actin (a-SMA) were performed on 4-micron thick sections. Fluorescence microscopy images of a central section of the spheroid indicate that these spheroids would be formed mainly by vascular smooth muscle cells in their central area, both those developed by hanging drop (Fig. 3Fig. 3A) and those from low-attachment plaques of larger (Fig. 3B-1) and smaller size (Fig. 3B-2). In addition, a minimal percentage of endothelial cells is observed in their nucleus measured as CD31+ cells/total cells that make up the nucleus of the spheroid (Fig. 3C). In the case of smaller spheroids developed in low-attachment plaques, the percentage of CD31+ cells is significantly lower (P < .01) (Fig. 3C). In all cases, a monolayer of CD31+ cells is observed in the external part (Fig. 3A).
Cellular structure of inverted blood vessel from spheroids in hanging drop and in low-attachment plate. A) Representative immunofluorescence images of HUVEC-HUASMC spheroids in hanging drop and in low-attachment plate B) both sizes; larger (1) and smaller (2). The endothelial markers CD31 (green), vascular smooth muscle cell α-SMA (red), as well as nuclear staining (DAPI) are observed. MERGE, all channels together. C) Quantification of the percentage of CD31+ cells in the total number of cells in the spheroid core. Scale bars 50 μm. n = 3 spheroids per condition. t-Student. **P < .01. The colour of the figures can only be seen in the electronic version of the article.
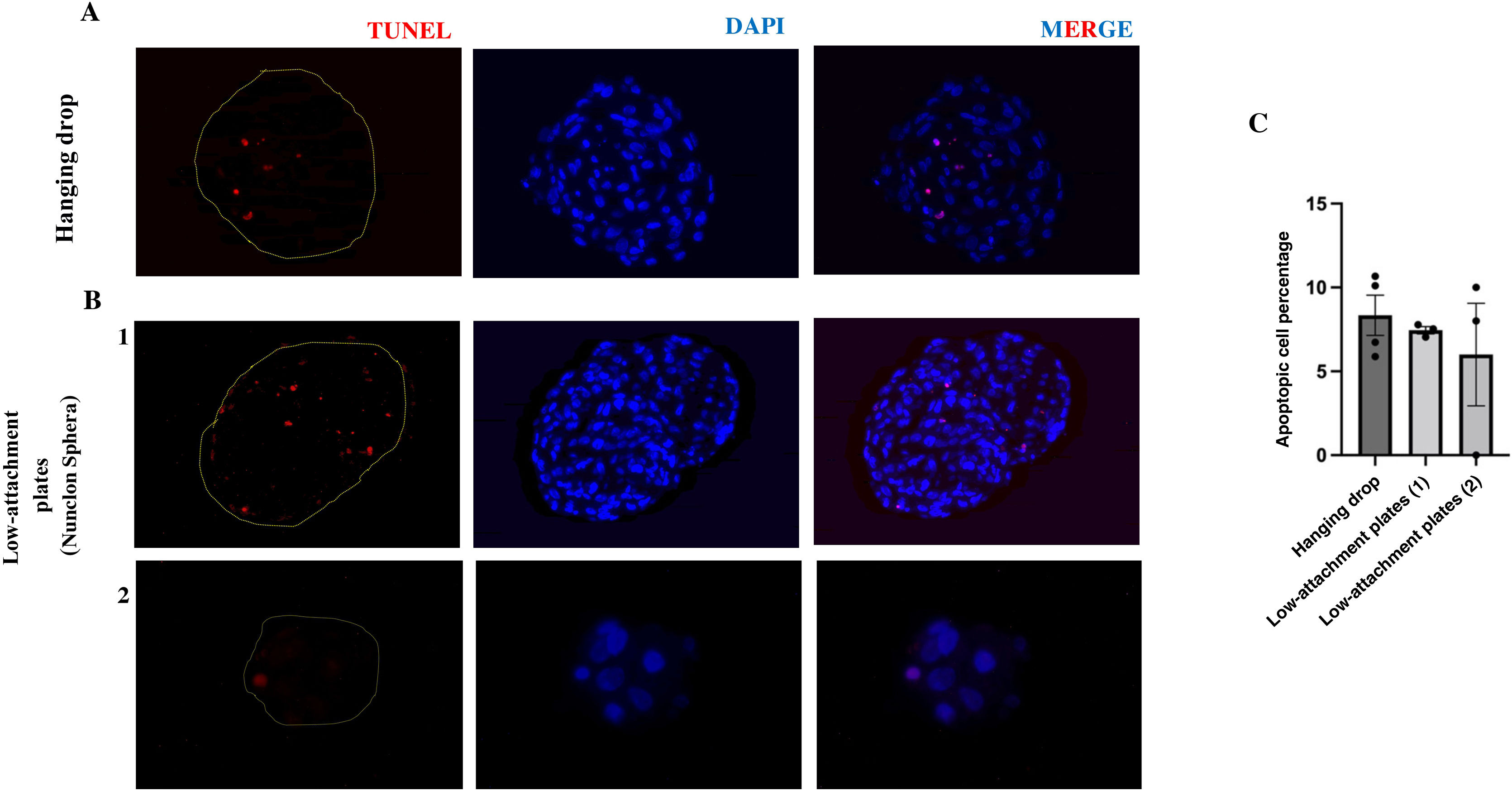
In addition, to analyse cell viability in the spheres grown under both buoyancy conditions, the spheres were stained with the in situ apoptosis detection kit. No significant differences were observed in the number of apoptotic cells at 72 h between the spheroids formed in hanging drop (8.34%) and the spheroids formed in low attachment plate (6.73%). In addition, these percentages indicate the viability at this time of both growth methods (Fig. 4A and B).
Presence of apoptotic cells in vasculoids grown in hanging drop or low-attachment plate. Representative images of histological analysis of 3D spheroids of HUVEC-HUASMC grown in hanging drop A) or in low-attachment plate B) 1,2) stained with TUNEL. Scale bars 50 μm. C) Graph of the percentage of apoptotic cells in each group n = 3–4 spheroids per condition.
This paper has shown how the tool of co-culivation of 3D spheres of HUVEC and HUASMC in a hanging drop12 could be an in vitro exploration strategy for the study of vascular communication in pathological vascular remodelling processes. We describe here that the circumstances of cell floatability in a low-attachment plate also generate the development of these spheres. Both culture methods generate a mimetic representation of the structure of an inverted blood vessel, observing an external layer of HUVEC and a majority core of HUASMC. Likewise, it is observed that these spheres are viable 72 h after their development and can be used in experiments of this specific time range. However, the development of spheroids in a hanging drop presents a greater risk of loss due to handling compared to spheroids developed in a low-attachment plate. The latter pose a risk in the analysis related to the heterogeneity in spheroid sizes, in addition to added economic costs.
The progress in the development of 3D organotypic culture technologies is currently a methodology that in CVD has focused on tissue design (cardiac and muscular), the development of nanobiomaterials, microfluidics, 3D printing, etc., and in any case, it represents a great step forward in relation to traditional 2D cell culture. In this paper, we observed that HUVEC-HUASMC spheroids in hanging drop and low-attachment plate conditions form spheres with structures mimetic to an inverted blood vessel. In addition, these co-culture spheres are viable for up to 72 h of development, as observed in the low number of apoptotic cells in their nucleus. However, cell co-culture under hanging drop conditions makes the spheres resulting from cell aggregation more homogeneous in area and cellularity than those generated in low-attachment plates, where cell clusters of various sizes are observed, which could be a key factor in cell communication studies due to the difference in cell number between spheroids. Studies carried out in 3D single-cell spheroidal cultures have shown that gene expression,13,14 metabolism,10 cell motility15 and cell polarity16 in the spheroid are different from those in 2D monolayer cultures, with a nature closer to native tissue. Studies have been described on HUVEC-HUASMC spheres in hanging drop form to evaluate the angiogenic impact of certain cytokines by culturing them in collagen matrices12 and have provided information on the importance of co-culture in contact with both cells, observing that it favours a state of cellular rest similar to what occurs in adult vasculature.17 In addition, 3D spheroid models of foam cells18,19 or VSMC20 have been used in order to reproduce the structural circumstances and the microenvironment for the study of lipid metabolism and the role of metalloproteinases in atherosclerosis processes, respectively. These examples would support the usefulness of these as a tool for studies in vascular remodelling pathologies. The compaction and rigidity of the structure of these spheroids could generate a hypoxic microenvironment in their nucleus over time, which may have consequences on their viability and the regulation of cell death proteins (e.g., caspase-3 in apoptosis).21 In this study, we observed viable spheres after 72 h of development both in a hanging drop and in a low-attachment plate, which would indicate viability of these at least in this time range. However, to achieve microenvironmental configurations similar to the in vivo situation of the vascular cells, the implementation of the development of these spheroids under dynamic flow conditions should be considered. In recent years, different groups have described the possibility of using flow bioreactors, both for the development of 3D structures and for their maintenance.22 However, this methodological objective represents a step forward to be developed in the case of vascular cell spheroids.
Taken together, these results indicate that co-culture in spheroids, due to their homogeneity and ease of development, would allow for high performance compared to other 3D models, making them an attractive model for the study of the mechanisms that regulate vascular remodelling. They could also be used to create patient-specific models for pharmacological screening purposes, as is already the case with other pathologies, allowing for possible precise planning of therapy.
LimitationsThe size of these co-culture systems (approximately .05–.1 mm2) could be one of the most important limitations for their use. Handling and experimentation with them requires careful pipetting and monitoring under a microscope, to avoid sample loss. Cell size (defined as the number of cells per spheroid) would also be a limitation for analysis, requiring optimisation of spheroid sets.
ConclusionsIn this article we have shown that the culture of human endothelial cells (HUVEC) and vascular smooth muscle cells (HUASMC) grown under low adherence conditions reproduces the self-assembly of these into viable spheroidal three-dimensional structures with an inverted blood vessel nature, as in a hanging drop, with the difference that in the low attachment plate there is no single grouping. This in vitro modelling could be used as a study tool to identify cellular and molecular mechanisms involved in the pathological and cellular communication processes underlying pathological vascular remodelling.
FundingThis research was supported by an FEA 2021 Grant for Basic Research in Arteriosclerosis from the Spanish Society of Arteriosclerosis. In addition, it received support from Health Research Funds, Carlos III Health Institute (ISCIII/FEDER)PI21/01126, PI19/00128, PI22/00233, LÓREAL Foundation for Women in Science 2021 Awards, and CIBERCV Madrid, Spain. NM-B has a Miguel Servet contract from ISCIII (MS19/00151), MJ F-G has a PFIS contract from ISCIII (FI22/00140) and I DSS-J a contract from CIBERCV.