Vitamin D (vitD) participates in phospho-calcium metabolism and exerts multiple pleiotropic effects. There is tissue 1-α (OH)ase that converts 25-OH cholecalciferol (25 (OH) D) in calcitriol that exerts autocrine and paracrine effects. 25 (OH)D deficiency could limit these tissue effects of vitD. The administration of nutritional vitD and the activator of the vitD receptor, paricalcitol, may promote beneficial effects on vascular and renal function. The objective of this work was to study in subjects with chronic kidney disease (CKD) the effect that the administration of different forms of vitD has on arterial function and albuminuria, and the possible relationship between the modifications of these variables.
Patients and methodsWe studied in 97 patients with CKD stages 3–4 the effect of the administration of cholecalciferol (group 2; n: 35) and paricalcitol (n: 31; group 3) on parameters derived from brachial blood pressure, aortic blood pressure and on aortic stiffness studied using carotid-femoral pulse velocity (Vpc-f), and on albuminuria. A group of patients with stages 3–4 CKD who did not receive vitD therapy served as a control group (n: 31; group 1). All parameters were studied at baseline and after the follow-up period which was 7±2 months.
ResultsIn the baseline phase, no differences were observed between the groups in brachial systolic blood pressure (bSBP), central systolic blood pressure (SBP), brachial pulse pressure (bPP), and central pulse pressure (pCP) or in aortic stiffness that was increased in all groups with a baseline Vpc-f value of 10.5 (9.2–12.1) m/sec. The baseline albuminuria value in the grouped patients was 229 (43–876) mg/g (median (interquartile range)), with no differences between the groups.
Serum calcium and phosphorus increased significantly in those treated with cholecal-ciferol (native vitD) and paricalcitol (active vitD). Parathormone (PTH) values decreased in those treated with paricalcitol. bPP and cPP decreased in all groups treated with native and active vitD. No significant changes in bPP and cPP were observed in the control group.
Vpc-f did not change significantly in any of the groups, although the variation was quantitatively greater in group 3 (11.2±2 vs. 10.7±1.6 (p:0.06)). No differences were observed in the changes in Vpc-f between the groups when adjusted to the baseline values of estimated glomerular filtration rate (eGFR), albuminuria, PTH, vitD, brachial and central blood pressure parameters, and their changes with treatment.
Those who received treatment with native and active vitD presented a significant decrease in albuminuria of 17% (group 2) and 21% (group 3) compared to a 16% increase in the untreated group (group 1) (p:0 .01). A decrease in albuminuria ≥30% was observed more frequently in the groups treated with some form of vitD (group 2: 23%; group 3: 45%) than in the control group (13%) (p:0.03). The decrease in albuminuria observed in the groups treated with any of the forms of vitD did not vary when the baseline values of the biochemical parameters of phosphorus-calcium metabolism, those of arterial function (PPb, PPc, Vpc-f) or its modifications were introduced as covariates. There was no significant correlation between changes in Vpc-f and albuminuria. In logistic regression, changes in arterial function parameters were also not explanatory for the ≥30% decrease in albuminuria.
ConclusionsIn patients with CKD stages 3–4, treated with RAS blockers and with residual albuminuria, the administration of or paricalcitol reduces brachial and aortic pulse pressures, and albuminuria. The decrease in albuminuria does not seem to be mediated, at least not decisively, by changes in central hemodynamics or aortic stiffness.
La vitamina D (vitD) ejerce efectos pleiotrópicos como son las modificaciones de la función arterial y descenso de la albuminuria. Existe 1-alfa-hidroxilasa tisular que convierte el 25-hidroxicolecalciferol (25(OH)D) en calcitriol que ejerce acciones autocrinas y paracrinas que intervienen en los efectos pleiotrópicos. El déficit de 25(OH)D podría limitar estos efectos tisulares de la vitD. La administración de vitD nutricional (colecalciferol) y de paricalcitol, puede promover beneficios en la función vascular y renal.
El objetivo fue estudiar el efecto que tiene, en la enfermedad renal crónica (ERC), la administración de diferentes formas de vitD sobre la rigidez aórtica y sobre la albuminuria, y la relación fisiopatológica entre las modificaciones de estas variables.
Pacientes y métodosEstudiamos en 97 enfermos con ERC estadios 3–4 y con albuminuria residual, el efecto de la administración de colecalciferol (grupo 2) y paricalcitol (grupo 3) sobre la rigidez aórtica estudiada mediante la velocidad de pulso carótida-femoral (Vpc-f), sobre la presión arterial braquial y aórtica (central), y sobre la albuminuria. Un grupo de enfermos con ERC estadios 3–4 que no recibió terapia con vitD sirvió como grupo control (grupo 1). Todos los parámetros se estudiaron basal-mente y tras un periodo de seguimiento de 7±2 meses.
ResultadosNo hubo diferencias entre los grupos en la rigidez aórtica que estaba aumentada en todos ellos con un valor basal de la Vpc-f de 10,5 (9,2–12,1) m/seg. Los valores basales de presión arterial sistólica braquial (PASb), presión arterial sistólica central (PASc), presión de pulso braquial (PPb) y presión de pulso central (PPc) fueron similares en todos los grupos.
El valor de albuminuria basal fue 198 (46–832) mg/g, sin diferencias entre los grupos.
La calcemia y fosforemia aumentaron significativamente en los tratados con colecalciferol y paricalcitol. Los valores de parathormona (PTH) disminuyeron en los tratados con paricalcitol.
La PPb y PPc disminuyeron en todos los grupos tratados con vitD nativa y activa y no se modificaron en el grupo control.
La Vpc-f no se modificó significativamente en ninguno de los grupos aunque la variación fue mayor en el grupo 3 (11,2±2 vs. 10,7±1,6 (p:0,06)).
Los que recibieron tratamiento con vitD presentaron un descenso de la albuminuria de 17 % (grupo 2) y 21% (grupo 3) frente a un aumento de 16% en el grupo no tratado (grupo 1) (p:0,01). Una reducción de la albuminuria ≥30% se observó más frecuente-mente en los grupos tratados con alguna forma de vitD (p:0,03).
No existió correlación significativa entre los cambios de la Vpc-f y los de la albuminuria ni participación de las modificaciones de la función arterial en la reducción ≥30% de la albuminuria.
ConclusionesEn la ERC estadios 3–4, con albuminuria residual, la administración de colecalciferol o paricalcitol reduce los índices de pulsatilidad arterial y la albuminuria. Este descenso de la albuminuria no parece estar mediado, al menos de forma determinante, por las modificaciones de la rigidez aórtica.
Vitamin D (vitD) is a prohormone present in some types of food and produced endogenously in the skin by photochemical reaction. The 2 major forms of vitD, ergocalciferol (vitD2) and cholecalciferol (vitD3), share metabolic pathways. Both are transported by vitD-binding protein (vitDBP). In the liver they are converted to 25(OH)D (calcidiol) which is transformed into the active form of vitD, 1,25 dihydroxy-vitamin D (1,25[OH)2D], calcitriol) by 1-alpha-hydroxylase (1α-OHase) located predominantly in the renal tubular epithelium and in other tissues.1,2
Normal renal function is essential to maintain the endocrine action of the calcitriol-vitD receptor complex (VDR), as the kidney is the main site of calcitriol production. In chronic kidney disease (CKD) there is a high prevalence of vitD insufficiency or deficiency that starts in its early stages.3 The progressive reduction of calcidiol secondary to cholecalciferol deficiency and to its lower tubular cell intake due to a decrease in glomerular filtration rate (GFR) in CKD aggravates the calcitriol deficit secondary to a lower concentration and activity of 1α-OHase due to loss of renal mass and to other factors, such as resistance to the action of parathormone (PTH), metabolic acidosis, and high values of fibroblast growth factor (FGF23), among others.4,5
VitD is involved in the maintenance of calcium and phosphorus homeostasis and bone-mineral metabolism. In addition, experimental data and observational studies show that vitD has various pleiotropic effects.6–8 Many of these involve active vitD generated in multiple tissues by local 1-α(OH)ase action that converts calcidiol to calcitriol, which exerts autocrine and paracrine effects.2 This process, which, therefore, is dependent on circulating 25(OH)D levels, may have important clinical implications in CKD where a deficit of 25(OH)D (substrate of active vitD) and a loss of renal 1αOHase activity often coexist. These pleiotropic effects may be involved in the association observed in the general population and in CKD subjects between reduced vitD values, cardiovascular morbidity and mortality, and various parameters of vascular and renal function, such as increased arterial stiffness and proteinuria.9,10
Very few studies have analysed the effect of vitD administration in CKD on arterial stiffness and results have been mixed.11–14 Studies of the effect of vitD on proteinuria in CKD have also yielded mixed results.15–20 In studies that have shown a decrease in albuminuria, various mediators have been proposed, but the mechanism of vitD's antialbuminuric effect has not been clarified. No studies have analysed the possible involvement of changes in arterial stiffness and central arterial haemodynamics in changes in albuminuria, an implication that can be inferred since an increase in aortic stiffness, common in CKD,21 may promote changes in central haemodynamics and microcirculation that may lead to impaired renal function and proteinuria,22 among other conditions.
The aim of the present study was to evaluate, in patients with stage 3–4 CKD and residual albuminuria, the effect of oral intake of different forms of vitD (cholecalciferol and paricalcitol) on arterial function (aortic stiffness and central blood pressure [BP]) and albuminuria, and the possible relationship between both parameters.
Material and methodsProspective observational study that included 99 patients with CKD with an estimated GFR (eGFR) according to the Modification of Diet in Renal Disease (MDRD) formula of between 60 and 15ml/min/1.73m2 (stages 3–4) that was stable over the last 6 months. Patients with glomerulonephritis requiring immunosuppressive therapy, those requiring the introduction or dose adjustments of RAS blockers in the last 3 months for BP control and those receiving therapy with any form of vitD were excluded.
The patients were examined and followed up in the nephrology outpatient clinic. According to clinical criteria based on an adaptation of the Kidney Disease Outcomes Quality Initiative (K-DOQI) guidelines for serum PTH, 25(OH)D and calcium values, patients were prescribed some form of vitD. Baseline serum calcium and phosphorus values had to be <10.2mg/dl and 4.6mg/dl, respectively. In the presence of serum 25(OH)D values <20ng/mL and PTH values 100–197pg/ml, cholecalciferol (drop formulation) was prescribed. Paricalcitol (capsule formulation) was prescribed if serum 25(OH)D values were between 20 and 30ng/mL and PTH values >146pg/mL. The dose of cholecalciferol and paricalcitol was variable, and was maintained or adjusted after 3 months according to calcaemia values. Patients with 25(OH)D >20ng/mL and PTH<146pg/mL did not receive vitD therapy and served as a control group.
All patients gave their informed consent for the study, which met all ethical criteria of the institution where the study was performed.
Laboratory parametersBiochemical parameters and markers of bone-mineral metabolism were measured in blood before initiation of vitD therapy and at the end of follow-up. PTH was tested using the electrochemiluminescence technique (ARCHITECT intact PTH, Abbott, Germany). These values are equivalent to 70 and 110pg/mL, respectively, when PTH determination is done by immunoradiometric assay (Nichols Institute diagnostic Inc., USA) used as reference range by K-DOQI.23
The month of 25(OH)D testing was noted (October to March was considered the period of least sunlight). According to 25(OH)D values, vitD status was defined as deficient (<20ng/mL), insufficient (21−29ng/mL), and normal (≥ 30ng/mL).24 The albumin/creatinine ratio was measured from first morning urine.
Haemodynamic parameters and aortic stiffnessThe study of central BP and carotid-femoral pulse wave velocity index (Pvc-f) was performed by flattening tonometry using a SphygmoCor device (AtCor Medical, Sydney, Australia) according to methodology previously described in detail.25 Briefly, from the pulse wave obtained by tonometry, central systolic BP (cSBP), central diastolic BP (cDBP), and central pulse pressure (cPP) (difference between cSBP and cDBP) were obtained. The same SphygmoCor device was used to determine Pvc-f. The pulse wave was obtained by sequential use of applanation tonometry over the common carotid artery and the femoral artery and the transit time between the 2 points was calculated from the difference between the R wave of the simultaneous electrocardiographic recording and the onset of the pulse wave at the respective arterial sites. From the measured Pvc-f values, the theoretical Pvc-f was obtained, which considers other variables that influence Pvc-f such as age, sex, BP, and heart rate. The Pvc-f index (Pvc-fi) was obtained from the measured Pvc-f and the theoretical Pvc-f. Tonometry to determine central haemodynamics and Pvc-f was always performed by the same investigator. The values of arterial parameters and pulse velocity were considered as the average of 2 recordings with a good quality index.
Study of vascular calcificationsBefore initiation of vitD therapy, 64 patients underwent lateral abdominal radiography to assess abdominal aortic calcifications. The degree of calcification (Kauppila index) was always read and scored by the same investigators following the described methodology26; the investigators were unaware of the laboratory and vascular function data.
Statistical analysisQualitative variables are expressed as absolute or relative frequencies and quantitative variables as mean±standard deviation or median (interquartile range) according to distribution. In some cases, non-normally distributed variables were converted into their logarithms. Comparison between qualitative variables was done using the χ2 test or Fisher's exact test, and the McNemar statistic was used to compare changes of these variables over time. The Student's t-test was used in the case of normal distribution, or Wilconson's test for non-normally distributed variables to compare pre- and post-treatment changes of quantitative variables within each group. Analysis of variance (ANOVA) was used for the comparative analysis between the different groups of normally distributed continuous variables. In the case of non-normally distributed variables, the Kruskal–Wallis test was used. For the analysis of the effect of different therapies on change (Δ) in arterial stiffness and albuminuria, analysis of covariance (ANCOVA) was used, considering Pvc-f and albuminuria as dependent variables, the treatment group as fixed factor, and baseline Pvc-f and albuminuria, presence of diabetes mellitus (DM), or cardiovascular disease (CVD) and changes in arterial function parameters as covariates. To assess possible independent effects of arterial function parameters on a ≥30% reduction in albuminuria, stepwise logistic regression was used. Analysis of the relationship between variables was performed using Pearson's or Spearman's correlation coefficient according to the distribution of variables. A p-value of <.05 was considered significant. All statistical analyses were performed with IBM SPSS version 25 for Windows (IBM North America, New York, USA).
ResultsAfter the initial visit, 2 patients were excluded because they were on calcitriol therapy in one case and a calcium/cholecalciferol combination in the other. A total of 97 patients aged 68 (59–72) years were included in the final analysis. Sixty percent of the patients were diabetic.
Follow-up time was 7±2 months.
Of the 97 patients, 31 did not receive vitD supplementation (group 1), 35 were treated with cholecalciferol (group 2) and 31 with paricalcitol (group 3). There were no significant differences in baseline clinical characteristics between the different groups (Table 1).
Baseline clinical characteristics.
| Group | 1 | 2 | 3 | |
|---|---|---|---|---|
| variable | No vitD supplementation | Cholecalciferol | Paricalcitol | Intergroup difference |
| N° | 31 | 35 | 31 | p |
| Age (years) | ||||
| Median (IQR) | 68 (60–70) | 69 (57–73) | 70 (61–73) | ns |
| Sex (%) | ns | |||
| Male | 71 | 43 | 64 | |
| Female | 29 | 57 | 36 | |
| BMI (kg/m2) | ns | |||
| Median (IQR) | 29 (27–32) | 28 (25–33) | 31 (28–32) | ns |
| Smoking (%) | ns | |||
| Active smoker | 10 | 9 | 0 | |
| Ex-smoker | 42 | 17 | 29 | |
| Never smoked | 48 | 74 | 71 | |
| DM2 (%) | 55 | 63 | 61 | ns |
| HBP (%) | 97 | 100 | 100 | ns |
| Nephropathy (%) | ns | |||
| Nephang. | 55 | 43 | 52 | |
| Diabetic | 16 | 31 | 19 | |
| CGN | 16 | 8 | 23 | |
| Other | 13 | 18 | 6 | |
| Associated CVD (%) | ns | |||
| No CVD | 74 | 66 | 52 | |
| HHD | 6 | 11 | 19 | |
| IHD | 10 | 11 | 16 | |
| Stroke | 6 | 9 | 22 | |
| Arteriopathy | 10 | 9 | 3 | |
| Treatment (%) | ns | |||
| RAS blockade | 97 | 100 | 97 | |
| BB | 26 | 26 | 39 | |
| Calcium antagonists | 64 | 54 | 71 | |
| Nitrates | 6 | 3 | 3 | |
| Statins | 77 | 66 | 71 | |
| Antiaggregants | 52 | 57 | 61 | |
| Phosphorus binders | 10 | 14 | 16 | |
BB: Beta-blockers; CA: Calcium antagonists; CVD: Cardiovascular Disease; CGN: Chronic glomerulonephritis; DM2: Type 2 diabetes mellitus; HHD: Hypertensive heart disease; HBP: High Blood Pressure; IHD: Ischaemic Heart Disease; IQR: Interquartile Range; nephang: nephroangiosclerosis; RAS: Renin-angiotensin System; vitD: Vitamin D.
Baseline laboratory and arterial function parameters can be seen in Table 2. Since vitD and PTH values served to categorise the groups, the values of these variables significantly differed between them. The baseline albuminuria value of all patients grouped together was 198 (46–832) mg/g and no significant differences were observed between groups.
Baseline laboratory and arterial function parameters.
| Group | 1 | 2 | 3 | |
|---|---|---|---|---|
| Variable | Without vitD supplementation | Colecalciferol | Paricalcitol | Intergroup difference |
| N° | 31 | 35 | 31 | p |
| Laboratory parameters | ||||
| GFR (ml/min/1,73 m2) | ||||
| Median (IQR) | 36 (27–41) | 31 (24–35) | 26 (20–31) | 1 vs. 2: .04 |
| Calcium (mg/dl) | 9.70 ± .4 | 9.62 ± .3 | 9.52 ± .4 | 1 vs. 3: .001 |
| Phosphorus (mg/dl) | 3.42 ± .5 | 3.34 ± .5 | 3.37 ± .5 | ns |
| PTH (pg/mL) | ||||
| Median (IQR) | 88 (72–126) | 134 (102–175) | 246 (166–294) | 1 vs. 2: .04 |
| 1 vs. 3: .001 | ||||
| 25-(OH)D (ng/mL) | 2 vs. 3: .00 | |||
| Median (IQR) | 26 (21–33) | 18 (12–23) | 27 (22–29) | 1 vs. 2: .001 |
| 2 vs. 3: .001 | ||||
| VitD status (%) | .001 | |||
| Deficient | 16 | 66 | 16 | |
| Insufficient | 55 | 34 | 64 | |
| Normal | 29 | 0 | 20 | |
| uCPR (mg/dl) | ||||
| Median (IQR) | .24 (.09–.50) | .25 (.13–.44) | .34 (.13–.46) | ns |
| Alb/creat. (mg/g) | ||||
| Median (IQR) | 182 (43–406) | 180 (37–714) | 359 (60–1206) | ns |
| Arterial function parameters | ||||
| bSBP (mmHg) | 140±14 | 145±19 | 149±18 | ns |
| bPP (mmHg) | 65±15 | 70±18 | 68±16 | ns |
| cSBP (mmHg) | 130±13 | 134±17 | 138±19 | ns |
| cPP (mmHg) | 54±14 | 57±17 | 6±17 | ns |
| Pvc-f (m/s) | ||||
| Median (IQR) | 10.9 (8.9–12.6) | 10.2 (9.1–11.1) | 11.5 (9.7–12.1) | ns |
| Median Kauppila index (IQR) | 7 (.5–11) | 5.5 (0–10.2) | 5 (2–11) | ns |
Alb/creat.: Urine albumin to creatinine ratio; cPP: Central pulse pressure central; cSBP: Central systolic blood pressure; GFR: Glomerular Filtration Rate; 25(OH)D: 25 hydroxycholecalciferol; IQR: Interquartile Range; ns: not significant; PP: Pulse Pressure; PTH: Parathormone; Pvc-f; carotid-femoral pulse wave velocity; SBP: Systolic Blood Pressure; uCRP: ultrasensitive C-reactive protein.
No significant differences were observed in baseline brachial and central BP parameters and aortic stiffness, which was elevated in all groups. There were no differences in Pvc-fi between groups.
At baseline, in all patients there was a significant direct correlation of Pvc-f with age (.407; p<.01), bSBP (.545; p<.01), cSBP (r: .503; p<.01), bPP (.435; p<.01), cPP (r: .389; p<.01), Kauppila index (.598; p<.01), and albuminuria (r: .262; p<.05).
The mean dose of cholecalciferol in group 2 was 1256±511 U/day and that of paricalcitol in group 3 was .69±.25μg/day.
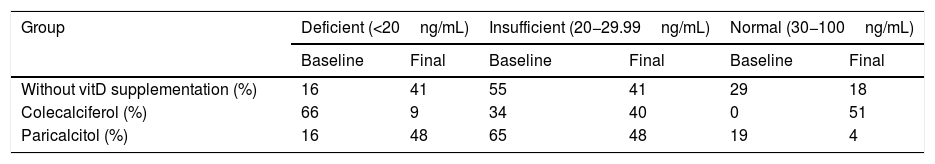
Fifty-six per cent of the baseline and 41% of the post-treatment 25(OH)D tests were performed in the months with less sunlight. After cholecalciferol therapy, 51% of patients in group 2 had normalised serum vitD values, which, however, decreased in the paricalcitol-treated group and in the control group (Table 3).
Vitamin D status before and after treatment in the different groups.
| Group | Deficient (<20ng/mL) | Insufficient (20−29.99ng/mL) | Normal (30−100ng/mL) | |||
|---|---|---|---|---|---|---|
| Baseline | Final | Baseline | Final | Baseline | Final | |
| Without vitD supplementation (%) | 16 | 41 | 55 | 41 | 29 | 18 |
| Colecalciferol (%) | 66 | 9 | 34 | 40 | 0 | 51 |
| Paricalcitol (%) | 16 | 48 | 65 | 48 | 19 | 4 |
vitD.: Vitamin D.
Table 4 shows the values of laboratory and arterial function parameters before and at the end of treatment. Calcaemia and phosphataemia increased significantly in the groups treated with native and active vitD. PTH values increased in the control group, were unchanged in group 2 (cholecalciferol), and significantly decreased in group 3 (paricalcitol). The percentage change (increase) in calcaemia and PTH (decrease) were higher in the paricalcitol group than in the cholecalciferol group. In diabetic subjects, glycosylated haemoglobin values did not change significantly in either group.
Laboratory and arterial function parameters before (baseline) and after (post) treatment and their percentage variation (Δ).
| Parameter | Without vitD supplementation (group 1) | Cholecalciferol (group 2) | Paricalcitol (group 3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | P | Post | Baseline | p | Post | Baseline | P | Post | |
| Laboratory parameters | |||||||||
| Calcium (mg/dl) | 9.7±.4 | ns | 9.8 ± .3 | 9,6 ± .3 | .02 | 9.8 ± .4 | 9.5 ± .4 | .01 | 9.8±.5 |
| Phosphorus(mg/dl) | 3.4±.5 | ns | 3.4 ± .4 | 3.5 ± .5 | .04 | 3.7 ± .8 | 3.4 ± .7 | .03 | 3.6 ± .7 |
| vitD (ng/mL) | 24 (20−32) | .07 | 21 (17−28) | 18 (12−23) | .001 | 30 (25−34) | 27 (22−29) | .001 | 20 (17−25) |
| PTH (pg/mL) | 88 (69−123) | .001 | 118 (84−148) | 134 (100−180) | ns | 130 (87−215) | 246 (166−294) | .001 | 128 (90−192) |
| AP (U/l) | 88±31 | ns | 84±38 | 96±29 | ns | 95±29 | 84±20 | .009 | 75±26 |
| eGFR (ml/min/1,73 m2) | 35±11 | .01 | 31±9 | 30±8 | .04 | 27±8 | 26±7 | ns | 25±7 |
| HbA1c (%) (only diabetics) | 7.5±.5 | ns | 7.4 ± .5 | 7.5 ± .4 | ns | 7.4 ± .4 | .5 ± .4 | ns | 7.3±.5 |
| Alb/creat (mg/g) | 182 (43−406) | ns | 135 (47−893) | 233 (48−736) | .006 | 207 (35−674) | 359 (60−1206) | .006 | 230 (38−1150) |
| Arterial function parameters | |||||||||
| bSBP(mmHg) | 140±14 | ns | 139±13 | 145±19 | ns | 141±20 | 149±18 | .02 | 141±16 |
| bPP (mmHg) | 66±16 | ns | 66±16 | 70±18 | .02 | 65±18 | 68±16 | .03 | 63±14 |
| cSBP (mmHg) | 132±12 | ns | 127±13 | 134±17 | ns | 129±21 | 136±18 | .04 | 129±16 |
| cPP (mmHg) | 55±15 | ns | 54±13 | 57±17 | .02 | 52±18 | 55±17 | .06 | 50±15 |
| Pvc-f (m/s) | 10.8±3 | ns | 10.6±3 | 10.7±3 | ns | 10.5±3 | 11.2±2 | .06 | 10.7±1.6 |
| Pvc-fi (%) | 4±23 | ns | 4±22 | 3±22 | ns | 2±23 | 4.2±15 | .051 | .65±13 |
| Δ of laboratory and arterial function parameters | Intergroup difference | |||
|---|---|---|---|---|
| Δ Calcium (x̄ ± SD) | 1.5±4 | 1.7±4 | 3.6±5 | 1 vs. 2.3: .04 |
| 2 vs. 3: .04 | ||||
| Δ PTH, median (IQR) | 35 (13−48) | −7 (−29 to 20) | −43 (−54 to −32) | 1 vs. 2.3: .001 |
| 2 vs. 3: .001 | ||||
| Δ Alb/creat, median (IQR) | 16 (−46 to 71) | −17 (−33 to 3) | −21 (−40 to 7) | 1 vs. 2,3: .01 |
| Δ Pvc-f (x̄ ± SD) | 1±13 | −1±15 | −2±15 | Ns |
Alb/creat.: Albumin/creatinine; AP: Alkaline Phosphatase; bPP: Brachial pulse pressure; bSBP: Brachial systemic blood pressure; cPP: Central pulse pressure; cSBP: Central systemic blood pressure; eGFR: Estimated glomerular filtration rate; HbA1c: Glycosylated haemoglobin; IQR: Interquartile Range; PTH: Parathormone; Pvc-f: Carotid-femoral pulse wave velocity; Pvc-fi:: Carotid-femoral pulse wave velocity index; vitD: Vitamin D; x̄ ± SD: Mean ± Standard Deviation.
eGFR decreased significantly in all groups except group 3, where the change was not significant. There was a significant decrease in albuminuria in the groups treated with any form of vitD compared to those who did not receive vitD (p=.001). This reduction remained significant when the presence of DM or CVD, baseline values of albuminuria, eGFR and Pvc-f, or changes in weight, Pvc-f, and other arterial function parameters were entered into the model as covariates.
A significant decrease in bPP and cPP was observed in the groups treated with some form of vitD (groups 2 and 3). Pvc-f and Pvc-fi did not change significantly in any of the groups, although a quantitatively larger decrease was observed in group 3 than in the other groups (Table 4).
There was a significant direct correlation between change (Δ) in Pvc-f and change in bSBP (r: .494, p=.001), bPP (r: .381, p=.01), cSBP (r: .399, p=.01) and cPP (r: .288, p=.02). None of the changes in vascular function parameters correlated with changes in albuminuria.
A decrease in albuminuria ≥30% was observed in 13% of those who did not receive vitD therapy and in 24% and 45% of those who received cholecalciferol or paricalcitol, respectively (p=.04). In logistic regression, neither baseline albuminuria nor changes in eGFR, uCRP, or arterial parameters were explanatory variables for the ≥30% decrease in albuminuria.
DiscussionIn this study we analysed the effect of the administration of different forms of vitD in patients with stage 3–4 CKD, treated with RAS blockers and with residual albuminuria, on aortic stiffness and central arterial haemodynamics, and the possible involvement of these in changes in albuminuria. The study demonstrates that a 7-month course of nutritional vitD or the VDR activator paricalcitol induces a significant decrease in brachial and central SBP and pulse pressure, a non-significant reduction in aortic stiffness, and a significant decrease in albuminuria in which changes in arterial function parameters are not substantially involved.
Our research design to compare the effect of these 2 forms of vitD and a control group is based on several facts. Firstly, increased aortic arterial stiffness is very common in CKD and can be associated with changes in renal function because a stiff aorta does not exert the normal buffering effect and allows the transmission of central BP to the glomerulus, favouring glomerular barotrauma and, consequently, albuminuria.22,27 On the other hand, it is important to have sufficient serum 25(OH)D, as there is wide tissue distribution of VDR together with 1α-OHase and it is estimated that 85% of serum 25(OH)D is used by tissues for its activation to calcitriol, whose local production is substrate-dependent28 and independent of the action of PTH, FGF-23 and calcitriol itself, as occurs with renal 1α-OHase. Many of the non-classical effects of vitD, including its actions on arterial and renal function, and its immunomodulatory and anti-inflammatory effects, are attributed to this autocrine/paracrine system. Paricalcitol also has pleiotropic effects beyond PTH reduction.8 It seemed plausible, therefore, that administration of cholecalciferol or paricalcitol to patients with CKD would improve arterial function and consequently reduce albuminuria.
As in other studies,3 we found a high prevalence of vitD deficiency/insufficiency in CKD patients; 82% in our study. Contributing to this deficiency are the frequent dietary restrictions in these patients that affect nutritional vitD intake, the progressive loss of megalin that mediates tubular uptake of filtered calcidiol bound to vitDBP, a loss that is greater in the presence of proteinuria,29,30 and the high values of FGF23 in CKD, which induce 24-hydroxylase activity, promoting calcitriol and calcidiol catabolism.5
Effect on central haemodynamics and arterial stiffnessWith all the forms of vitD therapy we observed as the only haemodynamic effect a significant decrease in bPP and cPP. bSBP and cSBP decreased in all vitD-treated groups, but the reduction only reached statistical significance in the paricalcitol-treated group. Although a direct significant correlation between changes in BP and changes in aortic stiffness was evident, the decrease in SBP and PP do not appear to be solely mediated by changes in Pvc-f, as the latter decreased non-significantly. It is possible that vitD reduces SBP due to its inhibitory effect on renin synthesis31 exerting synergy with the RAS blockers our patients were receiving and its beneficial action on endothelial function.32
There are very few studies on the effect of vitD on central haemodynamics in CKD. In 2 studies with a very small number of subjects with CKD and vitD deficiency, the administration of cholecalciferol and paricalcitol was not associated with a reduction in central SBP or PP.11,19 The difference in the number of patients, a shorter follow-up time, and the exclusion of subjects with DM may explain the differences with our results.
In our study, the administration of any form of vitD was associated with a non-significant decrease in Pvc-f. The correlation between the observed decrease in SBP and PP, and that of Pvc-f, confirms the interdependence of these variables. The observation that in the paricalcitol group the decrease in Pvc-f and Pvc-fi was quantitatively greater than in the other groups, and close to statistical significance, suggests an additional effect of paricalcitol on aortic stiffness independent of changes in BP. Paricalcitol may have added vascular protective effects mediated by a decrease in vascular calcium and phosphorus deposition (a relevant factor in aortic stiffness), factors promoting osteoblastic transformation of vascular smooth muscle cells33,34 and PTH, and induction of klotho, which has protective effects on the vessel,35 among others. The greater increase in calcium that we observed in the group that received paricalcitol could attenuate its possible benefits on vascular stiffness.
To our knowledge, only one study with cholecalciferol in relatively young subjects with CKD and normal baseline aortic stiffness (baseline Pvc-f: 8m/s) and another with paricalcitol involving 74-year-old subjects with CKD and high baseline Pvc-f (11.8m/s) showed a significant decrease in Pvc-f.12,14 It is possible that the clinical differences in the patients included, the higher dose of cholecalciferol and the longer duration of treatment with paricalcitol in these studies contribute to the differences compared to the present study. Other studies did not show any benefit of cholecalciferol and paricalcitol on aortic stiffness in subjects with CKD and none compared the 2 forms of vitD.11,36 In any case, it is reasonable to believe, given the magnitude of aortic calcification found in our study, it is difficult, at least in the medium term, to reverse the increase in aortic stiffness despite the effects proposed as mediators of the vascular benefits of any form of vitD (decrease in PTH, anti-inflammatory action, direct effect on aortic calcification, anti-inflammatory effect, direct effect on aortic stiffness), anti-inflammatory action, direct effect on vascular smooth muscle fibre, improved endothelial function, activation of matrix Gla protein which inhibits vascular calcification, decreased expression of osteogenic factors in vascular smooth muscle fibre, among others).
Effect on albuminuriaCompared to the untreated group, a significant reduction in albuminuria was observed, which was of a similar magnitude in the groups receiving either form of vitD. In a systematic review of randomised studies37 vitD analogues in CKD subjects induced a decrease in residual proteinuria of 16%. In our study the decrease in albuminuria in the cholecalciferol and paricalcitol treated group was 17% and 21%, respectively, compared to a 19% increase in the control group. Although baseline albuminuria was only moderately elevated in our patients (treated with RAS blockers), the reduction observed with vitD therapy may be clinically relevant. We observed a reduction of ≥30% in albuminuria in a considerable percentage of subjects treated with one of the forms of vitD. A decrease in albuminuria of this magnitude is associated, in diabetic and non-diabetic CKD subjects, with a reduction in cardiovascular events and CKD progression.38
Several studies that have analysed the effect of different forms of vitD on proteinuria/albuminuria in non-dialysis-treated CKD subjects15–20 have recorded varying results, with significant reductions in albuminuria in some and no effect in others. It is possible that factors such as underlying renal disease and coexisting comorbidities, additional therapy with RAS blockers, follow-up time, baseline 25(OH)D values, the dose used of the various forms of vitD, and differences in sodium intake, among others, contribute to this variety of response, although none compare the effects of administration of nutritional vitD and paricalcitol.
Compared to other studies, the dose of cholecalciferol we used can be considered moderate-low, with 51% of subjects having normal 25(OH)D values at the end of the study. The dose of paricalcitol was also low-moderate. Nevertheless, and despite the fact that almost all of our patients received RAS blocker therapy, we observed a significant decrease in albuminuria.
The mechanism by which the different forms of vitD induce a decrease in albuminuria is unclear. Anti-inflammatory and antifibrogenic effects, modulation of nitric oxide and RAS, prevention of protein loss from the inter-podocyte slit diaphragm and podocytes, modification of renal haemodynamics, and synergy with RAS blockers with decreased hyperfiltration, among others, have been proposed.15,17,19,39
In our study, uCRP values did not change with therapy with any of the forms of vitD and there was no relationship between changes in uCRP and albuminuria. A decrease in eGFR could decrease the filtered albumin load and albuminuria. However, this is unlikely because, on the one hand, there was no correlation between changes in eGFR and albuminuria and, on the other, eGFR decreased in the control group with no significant change in albuminuria and was unchanged in the paricalcitol group where albuminuria was reduced. There not being a significant decrease in eGFR in our study in the paricalcitol group suggests a possible protective role of paricalcitol on eGFR, an effect that cholecalciferol would not have. Experimental data show that paricalcitol has renal anti-fibrotic effects mediated by its inhibitory action on RAS, inflammation, and epithelial-mesenchymal transition.40 An additional nephroprotective effect of paricalcitol versus cholecalciferol is also indicated by the higher percentage of patients achieving a reduction in albuminuria ≥30% which was quantitatively greater and close to statistical significance (p=.054) in the paricalcitol-treated group.
Despite clinical and experimental studies to establish the metabolic molecular basis and mediators of the antialbuminuric effect of vitD we were not able to establish a clear mechanism and no previous study has investigated the possible involvement of vitD-induced changes in central haemodynamics and arterial stiffness in its antialbuminuric effect.
In our study, at baseline, we observed a significant direct correlation between albuminuria and various arterial function parameters such as bSBP, cSBP, and arterial stiffness, which would reflect the association between systemic and glomerular haemodynamics. An increase in cSBP in a stiff aorta would allow the transmission of this pressure to the glomerulus, favouring proteinuria, especially in the presence of impaired renal autoregulation as occurs in nephropathies. However, although we observed a decrease in bPP and cPP in the groups treated with native and active vitD, there was no association between the changes in these arterial pulsatility parameters and the decrease in albuminuria. Neither in ANCOVA analysis nor in logistic regression were changes in central haemodynamics and aortic stiffness consistently involved in the observed changes in albuminuria. However, despite the absence of statistical association, it cannot be ruled out that reduction in SBP and PP and changes in Pvc-f contribute to the reduction in albuminuria. The observation that the paricalcitol group, compared to the cholecalciferol group, showed a quantitatively greater decrease in Pvc-f and a higher percentage of subjects with a reduction in albuminuria ≥30% (close to statistical significance, p=.054) suggests some possible involvement of changes in arterial function in those in albuminuria.
Effects on phospho-calcium metabolismVitD administration in CKD is primarily aimed at controlling secondary hyperparathyroidism (HPT). All forms of vitD induced an increase in calcaemia compared to the control group, which was greater in the paricalcitol-treated group. While PTH increased by 35% in the control group, it decreased in those treated with vitD, but did not reach statistical significance in those treated with cholecalciferol. Studies of the effect of cholecalciferol on PTH values in subjects with CKD have yielded inconsistent results, with decreases in some cases17 and no significant changes in others.41 Differences in the dose and duration of vitD supplementation may explain these different results. Adequate doses of cholecalciferol are required to achieve 25(HO)D values to maintain appropriate amounts of calcitriol and prevent HPT. In our study, although the cholecalciferol dose used was insufficient to achieve effective vitD values for significant PTH reduction after 7 months, a significant decrease in PTH was observed compared to the control group (−7.5% vs. 35%, p=.001).
In addition to a significant decrease in PTH in the paricalcitol group, in our study we observed a significant decrease in 25(OH)D values in the paricalcitol-treated group. Other studies have shown a variable effect of paricalcitol on 25(OH)D values.16,35,42 The decrease observed in our study may be due to the increase in FGF23 that has been observed after paricalcitol therapy.21,42 In addition to inhibiting 1-αOHase activity, FGF23 increases the expression of 24-OHase, which promotes the catabolism of 1,25(OH) 2D and 25(OH)D.5
Limitations and strengths of the studyOur study has limitations. Although it is a prospective study, group allocation was not randomised and was based on biochemical criteria of vitD and PTH values. It would be unethical to randomise and assign, for example, vitD therapy to a subject with normal vitD values without PTH. Due to the categorisation criteria, there is a difference in the baseline vitD and PTH values between the groups. However, the adjustment to the baseline values of these parameters neutralises their possible effect on the results obtained in changes in arterial function and albuminuria. The follow-up time of 7 months, although longer than in other studies, is not very long, and the dose of nutritional vitD was insufficient to normalise serum 25(OH)D values in all subjects. Moreover, although a low sodium diet was prescribed in all our patients, sodium intake was not strictly controlled. Sodium restriction enhances the antiproteinuric effect not only of RAS blockers, but also of VDR activators.
Our study also has strengths. It is a prospective study comparing 2 therapy groups with various forms of vitD (cholecalciferol and paricalcitol) and a control group in patients with stage 3–4 CKD and similar clinical characteristics. Central haemodynamics and aortic stiffness were studied using a rigorous methodology, considered standard, and the impact of changes in these on changes in albuminuria was analysed for the first time.
ConclusionsOur study concludes that in patients with stage 3–4 CKD, treated with RAS blockade and with residual albuminuria, the administration of moderate doses of nutritional vitD and paricalcitol decreases brachial and central SBP and PP, and reduces albuminuria. Changes in central haemodynamics and aortic stiffness are not involved, at least substantially, in this antialbuminuric effect.
Because therapy with the active vitD analogue (paricalcitol) is associated with a greater increase in calcium/phosphorus and a greater reduction in PTH, with no clear additional benefits on arterial function and albuminuria compared to cholecalciferol, the suggestion of the KDIGO guidelines is reinforced that in patients with stage 3–4 CKD, vitD deficiency/insufficiency should be corrected using nutritional vitD with the same strategies recommended for the general population, and reserve VDR activators for cases of severe and progressive HPT.43
Ethical considerationsAll patients gave their informed consent for the study, which met all the ethical criteria of the institution where it was performed.
FundingNo funding was provided by any institution for the study.
Conflict of interestsThe authors have no conflict of interests to declare.