Edited by:
José Antonio Sainz Bueno
Professor of Obstetrics and Gynecology, Faculty of Medicine, University of Seville, Seville, Spain Professor of Obstetrics and Gynecology, Faculty of Medicine, University of Seville, Seville, Spain Professor of Obstetrics and Gynecology, Faculty of Medicine, University of Seville, Seville, Spain Professor of Obstetrics and Gynecology, Faculty of Medicine, University of Seville, Seville, Spain Professor of Obstetrics and Gynecology, Faculty of Medicine, University of Seville, Seville, Spain
Eugenia Antolín Alvarado
Senior Consultant in Fetal Medicine, Department of Obstetrics and Ginecology, University La Paz Hospital; Member of the Obstetric Group in IdiPAZ- Biomedical Research Institute; Member of the SAMID network, Associate professor UAM University, Madrid, Spain
Last update: August 2024
More infoFetal cardiac evaluation in the first trimester should be evaluated systematically: 1st. Heart rate. 2nd. Situs. 3rd. Cardiac axis. 4th. 4 chamber view. 5th. Outflow tract. Although its mandatory visualization is a matter of controversy and would bring us closer to a maximum protocol, current technology allows it in most cases, either directly or indirectly thanks to the three-vessel view and facilitated by the systematic use of the Color Doppler. There is evidence of its importance in contributing to increasing detection rate. We must recommend attempting its systematic evaluation, although it is difficult to consider it mandatory.
La evaluación cardiaca fetal en el primer trimestre debe evaluarse sistemáticamente: 1°. Frecuencia cardiaca. 2°. Situs 3°. Eje cardiaco. 4°. Vista de 4 cámaras. 5°. Tracto de salida. Aunque su visualización obligatoria es motivo de controversia y nos acercaría a un protocolo de máximo, la tecnología actual lo permite en la mayoría de los casos, ya sea de forma directa o indirecta gracias a la valoración de tres vasos y facilitada por el uso sistemático del Doppler Color. Existe evidencia de su importancia para contribuir a aumentar la tasa de detección. Debemos recomendar intentar su evaluación sistemática, aunque es difícil considerarla obligatoria.
Before defining imaging systematics, it is advisable to briefly address some of the aspects related to congenital heart diseases (CHD), in order to gain perspective on the impact of this exploratory method.1–3
Incidence and impactCHD are the most frequent severe congenital anomalies and affect 8–9/1000 newborns. They are involved in 20% of neonatal deaths and 40% of perinatal deaths, and their diagnose leads to a high rate of termination of pregnancy (TOP). The majority require one or more neonatal surgeries in highly specialized centers, with a direct impact on personal and family matters on an emotional, socioeconomic, and healthcare level. Furthermore, many types of CHD have an impact on neurodevelopment such as possible neurological sequelae, related to hypoperfusion and chronic deficient oxygenation of some brain areas.
EtiologyUnknown in most cases. 6% are associated with chromosomopathies (trisomies, triploidies, monosomies and microdeletions), 3% with genetic syndromes and 2% with a diversity of maternal, drug, and environmental factors.
Risk groupsWe can classify them into 3 large groups. 1st. Maternal risk due to different diseases: pregestational diabetes, connective tissue diseases, exposure to teratogens, etc. 2nd. Family risk due to a history of CHD (children, father, mother) or syndromes or malformations associated with CHD. 3rd. Fetal risk associated with multiple conditions: Nuchal Translucency (NT)>p99, malformations, chromosomopathies, infections, etc. 90% of CHD occurs in a low-risk population, but the existence of any of these risk factors makes it possible to select a subgroup of pregnant women for an early thorough examination in the first trimester (1stTRI) and to schedule another scan at 16 weeks of gestation.
Association with other anomaliesThere is a high association with chromosomal anomalies, 15–25%, depending on the type of CHD, and the risk of other structural anomalies (SA) is 10 times higher, making hereby a thorough examination of the rest of the anatomy mandatory and vice versa, in the event of an extracardiac malformation, a detailed cardiac scan should be performed.
The advantages of prenatal and early diagnosis of CHD are unquestionable, like: time for complementary tests and consultations, planning delivery in a specialized hospital with cardiac surgery (this significantly improves the prognosis of some children), access to intrauterine surgery for the few indications that currently exist, and/or in those with a worse prognosis, parents can opt for TOP or prepare for the birth of a child with a CHD, etc.
Detection rate (DR)The DR for major CHDs in the different series of the 1stTR varies between 17% and 90%, while the DR for the total CHDs would be between 19% and 33%.3 The technological advances of our equipment, incorporation of the transvaginal route (TVR), better training of professionals, application of care protocols, etc., have made it possible to move the diagnosis of many SA forward to the 1stTRI, including CHD. A meta-analysis that covers six 1erTRI studies with 328,262 fetuses, provides a DR of CHD above 63.67% for the low-risk population and 79.86% for the high-risk population.4 Another recent study series obtained a DR of 58.3% for low risk and 93.5% for high risk.5 The International Society of Ultrasounds in Obstetrics and Gynecology (ISUOG) points out a wide variation in different geographical regions and dependence on the type of CHD, placing the DR below 50%.6 These numbers carry with them a consideration: if DR can reach 80–90% in high risk, meaning visualization of these CHD has been possible… so why can this not be carried out for the general population?
Is it possible to improve the DR?We must consider that from the structural point of view, the fetal heart has the same characteristics at 12–13 weeks as at 20 weeks of gestation7 and that the systematics and formations to be explored are the same in both trimesters, assuming, of course, the differences inherent to the size and the intrinsic limitations of embryological development, which may condition a more incomplete evaluation. We are used to doing the second trimester ultrasound (2nd TRI), so a first consideration to improve our DR could be to transfer the same knowledge, time and diagnostic mentality to the 1stTRI.
There are many other factors that alter the DR; the non-modifiable, associated with the organ that is to be scanned or the sound transmission and the modifiable, linked to the examination process itself: gestational age (GA), equipment, examination route, fetal position, TN>p99, others anomalies, etc., factors that stand in direct relation to the user: experience, training, general knowledge/embryology, sensitivity, dedication, etc., or to the Healthcare system: time, patient pressure, existence of protocols and controls, audits, etc.8 Our objective is not to analyze these factors, but the following are nonetheless worth mentioning, turning them into a useful tool for improvement of our DR: 1st. There is consensus that performing the examination according to standardized protocols and compliance is the most important factor.6,8,9 2nd. The systematic incorporation of the evaluation of the outflow tracts (OT) and color Doppler substantially improves DR.4 3rd. The Guide of the Spanish Society of Gynecology and Obstetrics (SEGO) specifies a list of structures that must be visualized in the 1stTRI ultrasound. Furthermore, for each organ or system it enlists others under the heading “it is also possible to visualize” which, although not mandatory for evaluation, is intended to stimulate its search and the carrying out of a broader and more complete scan.10 This concept was recently incorporated by the ISUOG by differentiating 2 levels of exploration; one considered “basic” and essential and another more advanced, detailed or “best practices” that will allow a greater number of SAs to be detected and recommends that as the user's experience increase, a progress from basic to advanced exploration must be seen.6
Visualization of cardiac structures in the 1stTRIRecent studies focus on the chronology of visualization of the different cardiac structures throughout the 1stTRI11 and others study the possibility of visualizing 4-chamber view (4CV) and OT in the routine examination of the 1stTRI: 4CV and OT (direct view of the OT or through the Three Vessel View (3VV), with/without Doppler, with/without transvaginal route), reaching rates of 97%, 99.2%, 97.9%.12-14
Color DopplerIts systematic incorporation into 1stTRI ultrasound (the embryo-fetus is especially vulnerable) has been a source of controversy due to fear of potential tissue damage as a result of its biological, thermal and mechanical effects, and its routine use was discouraged.15 The 2020 SEGO guideline on fetal heart examination stated: “observing due safety standards, we believe that there are at least two situations that could be considered clear indications for the use of color Doppler: inability to complete the examination (4CV and OT-3VV) and suspicion of CHD”.3 Due caution must be maintained, and fetal exposure limited by applying the ALARA (As Low As Reasonably Achievable) principle, but this does not justify giving up the unquestionable benefits that its systematic use brings us and thus in 2021, the 1stTRI SEGO guide, advocates “responsible use of Doppler in 1stTRI”, although “the use of spectral Doppler should be limited”.10 Subsequently, the new ISUOG recommendations were published, in line with SEGO and insisting that, with due caution, Doppler is indicated in the fetal period (11−13+6s) for screening for CHD and Trisomies, the time of exposure should be as short as possible and no longer than 5–10min (we believe that less than 5min is sufficient for systematic screening). Restriction is maintained in the embryonic period (up to 10+6s only when indicated) and the safety of uterine artery Doppler is remembered because the fetus is outside the Doppler ultrasound field.16
Systematics of the fetal anatomic scan in the first trimesterGuidelines and Scientific Societies differ, sometimes very significantly, in their recommendations regarding exploration systematics and structures that must necessarily be visualized. In many there is no list of structures and/or the recommendations are vague, non-existent or excessively basic, which contrasts strikingly with the protocols for the exploration of the 2ndTRI and also with other publications, where recommendations are extensive and concrete.10 The concept that screening for malformations should be carried out in the morphological ultrasound of the 2ndTRI is still too present, with a very limited role being granted to the 1stTRI for SA screening. This contrasts with the evidence, since the overall DR for major anomalies in the 1stTRI is greater than 50%10 and even more so with the DR for CHD, which, as we previously mentioned, can potentially exceed 60%. The objective data reinforce the concept that the user should undertake this examination with the same diagnostic mentality as of the 2ndTRI.
We find it worth mentioning the following aspects related to the exploration:
1st. Gestational age. There is consensus that the GA for 1stTRI ultrasound is between 11−13+6 weeks of gestation and also that the optimal GA for visualization of fetal anatomy would be 13 weeks of gestation and this is also assumed by ISUOG, SEGO, etc.6,10,17
2nd. Exploration path. The incorporation of the TVR continues to be a source of controversy, which we will not encourage, but we do consider: a/ It provides better image resolution. b/ Different studies suggest that optimal visualization rates are obtained by combining the abdominal and transvaginal routes”.8 c/ SEGO recommends combining both routes.10 d/ ISUOG quotes verbatim: “Detailed assessment of fetal anatomy is best achieved using high-resolution transabdominal and transvaginal transducers. Both approaches may be necessary to complete a systematic examination of the fetal organs and adequate time should be scheduled for this evaluation. “While a transvaginal examination is not mandatory, it may provide better image resolution for the evaluation of fetal anatomy”.6 e/ The only arguments against TVR would be the discomfort (most would accept when the objective is explained) and the additional time consumption (difficult to deny if it improves the DR of SA). If, in the professional's opinion, the addition of the TVR can improve the results of this examination, he or she should not resign from its use.
3rd. Allotted time. It is unusual for guidelines of different societies to opinionate on this matter and, accordingly, the ISUOG does not do so either: “the time must be appropriate”.6 The exploration of the 1stTRI is increasingly gaining more relevance and its objectives have changed a lot in recent years, going from being a very basic exploration to acquiring an importance nothing short of that of the 2ndTRI, which is why we think, in line with the SEGO, that the programmed time should be in accordance with your objectives and should be allowed 25min (the heart scan would take up an importance part of it).10 Nicolaides’ group considers that 30min should be allocated,9 adding 10min to their previous recommendations, in line, as we already mentioned, with the importance that this ultrasound has been acquiring in recent times.18
4th. Professional training. It is important to distinguish the training concept of “making someone fit”, which usually involves the possession of a certain specific accreditation, from that of competence or “aptitude or suitability to do something”. The professional should possess both qualities. However, in Spain there is no specific accreditation for any type of examination, as is standard in other countries, although it requires that the explorer “be specialized personnel, with preferential dedication to fetal/obstetric ultrasound and specific capacity for its practice”.10 Of particular interest is the concept already presented “of good practice” of trying to progress toward an “advanced” exploration.6,10
5th. Protocol compliance. In most cases, explorers must comply with examination protocols defined by national guidelines, scientific communities, hospital centers, etc. There is a consensus that this protocol must necessarily be followed, and if it is not possible, a new examination must be scheduled, requesting, if appropriate, the collaboration of another colleague.6,10
6th. Heart disease markers. The NT is the most important marker and maintains its value. Classic secondary markers, such as ductus venosus and tricuspid regurgitation, of undoubted value a few years ago, are losing interest as technological advances allow a good direct evaluation of the fetal heart structure and its possible alterations19–22). A study compares the performance of the application of markers vs. that of a 4-chamber view (4CV) and concludes that the 4CV is the most sensitive and specific for the detection of CHD in the 1stTRI.23
Deviation of the cardiac axis has been considered as another marker of CHD in early gestation and may be altered in two-thirds of fetuses carrying CHD.24
We share the opinion expressed in 2017 in a Congenital Anomalies Guide on obtaining secondary markers and assumed by the SEGO “the group of experts considers that it should not be part of a population-based screening strategy; although in the centers where they are available, you can use them both in research and as part of your daily routine”.10,25
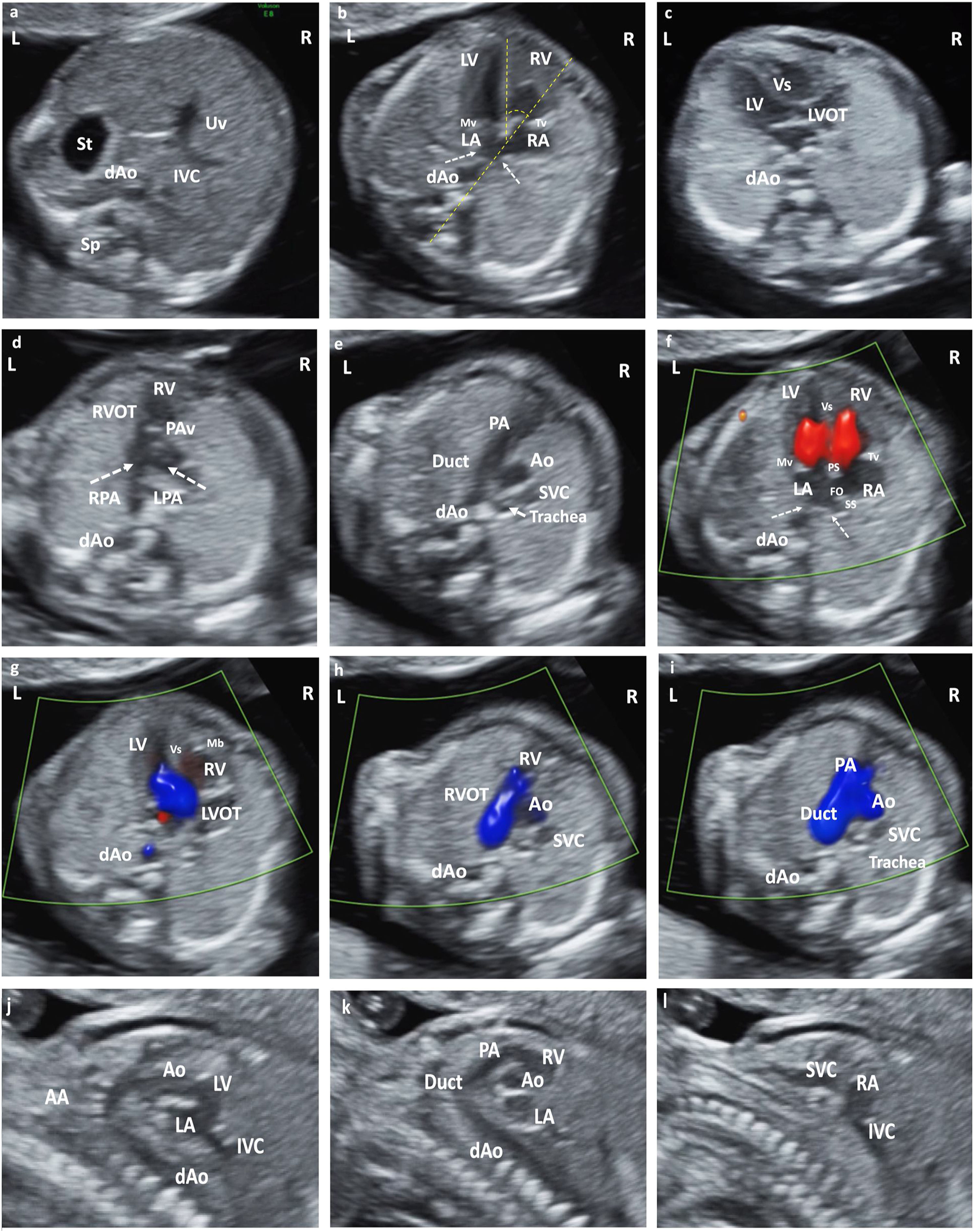
Systematics of exploration (Fig. 1)Structures that must be visualizedThey must be unequivocally defined, given that it is an examination subject to protocol. Remember the disagreement of the different scientific societies regarding the content of the exploration. The following should be systematically evaluated: 1st. Heart rate. 2nd. Situs 3rd. cardiac axis. 4th. 4CV. 5th. Outflow tract (OT). Although its mandatory visualization is a matter of controversy and would bring us closer to a maximum protocol, current technology allows it in most cases, either directly or indirectly thanks to the 3VV and facilitated by the systematic use of the Color Doppler. There is evidence of its importance in contributing to increasing DR.4 We must recommend attempting its systematic evaluation, although it is difficult to consider it mandatory. The indication to schedule a new examination would be less clear if a correct visualization of OT was not possible and given that too many variables are involved, it seems prudent for each institution to determine its need.
(a) Situs solitus. Axial section of the upper abdomen. (b) Apical section 4CV. Situs solitus. Observe pulmonary vein drainage (dashed arrows) in LA, moderator band in RV, and most apical insertion of the Tv. Cardiac axis, angle measurement (dashed yellow line). (c) LVOT section from LV in continuity with Mv and Vs. (d) Section RVOT from the RV. Observe the closed PAv and the bifurcation of the main pulmonary trunk in RPA and LPA. (e) Section of 3V trachea. From left to right and of decreasing size: PA and its continuity with the RA, Ao and SVC. Observe the Trachea that defines the position of the AA as left. (f) Color Doppler in apical 4CV section. Antegrade flow through the atrioventricular valves, with normal biventricular filling. AI with pulmonary vein drainage (dashed arrows). Observe PS, SS and FO. (g) LVOT color Doppler with laminar antegrade flow. (h) Color Doppler in RVOT with laminar antegrade flow. (i) Color Doppler in 3V trachea section. From left to right and of decreasing size: PA (continuity with the Duct), Ao and SVC. Anterograde laminar flow, observing that both PA and Ao code in the same color. (j) Sagittal section of the AA and LVOTT. (k) Sagittal section Ductal arch. (l) Bicave section. Abbreviations: St: Stomach. dAo: Descending aorta. IVC: Inferior Vena Cava. Uv: Umbilical vein. Sp: Spine. RA: Right atrium. LA: Left atrium. RV: Right ventricle. LV: Left ventricle. Mv: Mitral valve. Tv: Tricuspid valve. LVOT: Left ventricular outflow tract. Vs: Ventricular septum. RVOT: Right ventricular outflow tract. PAv: Pulmonary valve. LPA: Left pulmonary branch. RPA Right pulmonary branch. PA: Pulmonary Artery. Duct: Ductus arteriosus. Ao: Aorta. SVC: Superior vein cava. IVC: Inferior vein cava Trachea. PS: Septum primum. SS: Septum secundum. FO: Foramen ovale. AA: Supra-aortic trunks.
Contrast our proposal with that of ISUOG, which for the “basic” examination considers only “axial view of the heart at the level of the four chambers: Heart inside the thorax with regular rhythm” (it does not even explain whether the 4CV should be evaluated nor does it include situs, size or axis), unlike in the “detailed or advanced” examination that describes its content, so it can be understood that it is enough to see an intrathoracic heart with a rhythmic beat.6 With our current technology and supposed professional training for exploration, this proposal seems unaffordable and clearly insufficient.
General considerationsAll these structures that must be visualized are obtained, as in the 2ndTRI, by making the five axial Yagel views, from the upper abdomen to the mediastinum.26 b/ Remember that given the “horizontalization” of the heart in fetal life, the right chambers are the most anterior. c/ Prescriptive adjustments must be made to obtain a good visualization in both 2D and Doppler: size (1/2 to 1/3 of the screen), opening angle (limited to the heart), a single focus adjusted to the lowest point of study, color Doppler with high PRF, adequate gain, etc. d/ The systematic use of cine-loop is highly recommended to observe normal valve opening-closure and unidirectional blood flow without regurgitation.
LateralityBefore beginning the exploration of the heart, we must have a precise idea of how the fetus is positioned (laterality), including mentally placing ourselves in its position and using sagittal and axial planes we will have in mind the organs that we should find to the right and left.
1st. Fetal heart rate (FHR)Rhythmic FHR of 120–160beats per minute (bpm). It implies two concepts about deviations from normality that, although more frequent in 2nd TRI, we must consider: 1st. Rhythmic. There may be some alterations, the most common being “irregular” rhythms usually related to the loss or addition of a beat. They are usually benign and spontaneously resolving alterations and should only be taken into account if they are frequent or persistent. 2nd. Frequency. Transient episodes of bradycardia (≤110bpm) or tachycardia (>160–180bpm) are possible, which only require additional evaluation if they are persistent.
2nd. SitusVisceral situs is understood as the situation of the viscera and atria in relation to the sagittal plane of the organism. An axial section of the upper abdomen will show the stomach in its left upper quadrant and raising the transducer toward the thorax, the heart will be on the same side (2/3 of the heart on the left and 1/3 on the right) in levocardia, with its tip also directed to the left in levoapex, defining the normal situs solitus. If possible, it can be completed with observation of the prevertebral abdominal Aorta (Ao) on the left and the inferior vena cava in front and to its right.
3rd. Cardiac axis. Position of the heartIts subjective evaluation is sufficient, and it will only be measured when an abnormality is suspected. The normal axis forms an angle of 45°±20° to the left between 2 imaginary lines, one from the spine to the sternum and the other that would prolong the interventricular septum. Remember that the heart can be found in 3 positions within the thorax, regardless of situs and apex: a/ left hemithorax: Levocardia. b/ center of the Thorax: Mesocardia. w/ right hemithorax: Dextrocardia. The orientation of the cardiac tip is independent of the situs and the position of the heart: a/ tip to the left: Levoapex. b/ tip in the center: Mesoapex. w/ tip to the right: Dextroapex. An abnormal axis is a good marker of CHD, but it can also be altered by other extracardiac anomalies: diaphragmatic hernia, pulmonary hypoplasia or agenesis, omphalocele, gastroschisis, etc.
4th. Four chamber view (4CV). Size. StructureThe 4CV can be “apical” (spine forward or backward and ultrasound beam parallel to the interventricular septum) or “lateral” (spine to the right or left and ultrasound beam perpendicular to the septum). Although more so in 2ndTRI, in apical 4CV it is common to find an artifact that simulates a defect in the membranous portion of the septum, which disappears in lateral 4CV (good cut to assess the integrity of the septum) or other planes. In this plane, the cardiac size is also subjectively evaluated (only biometry if an anomaly is suspected), which occupies approximately 1/3 of the area of the chest or half of its circumference. Its transverse diameter (measured at the level of the atrioventricular (AV) valves) is slightly less than 50% of the transverse diameter of the thorax.
1st. Confirm the existence of 4 independent cavities: 2 Atria and 2 Ventricles of similar size and characteristics on both sides, separated by the interatrial partitions (formed by septum primum, foramen ovale, septum secundum) and interventricular (2/3 apical and muscular portion, membranous 1/3 proximal), with the thickness of the septum and the ventricular wall being very similar. It will be very difficult to evaluate contractility.
2nd. At this GA, it is not easy to confirm the integrity of the septa, but the crux cordis (convergence of the interventricular septum-septal insertion of the atrioventricular (AV) valves-septum primum) must be clearly identified.
3rd. Confirm the presence of 2 AV valves of similar size with complete and synchronous opening and closing (the valve disappears during diastole) and that the valve rings have a similar diameter.
4th. Behind the LA and in front of the spine, only the descending Ao should be seen and no other echonegative image that could suggest a collector. The existence of a small amount of pericardial fluid is physiological, which should not exceed the level of the AV valves.
In an advanced examinationIt will be possible to visualize on many occasions: a/ the foramen ovale occupies one third of the atrial septum and its valve flaps toward the left atrium (LA), where the entrance of the pulmonary veins can be observed. b/ The right ventricle (RV), more anterior and more triangular, can sometimes appear rougher (moderator band) and does not form an apex, while the left ventricle (LV) is smooth, more elongated and forms the cardiac apex. c/ It will be very difficult to see that the insertion of the septal leaflet of the tricuspid valve is more apical than that of the mitral valve.10 ISUOG includes in “advanced” situs, axis, position, size and 4CV.6
5th. Outflow tract (OT) view. Two-three vessel and three vessel and trachea viewIts systematic evaluation is controversial, but the recommendation is to always attempt its visualization. The OT view can be evaluated through direct visualization or indirectly through the 3 vessel view (3VV), sometimes of 2 vessels (2VV) if it is not possible to see the superior vena cava (SVC) and in other cases of 3V and trachea (3VT) when it is identified. The SEGO heart examination guide recommends “going increasingly toward advanced examination and training for the inclusion of direct or indirect evaluation of OT,3 a position that maintains the SEGO protocol for the 1stTRI and insists on ease to visualize with Doppler the “V” formed by Ao and Pulmonary Artery (PA) (11) Following the instructions of Yagel26 and starting from the 4C axial plane, a slow scan is made in a cephalic direction and the results are successively obtained. OT of Ao and PA, observing the symmetry of size and morphology and confirming that they follow “crossed” paths. Continuing the scan we obtain a cut of 3VV and 3VT.
Left ventricular outflow tract view (LVOT)The Ao leaves the LV in the center of the thorax, runs from left to right, follows a crossed path with respect to the PA and never bifurcates. The valve has normal opening and closing (it disappears during systole) and its size is similar or somewhat smaller than that of the PA.
Right ventricular outflow tract view (RVOT)The PA leaves the RV, more anterior in the thorax, from right to left and follows a crossed path with the Ao. Its valve has normal opening and closing (it disappears during systole) and its size is similar to or somewhat larger than that of the Ao. Sometimes we can see the bifurcation and the two branches of the PA.
2–3V/VT viewIt provides an indirect assessment of the OT, which is equally valid to indicate that they are crossed and of similar caliber. 3VV was described by Yoo in 1997 and 3VT by Yagel in 2002.26,27 They allow us to simultaneously evaluate the 3 vessels and their relationships in terms of number, size, arrangement and alignment: from left to right and from anterior to posterior, well aligned and of decreasing thickness appear: PA, Ao and SVC. In 2VV we visualize the convergence of both arches forming a “V”: ductal and transverse aortic, connecting the PA at the level of the ductus with the descending Ao at the level of the isthmus, which would be 3VV if the SVC is visualized and 3VT if we identify the trachea at the right of the Ao, which would indicate that the Ao arc is left. Obtaining all 3 vessels in a single image is very useful for the diagnosis of most CHDs that present with alterations of the OT and the great vessels. By applying color Doppler, it informs us of the perfusion (usually antegrade) of the large arteries.
In an advanced examinationIt will be possible to visualize on many occasions: a/ LVOT. The Ao appears between the two AV valves, it continues behind with the Mitral Valve (mitro-aortic continuity) and in front with the interventricular septum (septo-aortic continuity). Good plane for evaluation of the interventricular septum. b/ It will be difficult to confirm the ventriculo-arterial concordance of both TS. c/ On some occasions we can see the bifurcation and the two branches of the PA and less often, the thymus.10 ISUOG includes “advanced” LVOT, 3VT, absence of tricuspid regurgitation and positive a wave in the ductus venosus.6
Sagittal viewThey are not part of the systematic examination, but it is possible to obtain the sagittal sections of the Ao and Ductal arches, as well as the bicaval–parasagittal plane.
Doppler. Cine-loopWith due caution, the routine use of color Doppler in all sections, as well as cine-loop, is recommended since they help improve the DR of CHD. The Doppler will help to better anatomical assessment and identification of the different structures and will inform the direction and characteristics of the flow at the AV level with parallel, unidirectional flows without regurgitation, the same at the valve level in the OT and the “V” that should be anterograde and unidirectional without regurgitation. Of great support for the diagnosis of stenosis/atresias or valvular insufficiencies, ductus-dependence in cases of severe obstruction in the ventricular outflow tract, etc.
Possibility of CHD detection after compliance with the protocolThe professionals who perform this ultrasound, regardless of their level of training and/or experience, are required to have sufficient knowledge to detect that a certain structure is not normal. Although a diagnosis cannot be established, the suspicion of deviation from normality allows for a second opinion, at the center or another reference center, which will allow DR to be improved early, as shown in the CHD examples in Figs. 2 and 3.
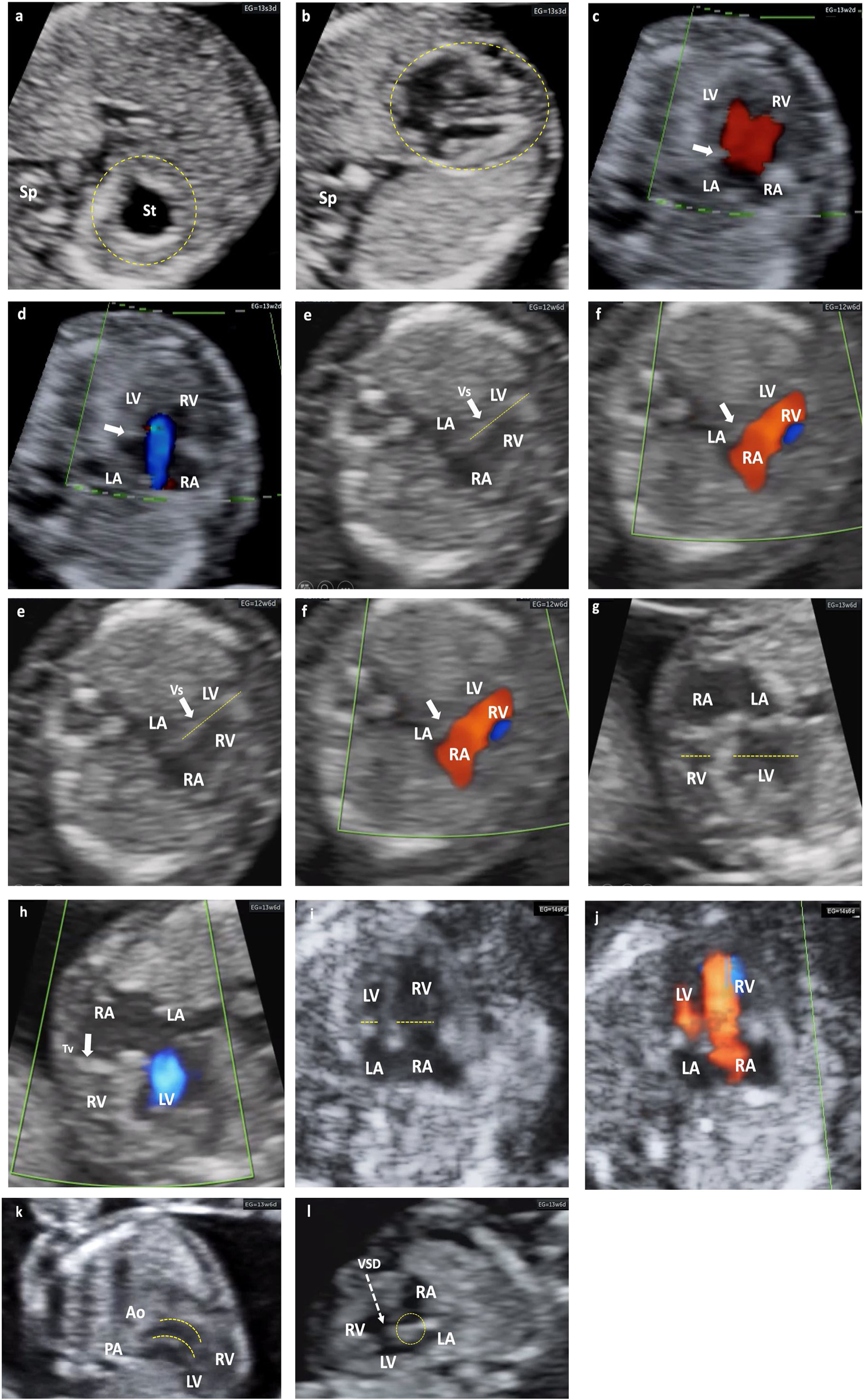
Different cardiac anomalies detected during the first trimester of pregnancy. Abbreviations: St: Stomach. dAo: Descending aorta. IVC: Inferior Vein Cava. Uv: Umbilical vein. Sp: Spine. RA: Right atrium. LA: Left atrium. RV: Right ventricle. LV: Left ventricle. Mv: Mitral valve. Tv: Tricuspid valve. LVOT: Left ventricular outflow tract. Vs: Ventricular septum. RVOT: Right ventricular outflow tract. PAv: Pulmonary valve. LPA: Left pulmonary branch. RPA Right pulmonary branch. PA: Pulmonary Artery. Duct: Ductus arteriosus. Ao: Aorta. SVC: Superior vein cava. Trachea. PS: Septum primum. SS: Septum secundum. FO: Foramen ovale. AA: Supra-aortic trunks. (a and b) Alteration of situs. Situs inversus. Note how the fetal heart and stomach (on the right side of the upper abdomen) are not on the same side. (c and d) Color Doppler. No visualization of the crux cordis and flow passage through a single valve orifice. Complete atrioventricular septal defect. (e and f) Mitral atresia. Striking asymmetry of cavities, the left ones being very small (thick arrow). Color Doppler: antegrade flow through the tricuspid valve and absent through the mitral valve. (g and h) Tricuspid atresia. Significant asymmetry between both cavities at the expense of less development of the RV (dashed lines). Color Doppler: absence of flow through the tricuspid valve, which appears hyperrefringent and thickened (thick arrow). Anterograde transmitral flow. (i and j) Suspected Coarctation of the Aorta, later confirmed. Marked asymmetry of cavities with predominance of right cavities (dashed arrows). Color Doppler with antegrade filling of both ventricular cavities, although asymmetric. (k) Transposition of great arteries. Exit of great vessels in parallel, with ventriculo-arterial discordance, such that the Ao leaves the anterior RV and the PA leaves the posterior LV (dashed lines). (l) Interventricular septal defect (dashed arrow). Observe the integrity of the Crux cordis.
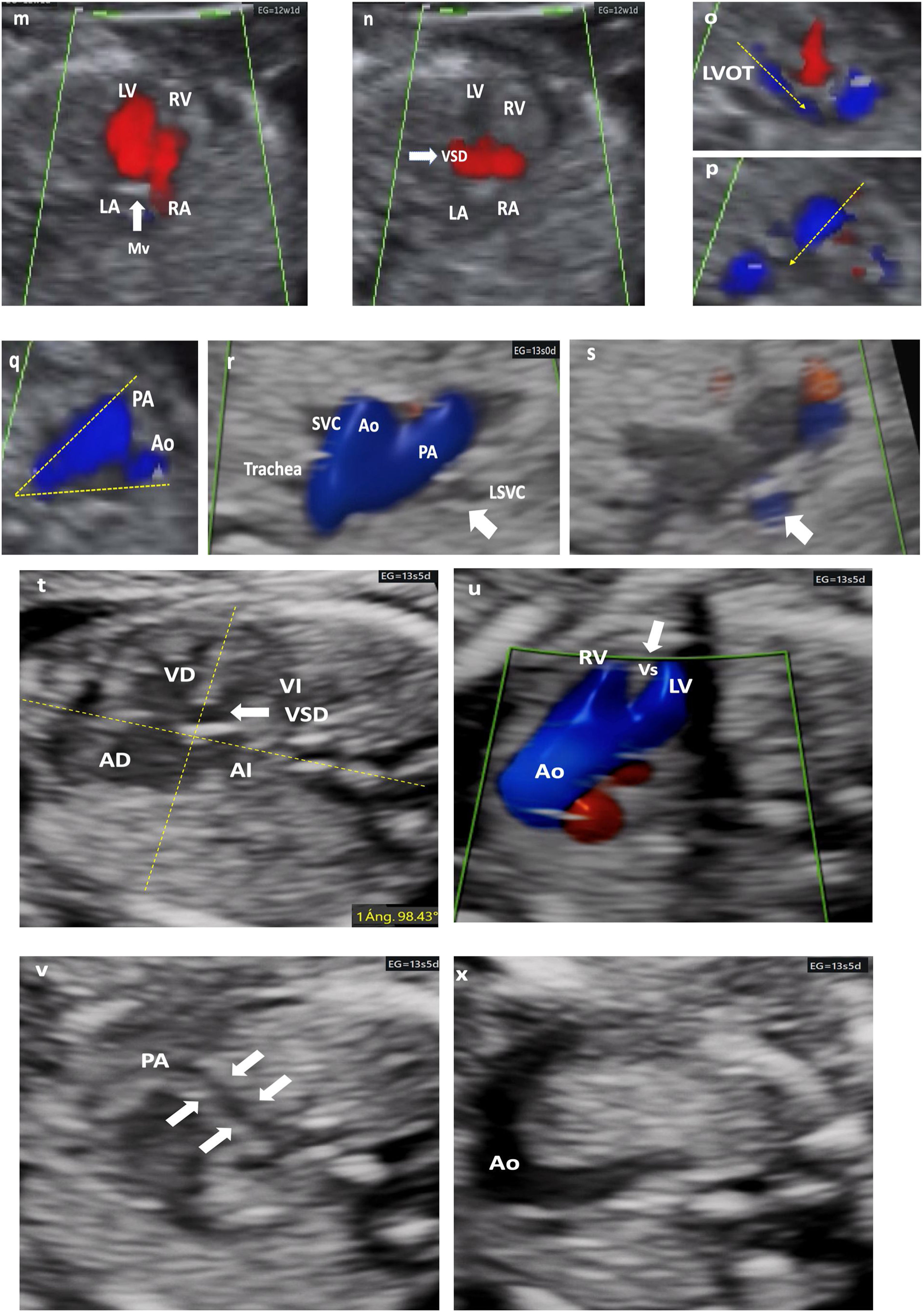
Different cardiac anomalies detected during the first trimester of pregnancy. Abbreviations: St: Stomach. dAo: Descending aorta. IVC: Inferior Vein Cava. Uv: Umbilical vein. Sp: Spine. RA: Right atrium. LA: Left atrium. RV: Right ventricle. LV: Left ventricle. Mv: Mitral valve. Tv: Tricuspid valve. LVOT: Left ventricular outflow tract. Vs: Ventricular septum. RVOT: Right ventricular outflow tract. PAv: Pulmonary valve. LPA: Left pulmonary branch. RPA Right pulmonary branch. PA: Pulmonary Artery. Duct: Ductus arteriosus. Ao: Aorta. SVC: Superior vein cava. Trachea. PS: Septum primum. SS: Septum secundum. FO: Foramen ovale. AA: Supra-aortic trunks. (m–q) Mitral atresia. (m and n) 4C view. Color Doppler. 4C section demonstrating an interventricular septal defect (VSD) that allows LV filling, despite the absence of flow passage through the mitral valve, which appears hyperrefringent and thickened (thick arrow). either. LVOT with a very small caliber Ao (dashed arrow). (p) RVOT, normal caliber (dashed arrow), with crossed paths. (q) 3V view (2V in this case) framed by dashed lines (V) with an Ao of very small caliber. (r and s) 3VT view. Color Doppler. LSVC persistence (thick arrow). Confluent antegrade flow. From left to right and from largest to smallest caliber. PA, Ao, SVC. The aortic arch is left. (t–x) Tetralogy of Fallot. (t) Very horizontal heart. Measurement of cardiac axis at 98° (dashed yellow lines. Interventricular septal defect (thick arrow). (u) LVOT with color Doppler. Ao rides over the septum and fills both ventricles through subaortic interventricular septal defect. (v) RVOT exiting of the RV with severe stenosis (filiform). (x) Very striking transverse aorta, no possible identification of normal 3V section. Note the great asymmetry of the great vessels.
The authors declare that no experiments have been carried out on humans or animals for this research.
Data confidentialityThe authors declare that they have followed their workplace's protocols regarding the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis article is not funded.
Patient consentIn the work, the clinical management protocols of the H U. Cabueñes of Gijón and Valme of Seville have been followed and the consent of the patients is obtained for its publication.
Conflict of interestThis article does not present any conflict of interest.