Patients with heart disease frequently have renal dysfunction manifested by a decrease in glomerular filtration rate (GFR) and/or increase of albuminuria.
ObjectivesThe objective was to study the possible role of increased aortic stiffness in the presence and extent of coronary artery disease (CAD) and kidney dysfunction in a group of patients with suspected CAD.
Patients and methodsWe studied forty-eight patients undergoing coronariography for suspected coronary disease (CAD). Using applanation tonometry on the radial artery and applying a transfer function, central blood pressure values were calculated. The study of aortic stiffness was done by determining the carotid-femoral pulse velocity (Pvc-f).
ResultsOf the 48 patients, 11 had no significant coronary lesions, 24 showed significant lesions in 1 or 2 coronary arteries and 13 in ≥ 3 arteries.
The group with a higher degree of CD had significantly higher cPP values than the group without CD. The Pvc-f increased progressively and significantly with the degree of CD. The logistic regression showed that Pvc-f independently predicted the presence of CD. The relative risk of CD increased 2.5 times for each meter of increase in Pvc-f. The GFR was negatively and significantly correlated with age and Pvc-f was associated with albuminuria.
ConclusionsIn patients with stable CD, Pvc-f, expression of aortic stiffness, is independently associated with the existence of CD and its degree of extension. The increase in arterial stiffness also participates in the decrease in GFR and in the increase in albuminuria.
Con frecuencia, los pacientes con cardiopatía tienen disfunción renal manifestada por descenso del filtrado glomerular (FG) y/o aumento de la albuminuria.
ObjetivosEl objetivo fue estudiar el papel del aumento de la rigidez aórtica en la presencia y extensión de la enfermedad coronaria (EC) y en la disfunción renal en sujetos con EC.
Pacientes y métodosEstudio observacional transversal de 48 pacientes con sospecha de EC sometidos a coronariografía. Mediante tonometría de aplanamiento sobre la arterial radial y aplicando una función de transferencia se calcularon los valores de presión arterial central. El estudio de la rigidez aórtica se hizo mediante la determinación de la velocidad de pulso carótida-femoral (Vpc-f).
ResultadosDe los 48 pacientes, 11 no tenían lesiones coronarias significativas, 24 evidenciaron lesiones significativas en una o dos arterias coronarias y 13 en ≥ tres arterias.
El grupo con mayor grado de EC tenía valores de presión de pulso central (PPc) más altos que el grupo sin EC. La Vpc-f aumentaba de forma progresiva y significativa con el grado de EC. La regresión logística mostraba que la VPc-f predecía de forma independiente la presencia de EC. El FG se correlacionaba de forma negativa y significativa con la edad. La Vpc-f se asociaba a la albuminuria.
ConclusionesEn pacientes con EC estable, la Vpc-f se asocia de forma independiente con la existencia y extensión de la EC y se relaciona con la disminución del FG y el aumento de la albuminuria.
Heart disease (HD) and chronic kidney disease (CKD) are both highly prevalent.1,2 The association between heart and kidney diseases is common. Patients with cardiovascular disease (CVD) very frequently present renal dysfunction,3 and those with any manifestation of CKD, whether decreased glomerular filtration rate (GFR) and/or increased urinary albumin excretion, are at higher risk of CVD.4 This association is so consistent that the term “cardiorenal syndrome” has been coined to define cardiac disturbances secondary to renal dysfunction and renal abnormalities resulting from cardiac problems, or dysfunction of both organs with a common cause.5
This relationship between heart disease and kidney disease may be due to several factors. Both may result from the effect of common traditional vascular risk factors (ageing, hypertension [HTN], diabetes mellitus [DM], dyslipidaemia, among others). In moderate-severe CKD with decreased GFR, non-traditional risk factors (oxidative stress, pro-inflammatory mediators, alterations in phosphocalcic metabolism, among others) can also induce cardiovascular (CV) disorders.4 There may also be proinflammatory mediators and neurohormonal stimuli in the context of heart disease that promote kidney dysfunction.5
Structural and functional impairment of the large arteries is one of the pathogenic mechanisms that may underlie combined heart and kidney damage. Both traditional vascular risk factors and phenomena generated by heart and kidney dysfunction may contribute to vascular atherosclerosis with increased arterial stiffness and secondary haemodynamic disturbances with increased pulsatile mechanical load, which may contribute to heart and kidney damage.4–7
The aim of this study was to investigate the possible role of disturbances in aortic function and stiffness in the presence and extension of coronary lesions and renal function in a group of patients with suspected HD undergoing coronary angiography.
Material and methodsForty-eight subjects were studied who were admitted for scheduled coronary angiography, prescribed by a cardiologist, for probable HD based on age, sex, symptoms, and other cardiac tests. Patients admitted for acute coronary syndrome were excluded from the study. People with myocardial infarction and/or revascularisation that occurred at least one year prior to the study could be included. All patients gave their informed consent for coronary angiography and other non-invasive arterial haemodynamic and laboratory tests. The study met all the ethical criteria of the institution where it was conducted.
Fasting blood was taken for laboratory testing (haemoglobin, creatinine, lipid profile, inflammation markers, uric acid, glycaemia, homeostasis model assessment-insulin resistance index (HOMA-IR) prior to coronary angiography. The patients’ kidney function was assessed by estimated glomerular filtration rate (eGFR), obtained using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula,8 and the albumin/creatinine ratio in first morning urine.
Non-invasive haemodynamic studiesTwelve hours before the coronary angiography, after 15 min rest in the supine position, brachial blood pressure (BP) was measured (an average of three readings) using an Omron M3 IT blood pressure monitor (Omron electrónica Iberia S.A.U., Madrid).
Central BP (cBP) was taken from the same arm as the bBP. The pulse wave was obtained in the radial artery by applanation tonometry using a micromanometer (Millar instruments) connected to a SphygmoCor device (AtCor Medical, Sydney, Australia). The bBP readings were used to calibrate the system. The device captures a series of waves for 10 s; these are digitised at 128 Hz and assembled into an average wave. The system uses a transfer function to convert the radial pressure wave to aortic (central) pressure. Only readings with a quality index, given by the device itself, higher than 85% were accepted. The value of the central parameters was considered the average of those from two readings with a good quality index. From the central pressure wave, we obtained the central systolic pressure (cSBP), the central diastolic blood pressure (cDBP), the central mean arterial pressure (cMAP), the central pulse pressure (cPP) (difference between cSBP and cDBP) and the augmentation index (AI) (difference between the first and second systolic peak), which is expressed as a percentage of cPP. Since the AI is influenced by heart rate (HR), the system provides the AI value normalised to an HR of 75 bpm (AI75).
The same SphygmoCor device was used to determine the carotid-femoral pulse wave velocity (c-fPWV). The pulse wave was obtained by sequential applanation tonometry over the common carotid and femoral artery. The transit time between the two arterial sites was calculated from the difference between the R-wave of the simultaneous electrocardiographic recording and the onset of the pulse wave at the respective arterial sites. The distance between the two arterial points was carefully measured with a tape measure by subtracting the distance between the carotid point and the jugular notch of the sternal manubrium from the distance between the latter and the femoral point. The c-fPWV was calculated from the distance travelled by the pulse wave (m) divided by the delay time (s) between the two arterial points. Thus, c-fPWV is expressed in metres per second (m/s).
The resulting c-fPWV values were compared with the European Society of Cardiology (ESC) reference values observed in 16,867 subjects, which consider age and BP, and which use c-fPWV values obtained by direct measurement of the distance between the carotid and femoral artery.9 Therefore, the subtracted distance using our method was converted to direct distance using the equation10: direct distance = (.45 × subtracted distance) + (.21 × height) + .08. Since the use of direct distance overestimates c-fPWV (when obtained by invasive methods or by magnetic resonance imaging [MRI]), the c-fPWV value was multiplied by .8.11 The c-fPWV index was obtained from the resulting c-fPWV and the reference c-fPWV according to the SEC (c-fPWVi) = (measured c-fPWV – reference c-fPWV) ÷ (reference c-fPWV) × 100.
The theoretical c-fPWV index (theoretical c-fPWVi) was also calculated, which considers other variables (age, sex, BP, and HR) that influence c-fPWV. c-fPWVi = (measured c-fPWV − theoretical c-fPWV) (theoretical c-fPWV) × 100, where theoretical c-fPWV (m/s) = (.0793 x age) + (.0427 × MAP [mean arterial pressure]) − (.0014 × cardiac period [ms]) − (.415 × sex) + 2.934 (sex = 1 male; 2 female).12
Using the same technique (except for the points to determine distance), carotid-radial pulse wave velocity (c-rPWV) was determined as an expression of peripheral muscular artery stiffness. The c-fPWV/c-rPWV ratio was calculated to assess the central/peripheral arterial stiffness gradient.13
The tonometry to determine central haemodynamics, c-fPWV and c-rPWV was always performed by the same investigator (PGF).
Coronary angiographyTwelve to 14 h following hospital admission, all the patients underwent coronary angiography via the radial or femoral route. All the angiograms were viewed by an experienced cardiologist-haemodynamicist (JOO) who did not know the results of the non-invasive haemodynamic studies. Significant HD was defined as the existence of at least one lesion with stenosis ≥60%. The extent of HD was defined by the number of arteries with significant obstruction, considering the three major coronary arteries: left anterior descending, circumflex, and right coronary artery. A significant lesion in the left main coronary artery was considered involvement of two arteries.
Statistical analysisCategorical variables are expressed as absolute and relative frequencies. Quantitative variables as mean ± standard deviation or as median (interquartile range [IQR]), depending on the distribution according to the Shapiro–Wilk test. In some cases, variables that were not normally distributed were converted into their logarithms. The X2 test was used to compare qualitative variables between groups. Analysis of variance (ANOVA) with Tukey’s test for post hoc multiple comparisons was used to contrast normally distributed continuous variables. The Kruskal–Wallis test was used for variables that were not normally distributed. The relationship between variables was analysed using Pearson’s or Spearman’s correlation coefficient according to the distribution of the variables. The independent relationship between variables was investigated by multiple linear regression, selecting potential covariates that previously showed significant correlation. Stepwise logistic regression was used to assess the independent effects of arterial stiffness parameters on CD risk. A p-value of p < .05 was considered significant. All statistical analyses were performed with IBM SPSS statistics version 25 for Windows.
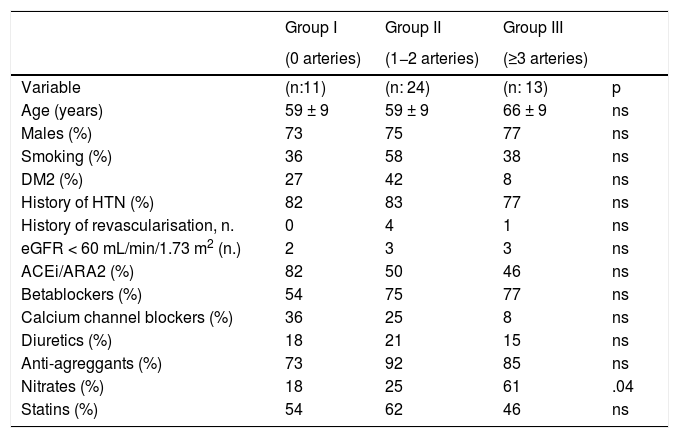
ResultsThe mean age of the subjects studied was 61 ± nine years, 64% were male. Of the 48 patients, 11 (23%) had no significant coronary lesions (group I), 24 (50%) showed significant lesions in one or two coronary arteries (group II) and 13 (27%) in ≥three arteries (group III) (Table 1). There were no significant differences between the three groups with respect to age, sex, prevalence of HT, DM2, smoking or kidney failure. The percentage of patients in group III receiving nitrate therapy was higher than in the other groups.
Clinical parameters in the groups classified by the number of coronary arteries with significant lesions.
| Group I | Group II | Group III | ||
|---|---|---|---|---|
| (0 arteries) | (1−2 arteries) | (≥3 arteries) | ||
| Variable | (n:11) | (n: 24) | (n: 13) | p |
| Age (years) | 59 ± 9 | 59 ± 9 | 66 ± 9 | ns |
| Males (%) | 73 | 75 | 77 | ns |
| Smoking (%) | 36 | 58 | 38 | ns |
| DM2 (%) | 27 | 42 | 8 | ns |
| History of HTN (%) | 82 | 83 | 77 | ns |
| History of revascularisation, n. | 0 | 4 | 1 | ns |
| eGFR < 60 mL/min/1.73 m2 (n.) | 2 | 3 | 3 | ns |
| ACEi/ARA2 (%) | 82 | 50 | 46 | ns |
| Betablockers (%) | 54 | 75 | 77 | ns |
| Calcium channel blockers (%) | 36 | 25 | 8 | ns |
| Diuretics (%) | 18 | 21 | 15 | ns |
| Anti-agreggants (%) | 73 | 92 | 85 | ns |
| Nitrates (%) | 18 | 25 | 61 | .04 |
| Statins (%) | 54 | 62 | 46 | ns |
ACEi: angiotensin-converting enzyme inhibitors; ARA2: angiotensin II receptor antagonists; DM2: diabetes mellitus type 2; eGFR: estimated glomerular filtration rate; HTN: hypertension; ns: not significant.
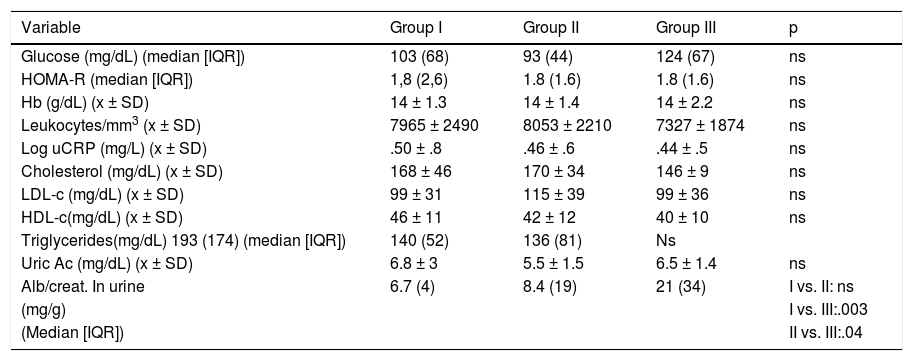
No significant intergroup differences were observed in lipid profile, eGFR, inflammation markers or insulin resistance. Albuminuria excretion, however, was significantly higher in group III (Table 2). Only four subjects in group II and five in group III had albumin/creatinine levels ≥30 mg/g.
Laboratory parameters.
| Variable | Group I | Group II | Group III | p |
|---|---|---|---|---|
| Glucose (mg/dL) (median [IQR]) | 103 (68) | 93 (44) | 124 (67) | ns |
| HOMA-R (median [IQR]) | 1,8 (2,6) | 1.8 (1.6) | 1.8 (1.6) | ns |
| Hb (g/dL) (x ± SD) | 14 ± 1.3 | 14 ± 1.4 | 14 ± 2.2 | ns |
| Leukocytes/mm3 (x ± SD) | 7965 ± 2490 | 8053 ± 2210 | 7327 ± 1874 | ns |
| Log uCRP (mg/L) (x ± SD) | .50 ± .8 | .46 ± .6 | .44 ± .5 | ns |
| Cholesterol (mg/dL) (x ± SD) | 168 ± 46 | 170 ± 34 | 146 ± 9 | ns |
| LDL-c (mg/dL) (x ± SD) | 99 ± 31 | 115 ± 39 | 99 ± 36 | ns |
| HDL-c(mg/dL) (x ± SD) | 46 ± 11 | 42 ± 12 | 40 ± 10 | ns |
| Triglycerides(mg/dL) 193 (174) (median [IQR]) | 140 (52) | 136 (81) | Ns | |
| Uric Ac (mg/dL) (x ± SD) | 6.8 ± 3 | 5.5 ± 1.5 | 6.5 ± 1.4 | ns |
| Alb/creat. In urine | 6.7 (4) | 8.4 (19) | 21 (34) | I vs. II: ns |
| (mg/g) | I vs. III:.003 | |||
| (Median [IQR]) | II vs. III:.04 |
Alb/creat.: albumin/creatinine; CKD-EPI: Chronic Kidney Disease Epidemiology; Hb: haemoglobin; HOMA-R: homeostatic model assessment insulin resistance; ns: not significant; uCRP: ultrasensitive C-reactive protein; IQR: interquartile range; x ± SD: mean ± standard deviation.
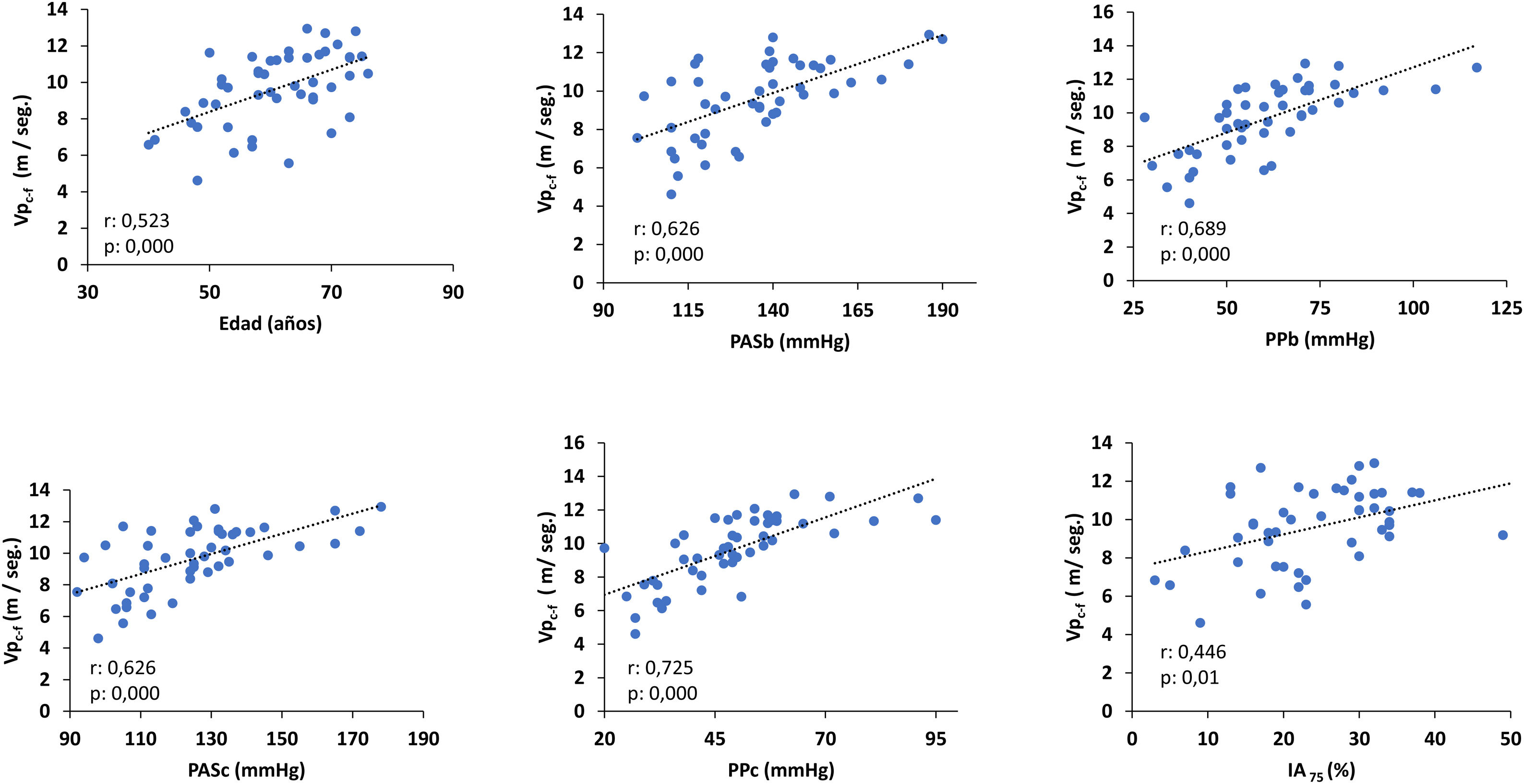
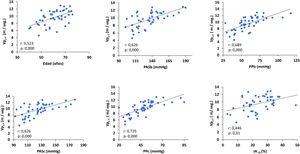
The c-fPWV correlated directly and significantly with age, brachial systolic blood pressure (bSBP), brachial blood pressure (bBP), cSBP, cPP and AI75 (Fig. 1). Only cPP and age were significant predictors of c-fPWV (regression coefficient ± SE: .09 ± .01; standardised ß: .72, p: .000 and .76 ± .02; ß: .35, p: .000, respectively).
Correlation of c-fPWV with age and arterial parameters.
AI75: augmentation index normalised to a heart rate of 75 bpm; bSBP: brachial systolic blood pressure; cSBP: central systolic blood pressure; bPP: brachial pulse pressure; cPP: central pulse pressure; c-fPWV: carotid femoral pulse wave velocity.
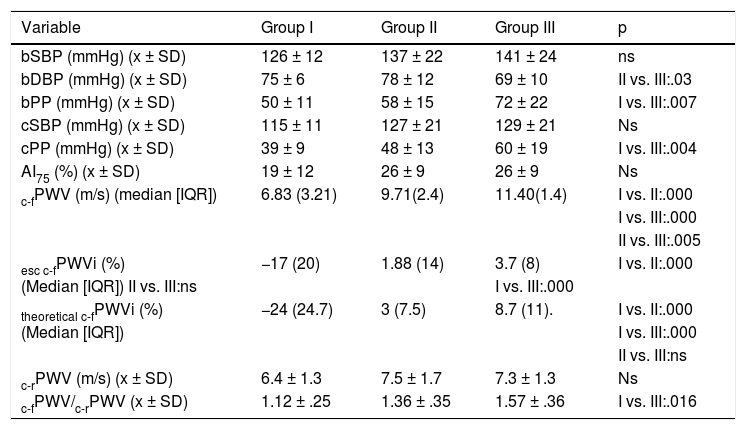
Table 3 shows the arterial function parameters in the three groups. Both bSBP and cSBP were quantitatively higher in the groups with more severe HD, although without reaching statistical significance. The group with more severe HD had much higher bPP and cPP values than the group without HD. c-fPWV and the c-fPWV indices increased progressively and significantly with the degree of HD. There was no difference between groups in c-rPWV, but the central arterial stiffness/peripheral arterial stiffness gradient (c-fPWV/c-rPWV) was higher in group III.
Arterial function parameters.
| Variable | Group I | Group II | Group III | p |
|---|---|---|---|---|
| bSBP (mmHg) (x ± SD) | 126 ± 12 | 137 ± 22 | 141 ± 24 | ns |
| bDBP (mmHg) (x ± SD) | 75 ± 6 | 78 ± 12 | 69 ± 10 | II vs. III:.03 |
| bPP (mmHg) (x ± SD) | 50 ± 11 | 58 ± 15 | 72 ± 22 | I vs. III:.007 |
| cSBP (mmHg) (x ± SD) | 115 ± 11 | 127 ± 21 | 129 ± 21 | Ns |
| cPP (mmHg) (x ± SD) | 39 ± 9 | 48 ± 13 | 60 ± 19 | I vs. III:.004 |
| AI75 (%) (x ± SD) | 19 ± 12 | 26 ± 9 | 26 ± 9 | Ns |
| c-fPWV (m/s) (median [IQR]) | 6.83 (3.21) | 9.71(2.4) | 11.40(1.4) | I vs. II:.000 |
| I vs. III:.000 | ||||
| II vs. III:.005 | ||||
| esc c-fPWVi (%) | −17 (20) | 1.88 (14) | 3.7 (8) | I vs. II:.000 |
| (Median [IQR]) II vs. III:ns | I vs. III:.000 | |||
| theoretical c-fPWVi (%) | −24 (24.7) | 3 (7.5) | 8.7 (11). | I vs. II:.000 |
| (Median [IQR]) | I vs. III:.000 | |||
| II vs. III:ns | ||||
| c-rPWV (m/s) (x ± SD) | 6.4 ± 1.3 | 7.5 ± 1.7 | 7.3 ± 1.3 | Ns |
| c-fPWV/c-rPWV (x ± SD) | 1.12 ± .25 | 1.36 ± .35 | 1.57 ± .36 | I vs. III:.016 |
AI75: augmentation index standardised to a heart rate of bpm.; bDBP: brachial diastolic blood pressure; bPP: brachial pulse pressure; bSBP: brachial systolic blood pressure; cPP: central pulse pressure; cSBP: central systolic blood pressure; c-fPWV: carotid-femoral pulse wave velocity; c-fPWVi: c-fPWV index; c-rPWV: carotid-radial pulse wave velocity; IQR: interquartile range; ns: not significant; esc: European Society of Cardiology; x ± SD: mean ± standard deviation.
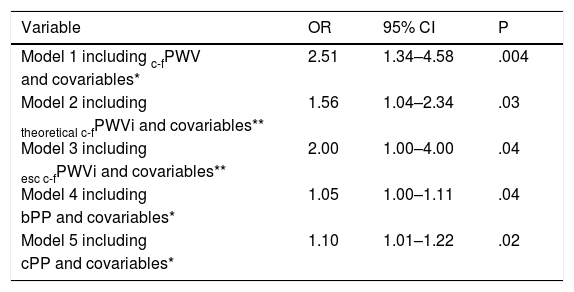
After adjustment for covariates such as age, sex, smoking, MAP, low-density lipoprotein (LDL-c), DM2 and CKD-EPI, c-fPWV independently predicted the presence of HD. The relative risk of HD increased 2.5-fold for each metre increase in c-fPWV (Table 4). Although cPP increased the risk of HD, this effect disappeared when c-fPWV was incorporated into the model. Both theoretical c-fPWVi and sec c-fPWVi independently predicted HD, but with lower power than c-fPWV (Table 4).
Predictors of heart disease.
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Model 1 including c-fPWV | 2.51 | 1.34–4.58 | .004 |
| and covariables* | |||
| Model 2 including | 1.56 | 1.04–2.34 | .03 |
| theoretical c-fPWVi and covariables** | |||
| Model 3 including | 2.00 | 1.00–4.00 | .04 |
| esc c-fPWVi and covariables** | |||
| Model 4 including | 1.05 | 1.00–1.11 | .04 |
| bPP and covariables* | |||
| Model 5 including | 1.10 | 1.01–1.22 | .02 |
| cPP and covariables* |
bPP: brachial pulse pressure; c-fPWV: carotid-femoral pulse wave velocity; c-fPWVi: carotid-femoral pulse wave velocity index; CI: confidence interval; cPP: central pulse pressure; sec: OR: odds ratio; esc: European Society of Cardiology.
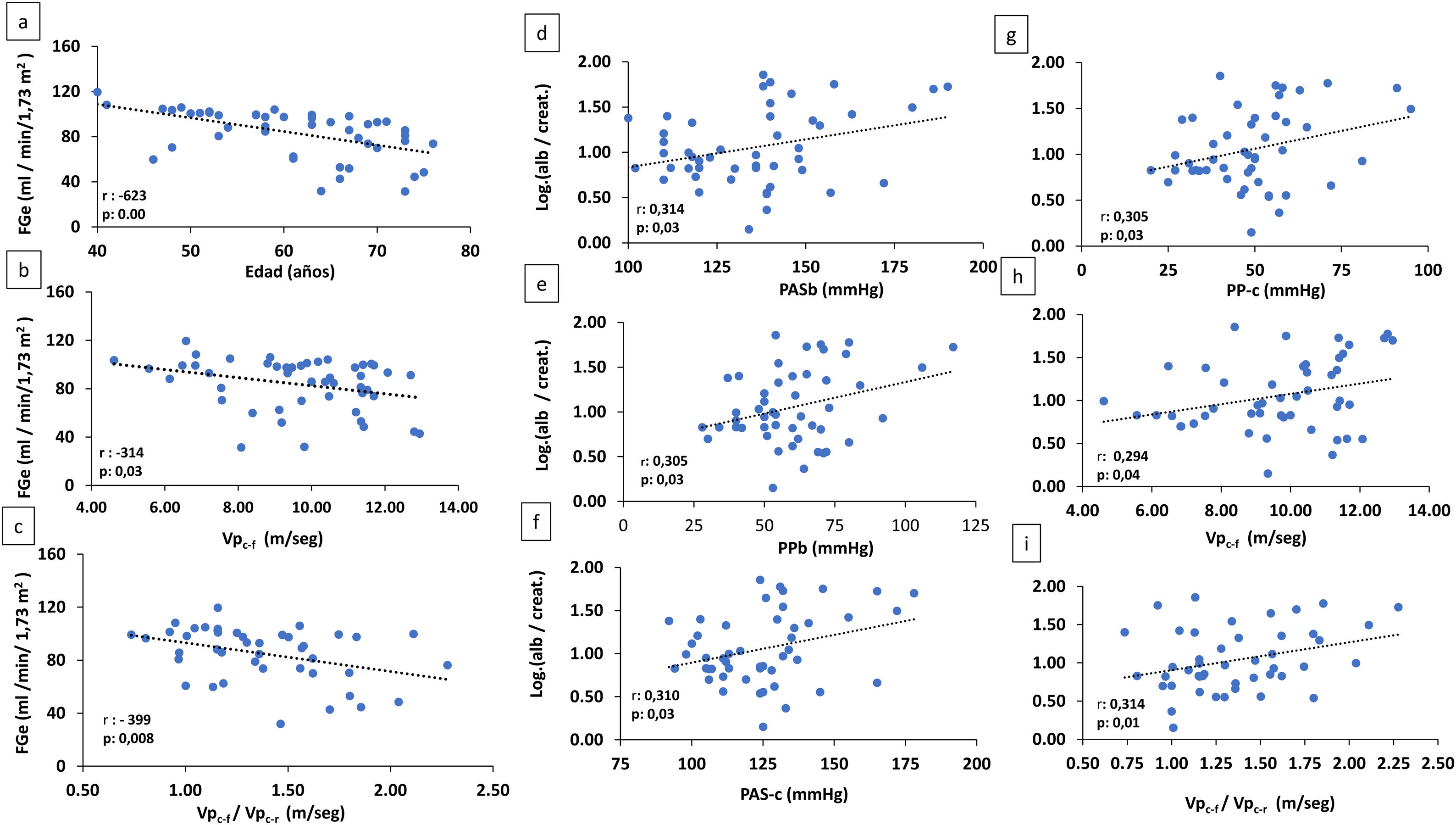
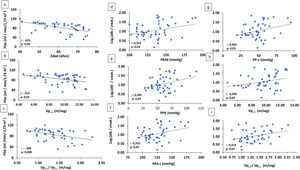
In the combined analysis of all the patients, eGFR correlated negatively and significantly with age (r: −623, p: .000), c-fPWV (r: −314, p: .03) and the c-fPWV/c-rPWV ratio (r: −399, p: .008) (Fig. 2).
(A–C) Correlation of eGFR with age and arterial stiffness parameters (c-rPWV: carotid radial pulse wave velocity) and c-fPWV/c-rPWV ratio. (D–I) Correlation of log alb./creat. With arterial parameters.
alb./creat.: albumin/creatinine in urine; eGFR: estimated glomerular filtration rate; bSBP: brachial systolic blood pressure; cSBP: central systolic blood pressure; bPP: brachial pulse pressure; cPP: central pulse pressure; c-fPWV/c-rPWV: carotid-femoral pulse wave velocity/carotid-radial pulse wave velocity.
There was a significant positive correlation between albuminuria and bSBP (r: .314, p: .03), bPP (r: .305, p: .03), cSBP (r: .310, p: .03), cPP (r: .305, p: .03) and c-fPWV (r: .294, p: .04) and the c-fPWV/c-rPWV ratio (r: .314, p: .01) (Fig. 2).
In the multiple linear regression analysis, the significant predictive power of c-fPWV on eGFR was lost when age, which was an independent predictor of eGFR, was incorporated into the model.
c-fPWV was associated with albuminuria (ß: .29, p: .04), but the relationship disappeared when arterial parameters conditioned by increased arterial stiffness (bSBP, cSBP, bPP and cPP), which did have the capacity to predict albuminuria, were introduced into the model.
The c-fPWV/c-rPWV gradient was associated with albuminuria independently of bSBP, cSBP, bPP and cPP (coefficient ß: .31, p: .03). The predictive capacity of these disappeared when the stiffness gradient was incorporated into the model.
Relationship between arterial function and the combination of heart disease (HD) and kidney disease (KD)To study this relationship, KD was defined as the presence of GFR < 60 mL/min/1.73 m2 and/or a urine albumin/creatinine ratio ≥30 mg/g. Four groups were defined: no HD and no KD (HD−/KD−); presence of HD and no KD (HD+/KD−); no HD and presence of KD (HD−/KD+); presence of HD and KD (HD+/KD+). c-fPWV in the HD+/KD+ (n: 12) (11.1 ± 1.6 m/s) and HE+/KD− (n: 25) (9.9 ± 1.4 m/s) groups was significantly higher (p: .000) than that of the HD−/KD− (n: 9) group (6.9 ± 1.7 m/s). c-fPWV in the HD−/KD + group was quantitatively higher (9.5 ± 0.4) than in the HD−/KD− group, but the low numerical representation of the latter (n: 2) does not allow statistical comparisons.
DiscussionThe main finding of our study is that in a group of patients with suspected stable HD referred for coronary angiography, c-fPWV is higher the more extensive the HD and also independently predicts the presence of significant coronary lesions. On the other hand, in patients with HD, an increase in c-fPWV and in central stiffness/peripheral stiffness gradient may also contribute to a decrease in eGFR and increase in albuminuria induced, respectively, by age and arterial parameters related to increased central stiffness (brachial and central SBP and PP).
c-fPWV is considered the reference method to determine aortic stiffness.14 Arterial stiffness is considered a structural and functional marker that can incorporate the effect of all known traditional vascular risk factors and is a predictor of mortality and CV events.15,16
In our study, non-indexed c-fPWV was significantly and progressively higher in subjects with more extensive HD. It is noteworthy that this occurred even though group III, with lesions in three or more arteries, were treated most with nitrates, which can reduce arterial stiffness.17
Given that age, sex, and BP are important determinants of c-fPWV, we used the theoretical c-fPWVi and the sec c-fPWVi integrating these factors to assess the independent predictive effect of aortic stiffness on the presence of HD. Although the risk decreased relative to that obtained when unindexed c-fPWV was considered (odds ratio [OR] [95% CI]: 1.56 [1.04–2.34] and 2.00 [1.00–4.00] vs. 2.51 [1.34–4.58], respectively), its significant predictive value persisted and was also independent of the other traditional vascular risk factors. This raises the possibility that aortic stiffness recapitulates the effect of vascular exposure over time to known and possibly unknown traditional risk factors on vascular structure and function.
Other studies have demonstrated an association between c-fPWV and coronary atherosclerosis and a relationship between c-fPWV and the presence and severity of HD demonstrated by coronary angiography, as well as its independent capacity to predict HD.18–22
There are several mechanisms by which increased aortic stiffness can induce myocardial ischaemia and HD. A higher pulse rate favours early return of the reflex wave and greater AI. This results in an increase in cBP, a decrease in DBP and an increase in PP. As a result of these changes, cardiac hypertrophy and myocardial ischaemia occur, with perfusion occurring predominantly during diastole.23,24 In our study, we observed quantitatively higher AI75 and cSBP values in the HD group, but which did not reach statistical significance, probably because of the small number of cases, and a significant increase in bPP and cPP in subjects with higher HD burden.
This increased pulsatility may also contribute to atherosclerosis. A great deal of experimental evidence and clinical data support the participation of the pulsatile component of BP in the genesis of atherosclerosis. In experimental animals, increased BP favours the activation of pro-inflammatory phenomena and the influx of oxidised low-density lipoproteins into the arterial wall.25 In experimental models of atherosclerosis, cyclic distension of the arterial wall stimulates cell proliferation and lipid uptake by the vascular wall.26 Endothelial cells subjected to cyclic strain increase the expression of selectins and intercellular adhesion molecules (ICAM-1), monocyte adhesion and superoxide anion production.27,28
Other studies have reported that cPP and cPP normalised to MAP (aortic pulsatility) are associated with the presence and extent of HD even after adjustment for other risk factors.29,30 Higher PP is also associated with peripheral arterial disease independent of age, smoking, DM, and dyslipidaemia.31 In the present study, cPP and bPP were also shown to be predictors of HD, irrespective of other risk factors. Increased arterial pulsatility contributes to atherosclerosis and atherosclerosis increases arterial stiffness and PP, and thus a bidirectional relationship is established.32
Increased central arterial stiffness promotes macrovascular lesions in the coronary bed and may also induce microvascular damage at the kidney level. In our study, there was a significant inverse correlation of eGFR with age and c-fPWV. In the regression analysis, it was observed that c-fPWV had predictive power for eGFR, however, this disappeared when age was included in the model. Our data suggest that age is the main determinant of eGFR in the population studied, and its harmful effect on GFR may be due to changes in arterial stiffness inherent to ageing. It is possible, however, that the absence of an independent association between arterial stiffness and GFR in our study is conditioned by the limited representation of subjects with significant deterioration in GFR (only 16% of the patients studied had a baseline eGFR < 60 mL/min/1. 73 m2).
Increased aortic stiffness and pulsatile aortic pressure inherent to ageing or enhanced by the presence of vascular risk factors results in structural and haemodynamic changes at the renal microvascular level that may lead to ischaemia and decreased GFR. A positive association has been demonstrated between the renal resistance index measured by Doppler ultrasound and aortic stiffness, and a negative correlation with GFR.33 Therefore, increased intrarenal resistance would not only be an intrinsic kidney disease, but also a pathophysiological adaptation to increased pulsatility linked to increased aortic stiffness.
In patients with CKD with severely reduced GFR, there is a consistent increase in c-fPWV 34,35 However, in mild to moderate kidney failure, cross-sectional studies that have analysed the relationship between aortic arterial stiffness and GFR have provided varied results. Some have found an association of the two variables irrespective of other risk factors,36,37 in others, the relationship was not independent,38–40 and some have found that the magnitude of the relationship between c-fPWV and GFR decreased considerably when age was incorporated into the model (OR 1.38 [95% CI 1.33–1.43] vs. 1.13 [95% CI 1.08–1.18]).41 It is possible that the variability of results is due to differences in the populations studied and in the methods of testing for arterial stiffness, and different adjustments to covariates. Other longitudinal studies have found that increased aortic stiffness was associated with a more rapid deterioration of GFR and a higher incidence of CKD.40–44 Therefore, it can be concluded that increased aortic stiffness, either directly or as a mediator of other risk factors, including age, is associated with lower GFR.
In addition to the decrease in GFR, another parameter that defines CKD is albuminuria. In our study, we observed that patients with a higher degree of HD were those with higher urinary excretion of albumin. We found a direct correlation between albuminuria, arterial stiffness and arterial function parameters related to albuminuria, such as bSBP, cSBP, bPP and cPP. In the regression analysis, the significant association between c-fPWV and albuminuria disappeared when bSP, cSBP, bPP or cPP were included in the model, which suggests their mediation of albuminuria related to increased arterial stiffness. Increased pulsatility and transmission of SBP not buffered by a stiff aorta to the glomerulus, especially in the presence of impaired kidney autoregulation, as may occur with the coexistence of nephropathy or vascular risk factors, can induce glomerular hyperperfusion, endothelial dysfunction, increased glomerular permeability and urinary albumin excretion, and, in the long term, glomerular structural damage. In our study, we found an increased c-fPWV/c-rPWV ratio in the group with more CD. On the other hand, the c c-fPWV/c-rPWV ratio correlated positively and significantly with albuminuria and, moreover, showed an independent predictive capacity for albuminuria. A higher c-fPWV/c-rPWV would express an increase in aortic stiffness in relation to the stiffness of peripheral muscular arteries. This increased central stiffness/peripheral stiffness gradient would induce a greater transfer of pulsatile energy to smaller arteries and the renal microcirculation.13,45
Many studies have demonstrated an association between albuminuria and CV morbidity and mortality, and a relationship between albuminuria and the presence and severity of HD independent of other vascular risk factors.46–48 As we observed in the present study, other cross-sectional studies have found a link between arterial stiffness and albuminuria, even with values lower than those considered as microalbuminuria.40,49,50 This observation is important, as the relationship between CV risk and albuminuria is continuous not dichotomous.51
Our study has some weaknesses: the small number of patients that may have conditioned the statistical power of some comparisons, the study was cross-sectional, which meant we could not analyse the prognostic impact of arterial stiffness on cardiorenal events, and the fact that all the patients were taking medication that could influence arterial stiffness. These limitations may be mitigated by the fact that all the patients included had a previous diagnosis of stable HD, excluding acute coronary events, the possibility of predictive analyses of cross-sectional association and the absence of significant differences between groups in the medication taken (intake of nitrates, drugs that can reduce arterial stiffness, was higher in the group with greater HD and greater aortic stiffness).
We believe our study also has some strengths: the design prior to its execution, an arterial function analysis and coronary angiography performed by two different investigators who did not know the results of the test they did not perform, and the use of c-fPWV as a method for studying aortic stiffness. This technique is recognised as a suitable procedure to study the relationship between arterial stiffness and CV events.52
Our study suggests a dynamic interaction between aortic function and HD, and between aortic function and kidney function, and suggests that increased aortic stiffness may be one of the underlying mechanisms of macro- and microvascular damage in HD. This is supported by the finding in our study of a significantly higher quantitative c-fPWV when combined with HD and KD.
Some applications for clinical practice can also be gained from the study. c-fPWV testing could complement other myocardial ischaemia tests to reduce unnecessary coronary angiographies as has been shown in another study.22 Furthermore, c-fPWV can be considered not only a biomarker for better risk stratification of heart and kidney events, but also as a potential therapeutic target to reduce cardio-renal events. Several therapeutic agents (renin-angiotensin system blockers, statins, antialdosterone agents and sodium-glucose cotransporter inhibitors [SGLT2i], among others) have shown the capacity to reduce aortic arterial stiffness.53–56
Conclusions- 1
In patients undergoing coronary angiography for suspected stable HD, c-fPWV, an expression of aortic stiffness is independently associated with HD and is related to its extension.
- 2
Increased aortic stiffness is associated with increased arterial pulsatility parameters.
- 3
Increased arterial stiffness is involved in the decrease in eGFR that accompanies ageing and as a pathogenic mechanism underlies the increase in albuminuria induced by increased pulsatile load. This increased pulsatile load would transmit more easily to the renal microcirculation due to an increase in the central/peripheral stiffness gradient.
- 4
In patients with HD, increased aortic stiffness that could integrate the effect, over time, of known and unknown vascular risk factors may be one of the underlying mechanisms of macro- and microvascular damage.
This work has received no funding.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Hidalgo-Santiago JC, Oneto-Otero J, Michán-Doña A, Gomez-Fernández P. Papel del aumento de la rigidez arterial central en el daño macro y microvascular en pacientes con enfermedad coronaria. Clin Investig Arterioscler. 2021;33:224–234.