To investigate the relationship between glomerular filtration rates (GFR), and homeostasis model assesment of insulin resistance (HOMA-IR), C-reactive protein (CRP) and neutrophil to lymphocyte ratio (NLR) in patients with polycystic ovary syndrome (PCOS).
Material and methodsThirty-one overweight and obese PCOS patients with body mass index (BMI)≥25kg/m2 and 25 non-obese PCOS patients with BMI<25kg/m2 were included into patients’ group, while 23 overweight and obese, and 25 non-obese age-and BMI-matched healthy individuals (aged between 18 and 40 years), were enrolled as controls. Levels of serum creatinine, glucose, insulin, CRP, and complete blood count were measured. eGFR, HOMA-IR and NLR were also calculated.
ResultsIn PCOS group, HOMA-IR (p=0.001), CRP (p=0.025) and waist hip ratio (WHR) (p=0.011) were higher than controls. In obese PCOS sub-group, HOMA-IR (p=0.004) and WHR (p=0.002) were higher than obese controls. In non-obese PCOS sub-group, HOMA-IR (p=0.001) were higher than non-obese controls. In obese PCOS sub-group; HOMA-IR (p=0.001) and CRP (p=0.001) levels were significantly higher than non-obese PCOS sub-group. In terms of other parameters, no significant difference was found between the groups. The analysis showed a negative correlation between GFR, and BMI and HOMA-IR in PCOS group, between GFR, WHR and insulin levels in obese PCOS sub-group, and between BMI, and HOMA-IR and NLR in non-obese PCOS sub-group.
ConclusionAlthough HOMA-IR and CRP were higher in PCOS group, there was no difference in NLR and GFR levels between those with PCOS and controls.
investigar la relación entre las tasas de filtración glomerular (TFG) y la evaluación del modelo de homeostasis de la resistencia a la insulina (HOMA-IR), la proteína C-reactiva (PCR) y la relación de neutrófilos a linfocitos (NLR) en pacientes con síndrome de ovario poliquístico (PCOS).
Material y métodosTreinta y un pacientes con PCOS con sobrepeso y obesidad con índice de masa corporal(IMC) ≥25 kg/m 2 y 25 pacientes con PCOS no obesos con IMC <25 kg/m 2 constituyeron el grupo de pacientes, mientras que 23 con sobrepeso y obesidad, y Se inscribieron como controles 25 sujetos sanos no obesos, todos con edad e IMC(edad entre 18 y 40 años). Se midieron los niveles séricos de creatinina, glucosa, insulina y PCR, y se evaluó el recuento sanguíneo completo; Se calcularon eGFR, HOMA-IR y NLR.
Resultadosen el grupo PCOS, HOMA-IR (p=0.001), CRP (p=0.025) y la relación cintura-cadera(WHR) (p=0.011) fueron más altos que los controles. En el subgrupo de PCOS obesos, HOMA-IR (p=0.004) y WHR (p=0.002) fueron más altos que los controles obesos. En el subgrupo PCOS no obeso, HOMA-IR (p=0.001) fue mayor que los controles no obesos. En el subgrupo de PCOS obesos; Los niveles de HOMA-IR (p=0.001) y CRP (p=0.001) fueron significativamente más altos que los del subgrupo PCOS no obeso. En cuanto a otros parámetros, no se encontraron diferencias significativas entre los grupos.
ConclusiónAunque los niveles de HOMA-IR y CRP se encontraron más altos en el grupo PCOS, no hubo diferencias en los niveles de NLR y GFR entre aquellos con PCOS y controles.
Polycsytic ovary syndrome (PCOS) is one of the most common endocrinologic disorders characterized by ovulatory dysfunction and hyperandrogenism in women at childbearing age with a worldwide prevalence of 6–18.1–3 Cardiovascular risk factors, such as obesity, glucose intolerance, dyslipidemia, obstructive sleep apnea and increased inflammation, are among the disorders commonly seen in patients with PCOS.4 In literature, it is asserted that C-reactive protein (CRP) playing an important role in the evaluation of subclinical low-grade inflammation is higher in patients with PCOS and has a significant correlation with insulin resistance (IR).5–10 Many recent studies demonstrated that neutrophil to lymphocyte ratio (NLR) is higher in patients with PCOS, and may be used as a sign of inflammation.9–13 Subclinical low-grade inflammation was also associated with obesity and metabolic disturbances, such as IR, dyslipidemia, glucose intolerance, hypertension in patients with PCOS, and these conditions may lead to changes in glomerular filtration rate (GFR). In literature, the number of studies investigating renal functions in PCOS patients is limited. In patients with PCOS, GFR was found to be significantly higher than normal subjects.14,15 On the other hand, in two different studies performed in rats, hyperandrogenemia in the presence of PCOS was shown to lead to a decrease between 40 and 60% in renal function,16,17 an increase in visceral obesity and IR,16 and also to cause glomerulosclerosis and interstitial sclerosis.17 In another study, no difference was found in GFR between patients with PCOS and controls.18
In the present study, we aimed at investigating estimated glomerular filtration rate (eGFR) in obese and non-obese PCOS patients and the association between eGFR, and metabolic and inflammatory parameters, such as HOMA-IR and CRP in these groups by comparing with two different age- and body mass index (BMI)-matched control groups.
MethodsThis prospective study was conducted in the clinics of Internal Medicine and Endocrinology and Metabolism at Konya Health Application and Research Center (previously entitled as Konya Training and Research Hospital), a tertiary care hospital, at University of Health Sciences between November 2016 and May 2017. An informed consent was obtained from each patient and control. The study protocol was approved by the Ethics Committee of Meram Faculty of Medicine of Necmettin Erbakan University on 7th October, 2016 (Number: 2016/693).
Thirty-one overweight and obese PCOS patients with BMI≥25kg/m2 and 25 non-obese PCOS patients with BMI<25kg/m2 were included into patients’ group (ranging between 18–40 years), while 23 overweight and obese, and 25 non-obese healthy individuals, all matched as to age and BMI, and without PCOS, diabetes mellitus (DM), any known heart, kidney, cardiovascular or atherosclerotic diseases were enrolled as the controls.
The diagnosis of PCOS was performed under the Rotterdam criteria 19 with at least two of the following three criteria: The existence of oligomenorrhea (cycles lasting longer than 35 days) or amenorrhea (less than two menstrual cycles over the past six months), clinical and biochemical hyperandrogenism and polycystic appearance of ovary on ultrasonography (USG), while other causes of hyperandrogenism, such as congenital adrenal hyperplasia, Cushing's syndrome, were accepted as exclusion criteria. Women with pregnancy or breast feeding; those with oral contraceptives or taking drugs affecting IR and leading to inflammation, such as estrogens, corticosteroids, immunosuppressions, insulin, thiazide or antidiabetic medications within the last 6 months; those with any known disorders, such as liver disease, cancer or rheumatological disease, history of active infection or febril illness within in the last 2 months; or those exposed to any surgical intervention within past 6 months, on illegal drugs, smoking cigarettes and consuming alcohol heavily were excluded from the study.
Height (m) and weight (kg) were measured with underwear clothing. BMI was calculated as weight (kg)/height square (m2). Waist circumference (WC) was measured as the minimum size between iliac crest and lateral costal margin, and hip circumference at maximum width of the buttocks. Waist/hip ratio (WHR) was calculated as the ratio of WC (cm) to hip ratio (cm). Blood pressure (BP) was measured from both arms in the seated position by using a standart mercury sphygmomanometer after 10-min rest, and average results were recorded. Blood samples were drawn after an overnight fasting to measure creatinine, insulin, glucose and CRP levels, centrifuged and stored in deep freeze at −80°C until being analyzed. Complete blood count was measured by Sysmex XE-2100 (Sysmex Corp, Kobe, Japan) with the fluorescence flow cytometry or electrical impedance method. Blood creatinine was measured with spectroscopic method by the Abbot C16000 autoanalyser (Abbot Laboratories, Abbot Park, IL, USA). Plasma glucose levels [normal range (NR), 70–105mg/dL] were measured using the hexokinase method with the Olympus AU 5800 device (Beckman Coulter Inc., CA, USA). Insulin levels [(NR), 6–27μlU/mL] were measured via the chemiluminescence method with the Immulite 2000 device (Siemens Healthcare Diagnostics, Germany). The analytic sensitivity of the assay was detected as 2μlU/mL. Serum CRP was measured using the nephelometric method via the Siemens Health Care Diagnostic BN II (Siemens Healthcare Diagnostics, Marburg, Germany). NLR was also calculated with the ratio of neutrophil counts to lymphocyte counts. IR was calculated by homeostasis model assesment of insulin resistance (HOMA-IR), based on the formula [fasting plasma glucose (mmol/L)×fasting serum insulin (μIU/mL)/22.5]. Additionally, eGFR (mL/min/1.73m2) was calculated using the Modification of Diet and Renal Disease formula.20
Statistical analysisContinuous variables were expressed as median (min–max) or mean±standard deviation (SD), while categorical variables were presented as frequency and related percentage values. The Mann–Whitney U and independent samples t tests were used for comparing groups. The chi-square with the Yates correction, the Fisher's exact, and the Fisher–Freeman–Halton tests were used to compare categorical variables. The Spearman's correlation coefficient was used to analyze the relationship between continuous variables. The Standard Package for Social Sciences for Windows version 21 (SPSS, IBM Inc. IL, USA) was used for analysis. An α level equal to 0.05 was accepted to be significant.
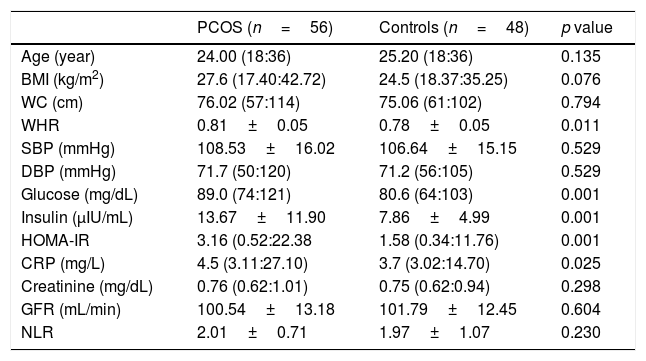
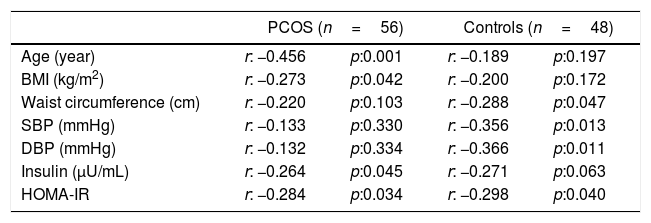
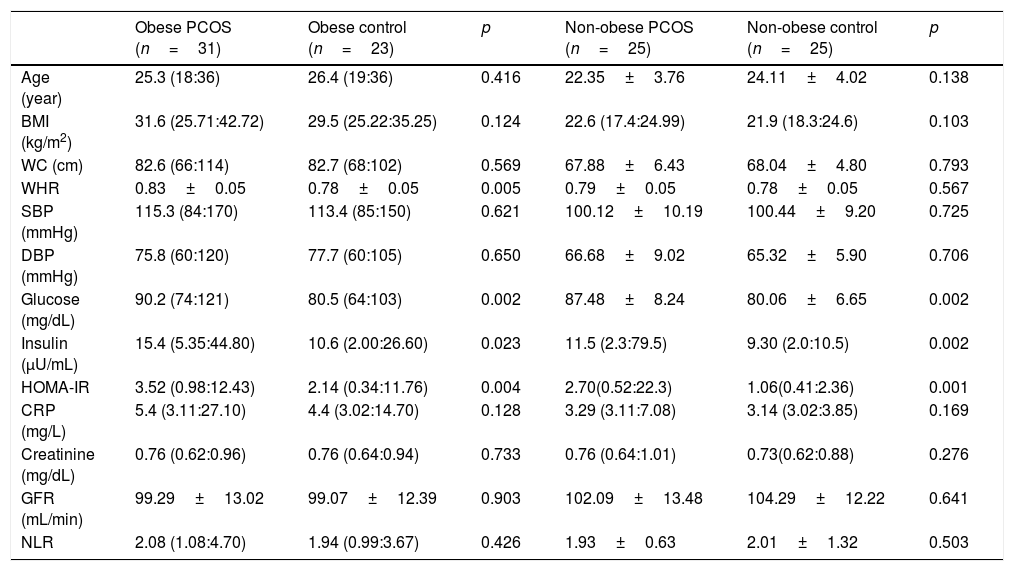
ResultsThe general characteristics of patients and controls are shown in Table 1. In PCOS group, glucose (p=0.001), insulin (p=0.001), HOMA-IR (p=0.001), CRP (p=0.025) and WHR (p=0.011) were higher than controls. No significant difference was found regarding eGFR and other parameters between both groups. In PCOS group, GFR was significantly correlated with age (r=−0.456, p<0.001), BMI (r=−0.273, p=0.042), insulin (r=−0.267, p=0.047) and HOMA-IR (r=−0.284, p=0.034) (Table 2). In obese PCOS sub-group, glucose (p=0.002), insulin (p=0.023), HOMA-IR (p=0.004) and WHR (p=0.002) were higher, compared with obese controls. In terms of other parameters, no significant difference was found between two groups. In non-obese PCOS sub-group, glucose (p=0.002), insulin (p=0.002) and HOMA-IR (p=0.001) were higher than non-obese controls. No significant difference was found between two groups in respect to other parameters.
Some anthropometric, metabolic and hormonal data of study population.
| PCOS (n=56) | Controls (n=48) | p value | |
|---|---|---|---|
| Age (year) | 24.00 (18:36) | 25.20 (18:36) | 0.135 |
| BMI (kg/m2) | 27.6 (17.40:42.72) | 24.5 (18.37:35.25) | 0.076 |
| WC (cm) | 76.02 (57:114) | 75.06 (61:102) | 0.794 |
| WHR | 0.81±0.05 | 0.78±0.05 | 0.011 |
| SBP (mmHg) | 108.53±16.02 | 106.64±15.15 | 0.529 |
| DBP (mmHg) | 71.7 (50:120) | 71.2 (56:105) | 0.529 |
| Glucose (mg/dL) | 89.0 (74:121) | 80.6 (64:103) | 0.001 |
| Insulin (μIU/mL) | 13.67±11.90 | 7.86±4.99 | 0.001 |
| HOMA-IR | 3.16 (0.52:22.38 | 1.58 (0.34:11.76) | 0.001 |
| CRP (mg/L) | 4.5 (3.11:27.10) | 3.7 (3.02:14.70) | 0.025 |
| Creatinine (mg/dL) | 0.76 (0.62:1.01) | 0.75 (0.62:0.94) | 0.298 |
| GFR (mL/min) | 100.54±13.18 | 101.79±12.45 | 0.604 |
| NLR | 2.01±0.71 | 1.97±1.07 | 0.230 |
PCOS: Polycystic ovary syndrome, BMI; body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, HOMA-IR: homeostasis model assessment of insulin resistance, CRP: C reactive protein, GFR: glomerular filtration rate, NLR: neutrophil to lymphocyte ratio, WC: waist circumference, WHR: waist to hip ratio. Results are given as; mean±standard deviation or median (min:max).
Correlation analyses between some parameters and GFR in PCOS and control groups.
| PCOS (n=56) | Controls (n=48) | |||
|---|---|---|---|---|
| Age (year) | r: −0.456 | p:0.001 | r: −0.189 | p:0.197 |
| BMI (kg/m2) | r: −0.273 | p:0.042 | r: −0.200 | p:0.172 |
| Waist circumference (cm) | r: −0.220 | p:0.103 | r: −0.288 | p:0.047 |
| SBP (mmHg) | r: −0.133 | p:0.330 | r: −0.356 | p:0.013 |
| DBP (mmHg) | r: −0.132 | p:0.334 | r: −0.366 | p:0.011 |
| Insulin (μU/mL) | r: −0.264 | p:0.045 | r: −0.271 | p:0.063 |
| HOMA-IR | r: −0.284 | p:0.034 | r: −0.298 | p:0.040 |
PCOS: Polycystic ovary syndrome, GFR: glomerular filtration rate, BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, HOMA-IR: homeostasis model assessment of insulin resistance.
In obese PCOS sub-group (Table 3), GFR was significantly correlated with age (r=−0.529, p:=0.002), WHR (r=−0.369, p=0.041) and insulin (r=−0.366, p=0.043). In non-obese PCOS sub-group, there was a negative correlation between eGFR, and such parameters as BMI (r=−0.501, p=0.011), HOMA-IR (r=−0.456, p=0.022) and NLR (r=−0.519, p=0.008).
Some anthropometric, metabolic, and hormonal data of the obese and non-obese subgroups.
| Obese PCOS (n=31) | Obese control (n=23) | p | Non-obese PCOS (n=25) | Non-obese control (n=25) | p | |
|---|---|---|---|---|---|---|
| Age (year) | 25.3 (18:36) | 26.4 (19:36) | 0.416 | 22.35±3.76 | 24.11±4.02 | 0.138 |
| BMI (kg/m2) | 31.6 (25.71:42.72) | 29.5 (25.22:35.25) | 0.124 | 22.6 (17.4:24.99) | 21.9 (18.3:24.6) | 0.103 |
| WC (cm) | 82.6 (66:114) | 82.7 (68:102) | 0.569 | 67.88±6.43 | 68.04±4.80 | 0.793 |
| WHR | 0.83±0.05 | 0.78±0.05 | 0.005 | 0.79±0.05 | 0.78±0.05 | 0.567 |
| SBP (mmHg) | 115.3 (84:170) | 113.4 (85:150) | 0.621 | 100.12±10.19 | 100.44±9.20 | 0.725 |
| DBP (mmHg) | 75.8 (60:120) | 77.7 (60:105) | 0.650 | 66.68±9.02 | 65.32±5.90 | 0.706 |
| Glucose (mg/dL) | 90.2 (74:121) | 80.5 (64:103) | 0.002 | 87.48±8.24 | 80.06±6.65 | 0.002 |
| Insulin (μU/mL) | 15.4 (5.35:44.80) | 10.6 (2.00:26.60) | 0.023 | 11.5 (2.3:79.5) | 9.30 (2.0:10.5) | 0.002 |
| HOMA-IR | 3.52 (0.98:12.43) | 2.14 (0.34:11.76) | 0.004 | 2.70(0.52:22.3) | 1.06(0.41:2.36) | 0.001 |
| CRP (mg/L) | 5.4 (3.11:27.10) | 4.4 (3.02:14.70) | 0.128 | 3.29 (3.11:7.08) | 3.14 (3.02:3.85) | 0.169 |
| Creatinine (mg/dL) | 0.76 (0.62:0.96) | 0.76 (0.64:0.94) | 0.733 | 0.76 (0.64:1.01) | 0.73(0.62:0.88) | 0.276 |
| GFR (mL/min) | 99.29±13.02 | 99.07±12.39 | 0.903 | 102.09±13.48 | 104.29±12.22 | 0.641 |
| NLR | 2.08 (1.08:4.70) | 1.94 (0.99:3.67) | 0.426 | 1.93±0.63 | 2.01±1.32 | 0.503 |
PCOS: Polycystic ovary syndrome, BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure: HOMA-IR: homeostasis model assessment of insulin resistance, CRP: C-reactive protein, GFR: glomerular filtration rate, NLR: neutrophil to lymphocyte ratio, WC: waist circumference, WHR: waist to hip ratio. Results are given as; mean±SD or median (min:max).
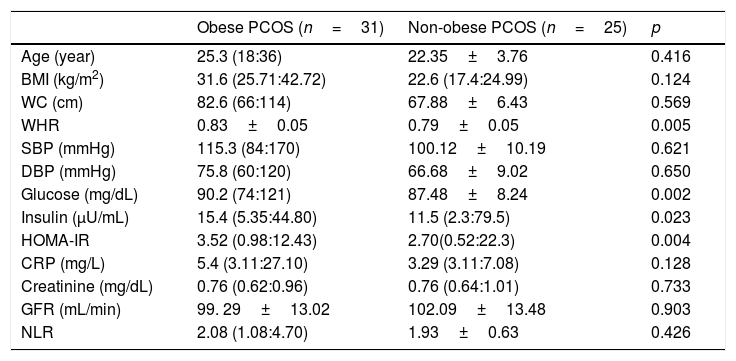
BMI (p<0.001), WC (p<0.001), WHR (p=0.004), systolic BP (p<0.001), diastolic BP (p<0.001), insulin (p=0.002), HOMA-IR (p=0.001) and CRP (p=0.001) were found significantly higher in obese PCOS sub-group, compared with non-obese PCOS sub-group. No significant difference was found between two groups in terms of other parameters (Table 4).
Some anthropometric, metabolic, and hormonal data of the obese PCOS and non-obese PCOS sub-groups.
| Obese PCOS (n=31) | Non-obese PCOS (n=25) | p | |
|---|---|---|---|
| Age (year) | 25.3 (18:36) | 22.35±3.76 | 0.416 |
| BMI (kg/m2) | 31.6 (25.71:42.72) | 22.6 (17.4:24.99) | 0.124 |
| WC (cm) | 82.6 (66:114) | 67.88±6.43 | 0.569 |
| WHR | 0.83±0.05 | 0.79±0.05 | 0.005 |
| SBP (mmHg) | 115.3 (84:170) | 100.12±10.19 | 0.621 |
| DBP (mmHg) | 75.8 (60:120) | 66.68±9.02 | 0.650 |
| Glucose (mg/dL) | 90.2 (74:121) | 87.48±8.24 | 0.002 |
| Insulin (μU/mL) | 15.4 (5.35:44.80) | 11.5 (2.3:79.5) | 0.023 |
| HOMA-IR | 3.52 (0.98:12.43) | 2.70(0.52:22.3) | 0.004 |
| CRP (mg/L) | 5.4 (3.11:27.10) | 3.29 (3.11:7.08) | 0.128 |
| Creatinine (mg/dL) | 0.76 (0.62:0.96) | 0.76 (0.64:1.01) | 0.733 |
| GFR (mL/min) | 99. 29±13.02 | 102.09±13.48 | 0.903 |
| NLR | 2.08 (1.08:4.70) | 1.93±0.63 | 0.426 |
PCOS: Polycystic ovary syndrome, BMI: body mass index. SBP: systolic blood pressure, DBP: diastolic blood pressure. HOMA-IR: homeostasis model assessment of insulin resistance. CRP: C reactive protein, GFR: glomerular filtration rate. NLR: neutrophil to lymphocyte ratio, WC: waist circumference, WHR: waist to hip ratio. Results are given as; mean±standard deviation or median (min:max).
In the present study, we showed that CRP, a sign of subclinical inflammation, and HOMA-IR were higher both in patients with PCOS than those without PCOS and also in overweight and obese PCOS patients than non-obese PCOS patients. However, there was no difference between the groups in terms of eGFR and NLR levels. IR and increased subclinical inflammation are among important characteristics of PCOS. In the study, we found HOMA-IR levels to be higher in all patients in PCOS group and in PCOS sub-group than non-obese PCOS group. We also found HOMA-IR levels to be higher in overweight and obese PCOS groups, compared to non-PCOS group. In one of our previous studies including 25 obese and 16 non-obese PCOS patients, and 16 and 14 age- and BMI-matched control patients respectively, HOMA-IR had been discovered to be higher in PCOS patients than controls.10 In literature, there are also studies reporting consistent results with those in our study. In a recent study,21 metabolic syndrome (MetS) and IR prevalences were reported as 10.4% and 27% in infertile 318 PCOS women respectively, and the presence of obesity and age over 30 were also reported as an independent risk factors for the development of MetS and IR. In the same study, while impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) was detected in 59.3% of patients with IR, IFG and IGT were found only in 10.3% of those without IR.
In our study, CRP was found to be higher in PCOS patients than those without PCOS, and also in overweight and obese PCOS sub-group than non-obese PCOS patients. However, CRP in both overweight and obese, and non-obese PCOS sub-groups was similar to non-PCOS BMI-matched control groups. It was reported that there was a significant relationship between increased CRP levels and IR in patients with PCOS.5 In another study by Elci et al., CRP was found significantly higher in both obese and non-obese PCOS patients than controls, and the difference was more pronounced, especially in obese PCOS group.7 In another study, Agacayak et al. found no difference between PCOS patients and controls in terms of CRP, neopterin, interleukin-6 and TNF, reporting CRP to be higher in obese PCOS patients than obese controls.9 In one of our previous studies, we had determined that high sensitivity CRP (hsCRP) were similar in PCOS patients and controls.10 In this study, according to the presence obesity, our overweight and obese PCOS group showed higher CRP levels than non-obese PCOS group. Obesity is well-known to be an important factor leading to an increase of CRP.8 The underlying mechanism of increased CRP in PCOS patients is yet to be elucidated, and whether it is related to PCOS itself or the accompanying obesity still remains uncertain.
NLR, another indicator of inflammation, was observed to be similar in PCOS patients and controls in our study, and no difference was found between PCOS patients and controls upon performing sub-group analyses. In the study by Cakıroglu et al., NLR was reported to be higher in 146 patients with PCOS, compared to age- and BMI-matched 146 healthy controls.12 Although the number of cases in their study was greater than the number of cases in our study, the fact that the subjects were not classified as obese and non-obese can be considered an important limitation of the study by Cakiroglu et al. In another study, Agacayak et al. found that NLR was significantly higher in PCOS patients, but no difference was detected when the patients were divided as obese (n=15) and nonobese (n=15) sub-groups.9 In the study by Keskin et al., NLR was found to be higher in both obese and non-obese PCOS patients than control subjects.13 In another recent study, platelet-lymphocytes ratio and NLR were shown to be correlated with Matsuda index and HDL-cholesterol levels, respectively, as independent of obesity.11
In a study we had conducted previously, NLR had been found higher in PCOS main group, compared to controls, and in non-obese PCOS group than non-obese controls.10 Contrary to the findings of abovementioned studies, we concluded that being NLR within normal limits in our current study could be due to lower number of samples.
It is a known fact that lower inflammation, IR, dyslipidemia, glucose intolerance, hypertension and obesity in PCOS patients may lead to changes in GFR. Elevated GFR (i.e. hyperfiltration) is seen in DM patients, as well as seen in pre-diabetic patients or those with MetS.22 In literature, there are limited number of studies evaluating glomerular functions in PCOS patients. In one of these limited number of studies, Gozukara et al. reported that eGFR values were significantly higher in spite of being within normal limits in 140 non-obese PCOS patients, compared to 60 healthy controls.14 In another study including small sample size (16 patients with PCOS and 15 healthy subjects) and performed by Lakhani et al., similar eGFR levels were reported between PCOS patients and controls.18 In a study where androgen replacement was conducted in rats from the early weeks to the late stage of life by Dalmasso et al. in order to mimic women with postmenopausal hyperandrogenism, it was detected that there was an increase in visceral obesity and IR, and abnormal glucose tolerance developed while BP increased, and a decrease occurred in GFR by 40%.16 In a recent study with similar design, Patil et al. demonstrated that in rats, hyperandrogenism was formed similar to that in PCOS, and the rates of GFR and renal plasma flow reduced by 60% and 40%, respectively; while urinary nitrate and nitrite excretion decreased, but focal segmental glomerulosclerosis, global sclerosis and interstitial fibrosis increased. Patil et al. also speculated that hyperandrogenemia was persistent even in the presence of PCOS in older women after menopausal period and elevated the risk of development of chronic kidney disease.17 In a retrospective cross-sectional study, Mu et al. detected that eGFR was higher among those with PCOS than non-PCOS patients (126.79mL/min/1.73m2 and 125.97mL/min/1.73m2, respectively); however, the researchers showed no reason why eGRF levels were high.15 In our study, no difference was found between both PCOS and control groups and between overweight and obese, and non-obese subgroups in terms of GFR levels. In addition, GFR was shown to be negatively correlated with age, BMI, insulin and HOMA-IR in PCOS group. However, we showed that GFR was negatively correlated with age, WHR and insulin in overweight and obese PCOS sub-groups, and negatively correlated with BMI, HOMA-IR and NLR in non-obese PCOS sub-group. We determined no correlation between CRP and eGFR in any of PCOS groups. Based on literature, an increase is observed in GFR in pre-diabetic patients or at the early developmental stages of DM due to increased hyperfiltration; however, as the disease progresses, a decrease develops in GFR, especially after the development of macroalbuminuria.23 In a limited number of studies evaluating GFR in PCOS patients,14,15 the fact that GFR was within normal limits, but higher in PCOS patients than controls may be attributed to the presence of the high prevalence of IFG-IGT or prediabetic features in such patients. In studies where old-age rats were used to investigate the effects of hyperandrogenism on GFR, the fact that a decrease was found in GFR by 40–60% can be evaluated as the long-term effects of glucose intolerance in old-age postmenopausal women on GFR.15,17 The inverse association between GFR, and HOMA-IR, NLR and BMI was an expected condition, especially in non-obese PCOS group.
Our study has also various limitations. The number of participants is limited, and the study was performed with the individuals from a narrow range between 18 and 40. We consider that if the more individuals with a larger age range had been included into the control group, the association between GFR and age could have been demonstrated.
ConclusionIn this study, although we demonstrated IR and inflammation levels to increase in PCOS patients, we found that NLR and eGFR were similar between PCOS patients and controls. Although eGFR was inversely associated with IR in PCOS group, we showed while performing sub-group analyses that such a negative correlation was only in non-obese PCOS group, and there was also a negative correlation between eGFR and NLR in non-obese PCOS group. However, considering that hyperandrogenemia leads to decreased renal function in PCOS patients especially at advanced age, it was concluded that further studies with larger population and including postmenopausal women are needed to elucidate the entity.
Compliance with ethical standardsAll procedures in the study involving human participants were performed in accordance with the ethical standards of the institutional research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consentAn informed consent was obtained from each participant included into the study.
FundingThe study was supported by the research fund of Konya Training and Research Hospital.
Conflict of interestNo competing financial interests exist. Authors also thank Numan Duran for language editing.