To investigate the frequency of hypogonadism and its relationship to inflammation and carotid intima-media thickness (CIMT) in male patients with predialysis chronic kidney disease (CKD).
MethodsA total of 105 patients with CKD, 55 (52.4%) as stage 3, 33 (31.4%) as stage 4 and 17 (16.2%) as stage 5, were enrolled into the study. Total testosterone (TT) and free testosterone (FT), interleukin 6 (IL-6), C-reactive protein (CRP) levels, and CIMT were measured.
ResultsAccording to TT and FT, hypogonadism was detected in 18 (17.1%) and 22 (20.9%) patients, respectively. There was no difference in terms of TT and FT, CIMT, CRP and IL-6 between the stages of CKD. According to TT, the patients with hypogonadism had significantly higher CRP and high-density lipoprotein cholesterol (HDL-cholesterol) levels (p=0.004 and p=0.005, respectively). There was no significant difference in other parameters. According to FT, the patients with hypogonadism had significantly higher CRP (p=0.017), and TT were negatively correlated with body mass index (BMI), waist circumference (WC), hip circumference, and CRP levels. FT was negatively correlated with age, waist circumference, systolic blood pressure, diastolic blood pressure (DBP) and CRP.
ConclusionsThe frequency of hypogonadism was found around 17–21% among the patients with CKD. Despite similar IL-6 and CIMT levels, CRP was found to be higher in the patients with hypogonadism. We consider that further studies with larger populations are needed to elucidate the entity.
Investigar la frecuencia de hipogonadismo y su relación con la inflamación y grosor de la íntima-media carotídea (CIMT) en varones con insuficiencia renal crónica (IRC) prediálisis.
MétodosSe incluyó en el estudio a un total de 105 pacientes con IRC, 55 (52,4%) en estadio 3, 33 (31,4%) en estadio 4, y 17 (16,2%) en estadio 5. Se midieron testosterona total (TT) y testosterona libre (TL), interleucina 6 (IL-6), niveles de proteína C reactiva (PCR), y CIMT.
ResultadosCon respecto a TT y TL, se detectó hipogonadismo en 18 (17,1%) y 22 (20,9%) pacientes, respectivamente. No se encontraron diferencias en términos de TT y TL, CIMT, PCR e IL-6 entre los diferentes estadios de IRC. Con respecto a TT, los pacientes con hipogonadismo tenían valores significativamente más altos de PCR y colesterol de lipoproteínas de alta densidad (HDL-colesterol) (p=0,004 y p=0,005, respectivamente). No se encontraron diferencias significativas en cuanto a otros parámetros. Con respecto a TL, los pacientes con hipogonadismo tenían valores significativamente más altos de PCR (p=0,017), y TT guardó una correlación negativa con el índice de masa corporal (IMC), perímetro de la cintura, perímetro de la cadera, y niveles de PCR. TL se correlacionó negativamente con la edad, perímetro de cintura, presión arterial sistólica (PAS), presión arterial diastólica (PAD) y PCR.
ConclusionesSe encontró frecuencia de hipogonadismo en cerca del 17-21% de los pacientes con IRC. A pesar de encontrar niveles similares de IL-6 y CIMT, los niveles de PCR fueron más altos en los pacientes con hipogonadismo. Consideramos que son necesarios más estudios, con poblaciones de mayor tamaño, para explicar esta entidad.
Chronic kidney disease (CKD) is a functional diagnosis characterized by a progressive and irreversible reduction in glomerular filtration rate (GFR), and may lead to fluid-electrolyte imbalance, metabolic disturbance and many endocrinological disorders, such as hypogonadism, infertility and sexual dysfunction.1,2 It is known that there is increased inflammation in the patients with CKD, and that an increase in the production and release of inflammatory cytokines occurs with the decrease in GFR.3 As CKD progresses over time, testicular atrophy and accordingly a decrease in testosterone production occurs, and primary hypogonadism develops.4 Low testosterone among the young or the elderly men was shown to increase the development of hypertension, type 2 diabetes mellitus (T2DM), metabolic syndrome (MetS) and cardiovascular disease (CVD), reducing lean body mass and increasing body fat mass.5–7 In addition, it was also shown in many studies that testosterone treatment has positive effects on MetS parameters and risk of atherosclerosis, and so reducing mortality due to the cardiovascular events.8-13
However, there are also studies showing that testosterone administered intramuscularly and transdermally has positive effects on glucose metabolism and indicators of endothelial dysfunction in the patients with T2DM and hypogonadism.14,15 Hypogonadism occurring in the patients with CKD may contribute to the development of atherosclerosis and inflammation. It is known that hypogonadism is seen in approximately half of the patients with non-dialysis CKD and end-stage renal disease (ESRD), and there is an inverse relationship between total testosterone (TT) and mortality rates.16-18 Based on literature, it was also shown that carotid intima media thickness (CIMT) values can be used as an early indicator of atherosclerosis.8 The aim of our study was to investigate the frequency of hypogonadism and investigate its relationship to the levels of interleukin-6 (IL-6), C-reactive protein (CRP) and CIMT in the male predialysis patients with CKD.
Material and methodsThis prospective study was conducted in the internal medicine and nephrology clinics of Konya Health Application and Research Center (previously Konya Training and Research Hospital) at University of Health Sciences, a tertiary healthcare center, between November 2014 and March 2015. A total of 105 male predialysis patients with CKD (eGFR <60mL/min/m2) between the ages of 18 and 65 years were enrolled into the study. An approval from the local ethical committee and written informed consent from each patient were obtained.
The height (m) and weight (kg) of each patient were measured with underwear. Body mass index (BMI) was calculated as weight (kg)/height squared (m2). Waist circumference (WC) was measured as the minimum difference between iliac crest and lateral costal margin and hip circumference at the maximum width of the buttocks. The waist/hip ratio (WHR) was calculated as the ratio of WC (cm) to hip ratio (cm). Blood pressure (BP) measurements were obtained from both arms in the seated position through a standard mercury sphygmomanometer after 10min of rest, and average results were recorded.
The patients on use of steroids, androgen, lipid-lowering drugs, gonadotrophins, gonadotrophin-releasing hormone agonists or antagonists; those with liver failure or any chronic inflammatory disease, prolactinoma, hypo or hyperthyroidism or adrenal enzyme deficiency; those with any malignancies, smoking cigarettes and consuming alcohol heavily; those undergoing coronary by-pass surgery, angioplasty or coronary stenting; those with peripheral arterial disease or experiencing stenting or operational revascularization surgery; and those under the ages of 18 and over 65 of years were excluded out of the study.
Venous blood samples were drawn to measure serum concentrations of creatinine, total cholesterol, high-density lipoprotein-cholesterol (HDL-cholesterol), low-density lipoprotein-cholesterol (LDL-cholesterol), triglyceride (TG), TT, free testosterone (FT), IL-6, and CRP. Blood samples were centrifuged and stored in a deep freeze at −80°C until analyzed. Blood creatinine was measured with the spectroscopic method by an Abbot C16000 autoanalyzer (Abbot Laboratories, Abbot Park, IL, USA). Total cholesterol [(NR), 110–200mg/dL] was measured by the spectroscopic method, while HDL-cholesterol [(NR), 40–90mg/dL] was measured with the immune reaction (antigen-antibody complex) by means of an Olympus AU 5800 device (Beckman Coulter Inc., CA, USA). TG [(NR), 0–200mg/dL] was also measured via a routine enzymatic method with an autoanalyzer using an Olympus AU 5800 device (Beckman Coulter Inc., CA, USA).
TT was measured by the chemiluminescence immunoassay method in an ADVIA Centaur XP (Siemens Healthcare Diagnostics, Camberley, UK). While TT more than 10nmol/L was accepted within normal limits, the patients with a value below 10nmol/L were accepted as those with hypogonadism. Plasma FT was measured by the radioimmunoassay method using the free testosterone-RIA-CT kit (DIAsource ImmunoAssays SA – Rue du Bosquet 2, B-1348 Louvain-la-Neuve, Belgium). For FT, age-adjusted reference ranges were used, and when the patients with hypogonadism were compared in terms of FT according to age levels, FT was found between 0.2–42.5pg/mL, 8.9–42.5pg/mL, 6.6–30pg/mL and 4.9–21.6pg/mL in the patients at the ages of <20, 20–39, 40–59 and >60, respectively. The values below the lower limit were accepted as hypogonadism. Serum CRP was measured using the nephelometric method via a Siemens Health Care Diagnostic BN-II (Siemens Healthcare Diagnostics, Marburg, Germany). IL-6 was also measured by IL-6-EASIA and ELISA kits (catalog number: KAP1261, DIAsource ImmunoAssays SA-Rue du Bosquet 2, B-1348 Louvain-la-Neuve, Belgium), and the units were expressed as pg/mL.
CIMT was measured as an indicator of subclinical atherosclerosis, and carotid arteries were measured, while the patients were in supine position using a real time triplex, high resolution B-mode ultrasonograph (AU4 Idea, Esaote Biomedica, Florence, Italy) with 7.5MHz probe. After the patients’ heads were extended in the supine position, the measurements were performed on both main carotid arteries (1cm proximal of the bulb). The highest values were obtained as two measurements, but no measurements were carried out from the atheroma plaque. The two echogenic lines between the intima-luminal surface and the media adventitia interfaces were evaluated as CIMT.
Statistical analysisThe Statistical Package for Social Sciences (SPSS) for Windows 15.0 program was used for statistical analyses (SPSS Inc., Chicago, IL, USA). The distributions within the normal limits were determined through the Kolmogorov–Smirnov and Shapiro–Wilk tests. While the student t test was used to compare the data with normal distribution between two groups, the Mann–Whitney-U test was utilized to compare the data without normal distribution between two groups. Also, the Pearson correlation test was used to examine the relationships between the parameters. The chi-square test was used to compare the qualitative data, and a p value of <0.05 was accepted significant.
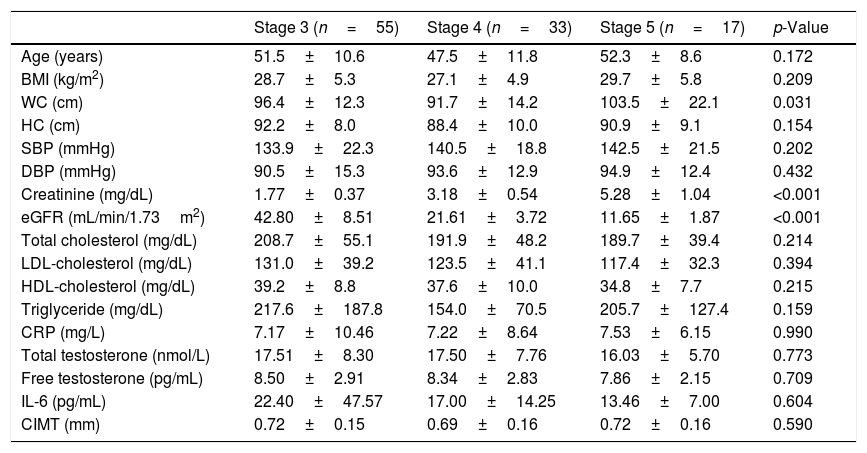
ResultsThe patients were grouped according to the stages of CKD. There were 55 (52.4%) patients in stage 3, 33 (31.4%) in stage 4, and 17 (16.2%) in stage 5. The general characteristics of these three groups are shown in Table 1. WC was also found higher in stage 5 patients than those in stage 4 (p=0.024).
Clinical and laboratory findings of male predialysis patients with CKD according to disease stages.
| Stage 3 (n=55) | Stage 4 (n=33) | Stage 5 (n=17) | p-Value | |
|---|---|---|---|---|
| Age (years) | 51.5±10.6 | 47.5±11.8 | 52.3±8.6 | 0.172 |
| BMI (kg/m2) | 28.7±5.3 | 27.1±4.9 | 29.7±5.8 | 0.209 |
| WC (cm) | 96.4±12.3 | 91.7±14.2 | 103.5±22.1 | 0.031 |
| HC (cm) | 92.2±8.0 | 88.4±10.0 | 90.9±9.1 | 0.154 |
| SBP (mmHg) | 133.9±22.3 | 140.5±18.8 | 142.5±21.5 | 0.202 |
| DBP (mmHg) | 90.5±15.3 | 93.6±12.9 | 94.9±12.4 | 0.432 |
| Creatinine (mg/dL) | 1.77±0.37 | 3.18±0.54 | 5.28±1.04 | <0.001 |
| eGFR (mL/min/1.73m2) | 42.80±8.51 | 21.61±3.72 | 11.65±1.87 | <0.001 |
| Total cholesterol (mg/dL) | 208.7±55.1 | 191.9±48.2 | 189.7±39.4 | 0.214 |
| LDL-cholesterol (mg/dL) | 131.0±39.2 | 123.5±41.1 | 117.4±32.3 | 0.394 |
| HDL-cholesterol (mg/dL) | 39.2±8.8 | 37.6±10.0 | 34.8±7.7 | 0.215 |
| Triglyceride (mg/dL) | 217.6±187.8 | 154.0±70.5 | 205.7±127.4 | 0.159 |
| CRP (mg/L) | 7.17±10.46 | 7.22±8.64 | 7.53±6.15 | 0.990 |
| Total testosterone (nmol/L) | 17.51±8.30 | 17.50±7.76 | 16.03±5.70 | 0.773 |
| Free testosterone (pg/mL) | 8.50±2.91 | 8.34±2.83 | 7.86±2.15 | 0.709 |
| IL-6 (pg/mL) | 22.40±47.57 | 17.00±14.25 | 13.46±7.00 | 0.604 |
| CIMT (mm) | 0.72±0.15 | 0.69±0.16 | 0.72±0.16 | 0.590 |
BMI: body mass index, CIMT: carotid intima-media thickness, CKD: chronic kidney disease, CRP: C-reactive protein, DBP: diastolic blood pressure, eGFR: estimated glomerular filtration rate, HC: hip circumference, HDL-cholesterol: high density lipoprotein cholesterol, IL-6: interleukin-6; LDL-cholesterol: low density lipoprotein cholesterol, SBP: systolic blood pressure, WC: waist circumference.
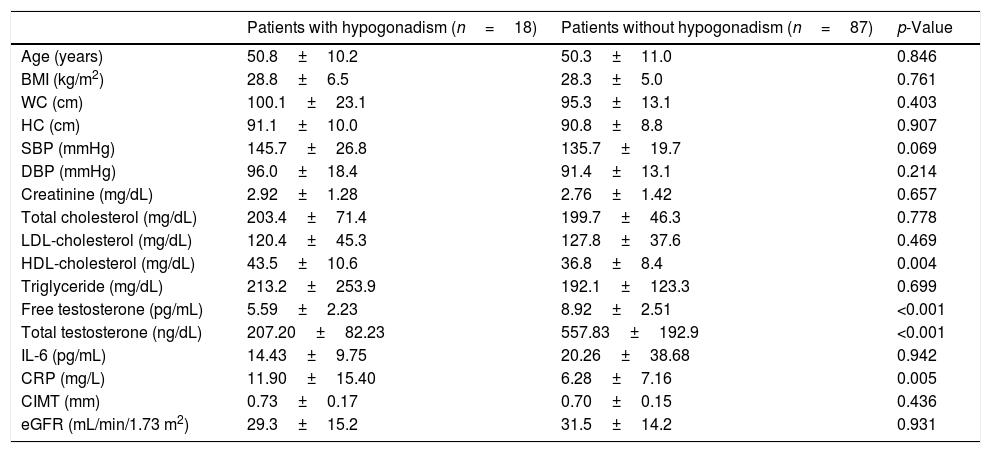
The patients were divided into two groups according to TT and FT with and without hypogonadism. According to TT, 18 (17.1%) of the patients had hypogonadism (Table 2). In terms of TT, hypogonadism was detected in nine (16.4%) of stage 3 patients with CKD, in six (18.1%) of stage 4 CKD patients, and in three (17.6%) of stage 5 CKD patients. Among the patients with hypogonadism, CRP and HDL-cholesterol were significantly higher than those without hypogonadism (p=0.005 and p=0.004, respectively), and no significant difference was found between anthropometric measurements and other parameters, such as IL-6 and CIMT.
Clinical and laboratory characteristics of male predialysis patients with CKD according to total testosterone levels.
| Patients with hypogonadism (n=18) | Patients without hypogonadism (n=87) | p-Value | |
|---|---|---|---|
| Age (years) | 50.8±10.2 | 50.3±11.0 | 0.846 |
| BMI (kg/m2) | 28.8±6.5 | 28.3±5.0 | 0.761 |
| WC (cm) | 100.1±23.1 | 95.3±13.1 | 0.403 |
| HC (cm) | 91.1±10.0 | 90.8±8.8 | 0.907 |
| SBP (mmHg) | 145.7±26.8 | 135.7±19.7 | 0.069 |
| DBP (mmHg) | 96.0±18.4 | 91.4±13.1 | 0.214 |
| Creatinine (mg/dL) | 2.92±1.28 | 2.76±1.42 | 0.657 |
| Total cholesterol (mg/dL) | 203.4±71.4 | 199.7±46.3 | 0.778 |
| LDL-cholesterol (mg/dL) | 120.4±45.3 | 127.8±37.6 | 0.469 |
| HDL-cholesterol (mg/dL) | 43.5±10.6 | 36.8±8.4 | 0.004 |
| Triglyceride (mg/dL) | 213.2±253.9 | 192.1±123.3 | 0.699 |
| Free testosterone (pg/mL) | 5.59±2.23 | 8.92±2.51 | <0.001 |
| Total testosterone (ng/dL) | 207.20±82.23 | 557.83±192.9 | <0.001 |
| IL-6 (pg/mL) | 14.43±9.75 | 20.26±38.68 | 0.942 |
| CRP (mg/L) | 11.90±15.40 | 6.28±7.16 | 0.005 |
| CIMT (mm) | 0.73±0.17 | 0.70±0.15 | 0.436 |
| eGFR (mL/min/1.73 m2) | 29.3±15.2 | 31.5±14.2 | 0.931 |
BMI: body mass index, CIMT: carotid intima-media thickness, CKD: chronic kidney disease, CRP: C-reactive protein, DBP: diastolic blood pressure, eGFR: estimated glomerular filtration rate, HC: hip circumference, HDL-cholesterol: high density lipoprotein cholesterol, IL-6: interleukin-6, LDL-cholesterol: low density lipoprotein cholesterol, SBP: systolic blood pressure, WC: waist circumference.
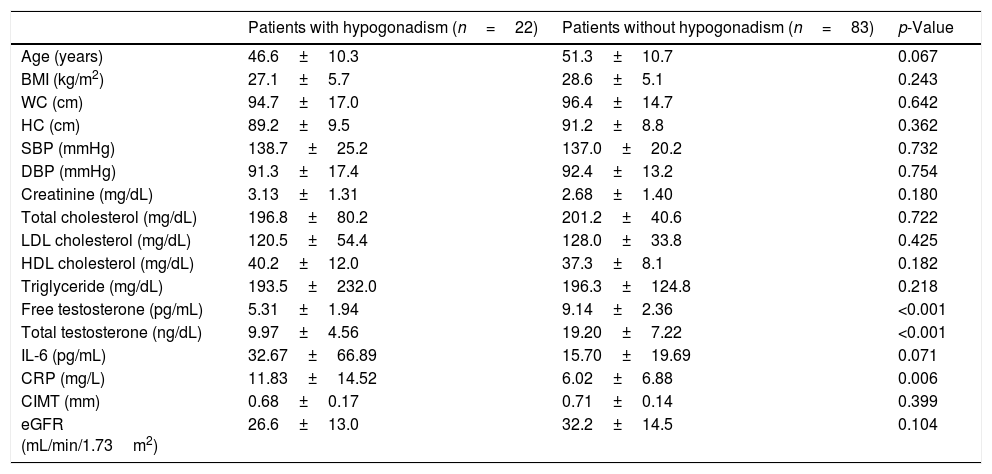
In terms of age-adjusted FT, 22 (20.9%) patients were diagnosed with hypogonadism. CRP values were significantly higher in the patients with hypogonadism (p=0.006) (Table 3). There was no significant difference with respect to other parameters in the patients with or without hypogonadism.
Clinical and laboratory characteristics of male predialysis patients with CKD according to free testosterone levels.
| Patients with hypogonadism (n=22) | Patients without hypogonadism (n=83) | p-Value | |
|---|---|---|---|
| Age (years) | 46.6±10.3 | 51.3±10.7 | 0.067 |
| BMI (kg/m2) | 27.1±5.7 | 28.6±5.1 | 0.243 |
| WC (cm) | 94.7±17.0 | 96.4±14.7 | 0.642 |
| HC (cm) | 89.2±9.5 | 91.2±8.8 | 0.362 |
| SBP (mmHg) | 138.7±25.2 | 137.0±20.2 | 0.732 |
| DBP (mmHg) | 91.3±17.4 | 92.4±13.2 | 0.754 |
| Creatinine (mg/dL) | 3.13±1.31 | 2.68±1.40 | 0.180 |
| Total cholesterol (mg/dL) | 196.8±80.2 | 201.2±40.6 | 0.722 |
| LDL cholesterol (mg/dL) | 120.5±54.4 | 128.0±33.8 | 0.425 |
| HDL cholesterol (mg/dL) | 40.2±12.0 | 37.3±8.1 | 0.182 |
| Triglyceride (mg/dL) | 193.5±232.0 | 196.3±124.8 | 0.218 |
| Free testosterone (pg/mL) | 5.31±1.94 | 9.14±2.36 | <0.001 |
| Total testosterone (ng/dL) | 9.97±4.56 | 19.20±7.22 | <0.001 |
| IL-6 (pg/mL) | 32.67±66.89 | 15.70±19.69 | 0.071 |
| CRP (mg/L) | 11.83±14.52 | 6.02±6.88 | 0.006 |
| CIMT (mm) | 0.68±0.17 | 0.71±0.14 | 0.399 |
| eGFR (mL/min/1.73m2) | 26.6±13.0 | 32.2±14.5 | 0.104 |
BMI: body mass index, CIMT: carotid intima-media thickness, CKD: chronic kidney disease, CRP: C-reactive protein, DBP: diastolic blood pressure, eGFR: estimated glomerular filtration rate, HC: hip circumference, HDL-cholesterol: high density lipoprotein cholesterol, IL-6: interleukin-6, LDL-cholesterol: low density lipoprotein cholesterol, SBP: systolic blood pressure, WC: waist circumference.
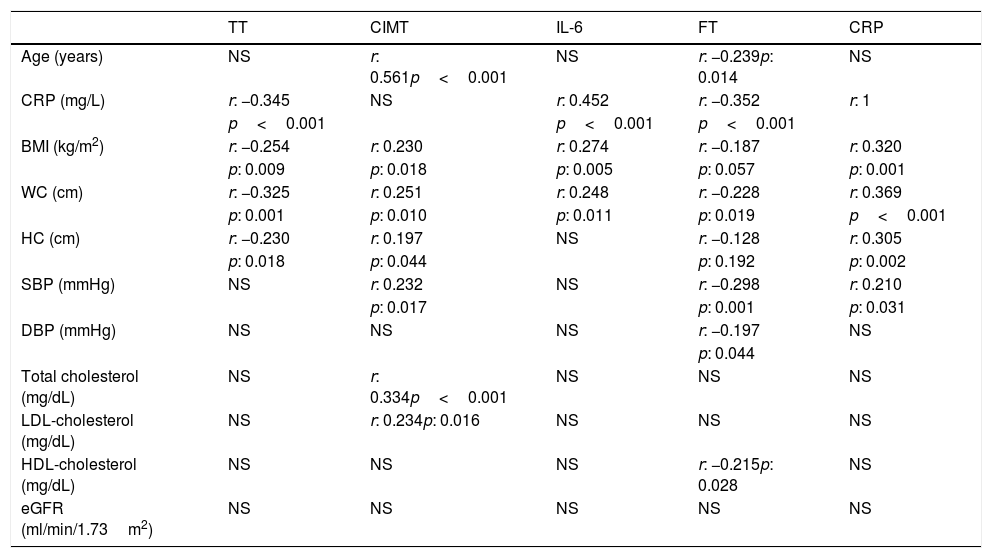
While performing the correlation analysis, a negative correlation was detected between TT and CRP, BMI, WC, and HC (Table 4). FT was also negatively correlated with age, WC, systolic blood pressure (SBP), diastolic blood pressure (DBP), HDL-cholesterol and CRP (Table 4).
Correlation analyses of some parameters in predialysis patients with CKD.
| TT | CIMT | IL-6 | FT | CRP | |
|---|---|---|---|---|---|
| Age (years) | NS | r: 0.561p<0.001 | NS | r: −0.239p: 0.014 | NS |
| CRP (mg/L) | r: −0.345 | NS | r: 0.452 | r: −0.352 | r: 1 |
| p<0.001 | p<0.001 | p<0.001 | |||
| BMI (kg/m2) | r: −0.254 | r: 0.230 | r: 0.274 | r: −0.187 | r: 0.320 |
| p: 0.009 | p: 0.018 | p: 0.005 | p: 0.057 | p: 0.001 | |
| WC (cm) | r: −0.325 | r: 0.251 | r: 0.248 | r: −0.228 | r: 0.369 |
| p: 0.001 | p: 0.010 | p: 0.011 | p: 0.019 | p<0.001 | |
| HC (cm) | r: −0.230 | r: 0.197 | NS | r: −0.128 | r: 0.305 |
| p: 0.018 | p: 0.044 | p: 0.192 | p: 0.002 | ||
| SBP (mmHg) | NS | r: 0.232 | NS | r: −0.298 | r: 0.210 |
| p: 0.017 | p: 0.001 | p: 0.031 | |||
| DBP (mmHg) | NS | NS | NS | r: −0.197 | NS |
| p: 0.044 | |||||
| Total cholesterol (mg/dL) | NS | r: 0.334p<0.001 | NS | NS | NS |
| LDL-cholesterol (mg/dL) | NS | r: 0.234p: 0.016 | NS | NS | NS |
| HDL-cholesterol (mg/dL) | NS | NS | NS | r: −0.215p: 0.028 | NS |
| eGFR (ml/min/1.73m2) | NS | NS | NS | NS | NS |
BMI: body mass index, CIMT: carotid intima-media thickness, CKD: chronic kidney disease, CRP: C-reactive protein, DBP: diastolic blood pressure, eGFR: estimated glomerular filtration rate, HC: hip circumference, HDL-cholesterol: high density lipoprotein cholesterol, FT: free testosterone, IL-6: interleukin-6, LDL-cholesterol: low density lipoprotein cholesterol, NS: not significant, SBP: systolic blood pressure, TT: total testosterone, WC: waist circumference.
In this study, the frequency of hypogonadism was found to be 17.1 and 20.9% in terms of TT and FT in the patients with CKD during predialysis period, respectively; however, the frequency of hypogonadism showed no difference according to CKD stages. Hypogonadism is a widespread entity in the patients with CKD.4,19–21 In a study by Gungor et al., hypogonadism frequency was reported to be 66% and testosterone to be within the normal limits in 10% of the patients with CKD and slightly lower than the normal limits in the rest of the cases.20 In another study performed by Khurana et al.,21 the frequency was reported as 53% in stage 3–4 CKD patients. Because testosterone was found to be 350ng/dL and below (approximately 12.15nmol/L) as the limit of hypogonadism by Khurana et al., and this level was over our hypogonadism limit (10nmol/L), we consider that the level in their study may have arisen from their higher hypogonadism limit. In our study, hypogonadism frequency was found to be 20.9%, compared with the age-adjusted FT. Although higher than that of hypogonadism in terms of TT, this frequency was not statistically different. In the study performed in 250 patients with 250 ESRD, Carrero et al. found testosterone deficiency to be <10nmol/L) in 44% of the cases, testosterone insufficiency to be 10–14nmol/L in 33% and testosterone within normal limits (>14nmol/L) in 23% of the cases.19 The same study also demonstrated that testosterone was inversely related to CRP, IL-6 and fibrinogen, and lower testosterone was associated with cardiovascular co-morbidity (OR 2.51) and mortality (OR 2).19 Although the frequency of hypogonadism was found lower in our study, compared with that in other studies, we consider that the probable cause of lower hypogonadim was due to our diagnostic and inclusion criteria. Because the lower limit accepted for hypogonadism was higher in various studies than our value, more patients were diagnosed with hypogonadism, and so the frequency of hypogonadism was found to be higher in those studies than that of our study.19,21
While the testosterone in circulation is due to the glycoprotein called sex hormone-binding globulin (SHBG) at a rate of 70%, it is less dependent on albumin, and only 1–3% is circulated freely.22 In fact, the active part of the testosterone is such free part in circulation. Although SHBG is increased with age, TT is decreased each year by 0.5–1%,23,24 and such a decrease is more pronounced in FT.25 In the patients with CKD, loss of urinary SHBG may have effects on FT; therefore, it may be misleading in diagnosing hypogonadism only by taking TT into account.2,26 In literature, there are also studies reporting a strong association between GFR and hypogonadism among the non-dialysis patients with CKD.4,21 In a study by Yilmaz et al., it was reported that hypogonadism was present in 34% of stage 3 CKD patients, 38% of stage 4 CKD patients and 57% of those with stage 5 CKD.4 On the other hand, considering FT, and that the cut-off value was 50pg/mL, Yilmaz et al. also stated that the frequency of hypogonadism was 80% on average, 75% in stage 1 CKD patients and 92% in stage 5 CKD patients, and that FT was much lower in dialysis patients, but the rate of hypogonadism was higher.4 In our study, we detected hypogonadism between average 16–18% in stage 3–5 CKD patients and found no difference between the stages in terms of hypogonadism frequency. Considering FT, however, we found the frequency of hypogonadism as 20.9%. While we diagnosed the patients with hypogonadism according to FT in our study, we used the lower limit of the reference value defined for age segments in the laboratory manual. Because the limit in our study was even lower than that (50pg/mL) by Yilmaz et al., the frequency of hypogondism may have been found lower as to FT. In addition, although the absence of assessing SHBG can be seen as a limitation of our study compared to other studies, we consider that the assessments performed for FT reduced the need for SHBG measurements.
Another important issue in the patients with hypogonadism is the risk of increased atherosclerosis and CVD. In many studies performed in general population and dialysis patients, such risks were shown to increase.27 In a study carried out in 2419 patients with stage 3–4 CKD, Khurana et al. reported that there was a relationship between TT and mortality rates in the CKD patients, and the mortality was increased in the presence of hypogonadism.21 In spite of limited number of studies related to the entity, however, the presence of similar risk was shown for non-dialysis CKD patients.4,20,21,28 In a study including 239 patients with hypogonadism, SBP, phosphorus and PTH were shown to be higher, while hemoglobin, calcium and estimated glomerular filtration rate (eGFR) were lower, cardiovascular events were more frequent, and flow-mediated dilatation was also lower.4 The lowest tertile testosterone was reported to increase the all-cause mortality rates by 2.03 times and CVD-induced mortality by 3.19 times in the dialysis patients with ESRD.27 CIMT values were shown to be used as an early indication of atherosclerosis risk in many studies.29 In our study, CIMT was found similar in the patients with and without hypogonadism. In also the presence of different stage CKD, no difference was observed in CIMT. As opposed to previous studies, we excluded the patients with any known CVDs out of the study; that is, we evaluated the very early stages of atherosclerosis, and therefore CIMT levels may have displayed similar results in the patients with hypogonadism, as different from the findings of previous studies.
However, a positive correlation was determined between CIMT, and age, BMI, WC, HC, SBP and LDL-cholesterol in our study. While emphasizing a positive relationship between CIMT, and age, BMI and WC, Pergola et al. reported a negative relationship between FT and WC.14 It is known that obesity is the main risk factor for atherosclerosis and CVDs.15 However insufficient BMI may be involved in demonstrating metabolic risk associated with obesity,16 we also used WC and HC measurements, as well as BMI, in order to evaluate the metabolic risk in our study. Although all of the three obesity indicators were negatively correlated with TT, FT was negatively correlated only with WC. In addition, in terms of these anthropometric measurements, no difference was found between the patients with and without hypogonadism. This entite was both interesting and a finding to be explained.
In our study, no difference was detected in terms of lipid parameters in the patients with and without hypogonadism, compared with FT; however, when the presence of hypogondism was assessed in terms of TT, HDL-cholesterol levels were found to be higher in those with hypogonadism. This entity was both intriguing and unclear finding to be elucidated. In addition, the negative correlation between FT and HDL-cholesterol was also another intriguing finding to be referred to. Dyslipidemia is an important factor for the development of atherosclerosis, and abnormal lipids increases were reported in the CKD patients with hypogonadism.20,28 In a study including 280 diabetic patients, although a positive associaton was reported between TT, and such lipids as HDL-cholesterol and apolipoprotein-A1 (APO-A1) with positive effects on metabolic and CVDs,6 the inclusion of limited number of controls (50 healthy controls) and of only the patients treated with statins into the study is a serious limitation. The statin treatment has been known to have some effects on both lipid profiles, inflammatory parameters and oxidative stress; consequently, this makes it impossible to interpret the association between TT and lipid profiles among the patients receiving statin treatment. In another study by Pottelbegh et al. with 715 healthy middle-aged men, although a positive relationship was reported between TT and FT, and HDL-cholesterol and APO-A1, which have positive effects on cardiometabolical disorders, it was stated that there is also a positive association between FT, and such negative lipid profiles as total cholesterol and apolipoprotein-B (APO-B) in terms of developing atherosclerosis; as a result, testosterone has complex effects on lipid profile, and so causing both a positive protective lipid profile against the development of atherosclerosis and a negative lipid profile increasing the risk of atherosclerosis.7 Fasting and satiety are two factors affecting lipid profile, and testosterone shows variations or is altered vary depending on the moment of the day. The fact that fasting/satiety measurements were not performed in drawing blood samples, and blood samples are taken at any time between 08:30 and 16:00 during the day can be postulated as limitations making the study by Pottelbegh et al. to be interpreted. Therefore, both including the patients taking statin into the study, and the fact that the results found between lipid and testosterone contradictory to ours, which were measured during at any time of the day without fasting are a matter of controversy. In our study, although HDL-cholesterol levels were found higher in those considered to have hypogonadism according to TT, no difference was observed between lipid profiles, compared the patients with and without hypogonadism according to FT. Most of the circulating testosterone is linked to SHBG, and TT is influenced by SHBG.22 The alterations in SHBG occurring in case of renal failure may have affected TT levels, and this may have caused the result difficult to be explained. One of our limitations is that we did not evaluate SHBG levels. In our study, BP was similar in the patients with and without hypogonadism, as consistent with the findings declared in other studies.4,14 In addition, there was a negative correlation between FT levels and SBP and DBP in our study.
Increased inflammation is a common condition encountered in the patients with CKD. As GFR decreases, the release of proinflammatory and inflammatory cytokines increases.3 The increased levels of inflammation, decreased albumin, CRP and fibrinogen are the features witnessed in the patients with CKD. Increased inflammatory cytokines and inflammatory diseases induce hypothalamic-pituitary-testis axis and cause testosterone deficiency/hypogonadism.17 Therefore, lower testosterone in such patients may be considered an indicator of increased inflammation. CRP and IL-6 levels, the indicators of inflammation in the patients with hypogonadism, were shown to be higher than those without hypogonadism.4,28,27 In the study performed by Gungor et al., when testosterone levels were divided into different tertiles in dialysis patients with CKD, high-sensitive CRP was reported to be similar in all tertiles.20 In our study, when the patients were diagnosed with hypogonazidm based on TT and FT, CRP was seen to be higher among those with hypogonadism. However, it was seen that no difference was present between those with and without hypogonadism in terms of IL-6. In the study where 50 eugonadal patients and 44 hypogonadotropic hypogonadism, all with T2DM, were evaluated, Dhindza et al. reported that those with hypogonadism had more visceral and subcutaneous fat; the rate of glucose infusion was seen to be 36% lower in euglycemic clamp test; and, when testosterone treatment was given as placebo or intramuscularly to those with hypogonadism at 250mg every two weeks for a 24-week period, the glucose infusion increased 32% in the testosterone group. The same study also reported that a reduction of 3.3kg was determined in subcutaneous adipose tissue, while an increase of 3.4kg occurred in lean mass, and that free fatty acid in the circulating blood, IL-1β, CRP, tumor necrosis factor-α and leptin were decreased, although the treatment caused an increase in insulin sensitivity.15 In a recently published study, when diabetic patients with functional hypogonadism were given transdermal or placebo testosterone treatment (50mg/day) for 9 months, thanks to the treatment, a decrease was stated to take place in the indicators of carbohydrate metabolism and endothelial dysfunction, such as WC, HOMA-IR, hemoglobin A1c, leptin, resistin, intercellular adhesion molecule-1 and CRP.16 In our study, no patients with hypogonadism were given any androgen replacement therapy. Therefore, the effects of the treatment on IR and inflammation markers could not be investigated.
ConclusionAs a result, the frequency of hypogonadism was found around 17–21% in the patients with CKD. No matter how similar IL-6 was found in the patients with and without hypogonadism, CRP was determined higher in men with hypogonadism. No association was observed between hypogonadism and CIMT. The fact that CIMT was not found to be different could be related to our inclusion criteria. The exclusion criteria for those with any known CVD may have led different CIMT values to be found in our study. Although HDL was found higher in those with hypogonadism considering TT, this difference was not observed in HDL-cholesterol, when a reclassification was performed according to FT. However, the presence of a negative relationship between FT and HDL-cholesterol was another intriguing finding to be elucidated in our study. We consider that further studies with larger populations are needed to enlighten the entity.
FundingThis study was funded by the research fund of Konya Training and Research Hospital.
Conflict of interestAuthors declare no conflicts of interest.
Authors thank Numan Duran for language editing.