To evaluate the clinical aspects, diagnoses, prognostic factors, and percent progression of plasmacytoma to multiple myeloma.
MATERIALS AND METHODS103 medical records of patients suspected of plasmacytoma were surveyed covering the period between 1950 and 1998, and 30 were selected for analysis. Patients were classified into 2 groups: patients who did (n = 17) and did not (n = 13) progress to multiple myeloma. Comparative statistics regarding a variety of clincial aspects were developed.
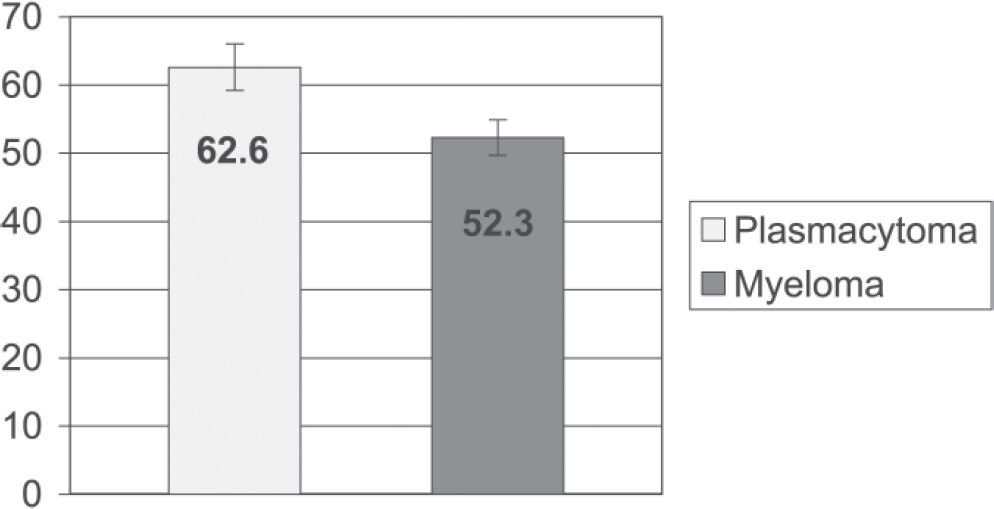
RESULTSPatients who progressed to multiple myeloma were younger than those who did not (52.3 ± 2.6 vs 62.6 ± 3.4 years; mean ± SEM; P = 0.02). There were no significant differences in gender between groups. A higher incidence of multiple recurrence was observed in patients who progressed to multiple myeloma (75%, P = 0.049). Both groups showed a prevalence of vertebral column injuries. No significant differences were found between groups regarding the disease period (from the onset of symptoms until diagnosis) (P = 0.20) and survival (P = 0.34). The average time to progression from plasmacytoma to myeloma was 41 ± 39 months (mean ± SD), and the progression rate was 57%.
CONCLUSIONPatients who progressed to multiple myeloma were younger than those who did not. No significant differences were found between groups regarding sex, time from symptom onset to diagnosis, and survival time. In both groups, the most affected anatomic location was the vertebral column, and most affected sex was male. The average time to progression to multiple myeloma was 41 months. It was not possible to determine the factors that influenced the survival of patients with plasmacytoma or for those who progressed to multiple myeloma.
Avaliar os aspectos clínicos, diagnósticos, fatores de prognóstico e porcentagem de evolução dos casos de plasmocitoma para mieloma múltiplo.
MATERIAS E MÉTODOSForam levantados 103 prontuários do Hospital das Clínicas da FMUSP, entre os anos de 1950 e 1998. Destes, 73 não foram utilizados por perda de seguimento ou por apresentarem diagnóstico diferente de plasmocitoma.
RESULTADOSConcluímos que a idade dos pacientes que evoluíram para mieloma múltiplo é inferior a dos pacientes que não evoluíram. A média do primeiro grupo foi de 52,3 ± 2,6 anos e a do segundo 62,6 ± 3,4 anos (média ± SEM; p=0,02). Não houve diferença estatística quanto ao sexo. Analisando pacientes com plasmocitoma que evoluiu para mieloma múltiplo, foi observada uma incidência maior de recidivas múltiplas (75%, p=0,049). Em ambos os grupos houve predominância de lesões da coluna vertebral. Não houve nenhuma diferença significativa entre os grupos com relação ao tempo de doença (desde o aparecimento dos sintomas até o diagnóstico) (p=0,20) e à sobrevida (p=0,34). Quanto ao tempo de evolução de plasmocitoma para mieloma, a média foi de 41 meses (DP=38,8), com uma taxa de evolução aproximadamente igual a 57%.
CONCLUSÃOOs pacientes que evoluíram para mieloma múltiplo são mais jovens. Não houve diferença significativa entre os dois grupos quanto ao sexo, tempo de doença e tempo de sobrevida. Em ambos os grupos a localização anatômica mais acometida foi a coluna vertebral. O tempo médio de evolução para mieloma múltiplo foi de 41 meses. Não foi possível calcular os fatores que influem na sobrevida dos pacientes com plasmocitoma e dos pacientes com plasmocitoma que evoluiu para mieloma múltiplo.
MacIntyre1 described in 1850 the first case of Mollities et Fragilitas Ossium, and in 1873 Rustizky2 wrote the first definitive pathological description of the disease, proposing the name myeloma, which was consolidated by Kahler3 in 1889. Multiple myeloma was recognized as having a combination of indicators, including osseous deformity, fragility and pain, cachexia, and Bence Jones protein in the urine.
Later, the use of radiography facilitated diagnosing the disease. Radiographic evaluation showed that the disease is characterized by well-defined osteolytic areas without the presence of bone proliferation or sclerosis.
According to the current definition issued by the World Health Organization, multiple myeloma is a malignant tumor with multiple or diffuse involvement of the bone with neoplastic plasma cells having different degrees of immaturity including atypical forms. Lesions are often associated with abnormal presence of proteins in blood and urine and, occasionally, with the presence of amyloid or para-amyloid deposits in the tumoral tissue or other organs.
The probable origin of plasmacytoma relates to mutagenic changes in the development of the B lymphocyte. Possibly, initial oncogenic mutations appear and establish themselves during the late periods of lymphocytic development (these lymphocytes multiply in large scale, forming clones, and their characteristics may also be stored in memory cells). The plasmocyte that originated from a mutated lymphocyte and had its characteristics altered then accommodates in the bone marrow.4 Radiation may induce mutations that activate oncogenes, leading to the development of multiple myeloma. This has been observed with the increased incidence of myeloma in Hiroshima atomic bomb survivors and in radiologists.5,6 Cytogenetical studies in patients with multiple myeloma showed that 68% of samples presented numerical chromosomal alterations (62%), structural alterations (31%), or both (7%).7
Multiple myeloma is generally associated with the production by tumor cells of IgG or IgA monoclonal immunoglobulin or of light chains (Bence Jones protein), while the polypeptidic M protein can be detected in serum and/or urine using immunohistochemical techniques.
As to incidence, in a review of cases of multiple myeloma studied between 1950 and 1975 in the USA, Blattner et al8 found an incidence of 2 or 3 cases of multiple myeloma per 100,000 people, with highest incidence among African American, whose mortality rate was also twice that reported for Caucasians.9 According to the SEER Program, multiple myeloma is responsible for 1.0% of all malignancies in Caucasians and 2% in African Americans. The yearly average (1970 US standard) incidence per 100,000 was 4.7 in Caucasian males and 3.2 in Caucasian females, while for African Americans incidence was nearly doubled, with 10.2 for men and 6.7 for women.10
In general, myeloma occurs in patients over 40, often between 50 and 70 years of age.9 Around 1973, most cases were in the 70 to 80 year age group, with no significant numbers of patients under 40 years of age.5 This difference is due to the improvement in diagnosis enabling patients to discover their disease earlier. Recently, computerized tomography and nuclear magnetic resonance imaging have detected abnormalities in bones that were deemed normal by simple radiographs.11
In the absence of treatment, the prognosis for multiple myeloma survival is lower than 2 years. Patients will eventually die due to renal failure, severe anemia, infections, or complications secondary to neurologic problems.9,12
Plasmacytoma or solitary myeloma is characterized by a single focus, generally in a long bone or a vertebral body. The difference between solitary myeloma and multiple myeloma is that the former does not present other radiologically proven lesions and does present an absence of changes in dysproteinemia and in proteinuria, as well as a negative biopsy from the sternal medulla.9 Other criteria are the absence of anemia, absence of hypercalcemia, and absence of renal involvement.11,13 Plasmacytoma may involve any bone, but it is more common in vertebrae (33.3% of patients).11,15 Plasmacytoma is more common in men. The age at the time of diagnosis has been reported to be less than the age of typical multiple myeloma patients11,14–15 (Figure 1). The therapeutic results are better and the prognosis is more favorable for plasamcytoma than for multiple myeloma.14
As to treatment, radiotherapy is the first modality used for plasmacytoma. The recommended dose of radiation is 5000-6000 cGy for 5 to 7 weeks. Regarding chemotherapy, Holland et al16 reported that it delays the conversion time of plasmacytoma to myeloma. With chemotherapy, the average time to conversion was 59 months, versus 29 months in the group without chemotherapy; however, its use did not reduce the conversion rate, since 64% of patients who received chemotherapy converted to myeloma, while 41% of patients who did not receive chemotherapy presented conversion. Moreover, after conversion, patients who received chemotherapy had the same survival time as patients who did not receive this therapy.16 Currently, surgery is also used as well as a combination of chemotherapy and radiotherapy.9
Another recent type of treatment is the autologous stem cell transplant, with a faster response rate and a longer disease-free survival time than with the conventional form of chemotherapy (alkylating agents or vincristine, doxorubicin and dexamethasone [VAD] alone) .17 Stem cell transplant is a form of treatment for patients over 65 years of age (50% of the patients with multiple myeloma) because high-dose chemotherapy is not recommended for these patients, due to age.18,19
The treatment for multiple myeloma has undergone 2 therapeutic advances, namely, the introduction of high-dose chemotherapy, which has proved to be more effective than conventional chemotherapy, and the use of biphosphonates, which decrease the odds ratio for vertebral fracture.20
In a study published by Bataille and Sany,13 53% of patients with plasmacytoma progressed to multiple myeloma, with the average progression time being 31 months. In some patients, the progression occurred only 17 years later.16 The 10-year survival reached 68.5% for the cases of plasmacytoma.21 This average survival time was better than that found for patients with multiple myeloma who were given alkylating agents only (average: 20 months)22 or a combination of antineoplastic drugs (average: 42 months).13,23
According to the clinical staging system developed by Durie and Salmon,10,13,24 the prognosis for survival is better for patients with plasmacytoma than for patients with Phase I multiple myeloma. Over a 10-year follow-up period, 85% of patients with plasmacytoma presented recurrences, either local, remote, or multiple, the latter being responsible for 58% of the episodes.13,24 Although most publications mention that only 15% of patients with plasmacytoma remain stable for over 10 years, there is evidence that about one third of patients remain free of multiple myeloma for 10 years.11,25–28 Among the prognostic factors are age, size of the injury, persistence of altered immunoglobulins after radiotherapy, axial injury, early diagnosis, and the treatment employed.14
Currently there are no studies in Brazil about the progression of plasmacytoma to multiple myeloma; hence the need for our study that aims to provide clinical and epidemiological data obtained in our service concerning this neoplasia.
MATERIALS AND METHODSA retrospective evaluation was performed that included 103 medical records of patients with anatomic pathological diagnosis for bone plasmocytosis between 1950 and 1998.
The parameters considered were age, sex, anatomic location, time of symptom onset, time to start of treatment, type of treatment given, type of recurrence, time and rate of progression to multiple myeloma, and survival time. Sixty-three medical records were not used because of having been lost to follow-up or for incompleteness; 10 were not used because the disease in question was not plasmacytoma (4 of them were already multiple myeloma at the time of diagnosis, and the others were cases of neuropathy, falciform anemia, reactional plasmocytosis, non-Hodgkin's lymphoma, and subacute osteomyelitis). Thirty medical records were used in the statistical analysis.
The statistical analysis included absolute (n) and relative (%) frequency distribution of the qualitative nominal parameters. The nominal sample data were presented as contingency tables for chi-square analysis (Table 1) and by means of sector graphs. For the comparison of frequencies between occurrences we used Fisher's exact test. (tables 1 and 2; figures 2 and 3). To describe samples of the quantitative ordinal parameters, we used descriptive statistics: mean (M), standard deviation (SD), standard error of the mean (SEM), maximum and minimum values, and the number of cases (N). Ordinal data were presented in statistical tables and represented by column graphs (mean ± SEM) (tables 3, 4, 5, 6, and Figures 1, 4, 5, 6). To compare average values (means), we used the Mann-Whitney U test in case of samples with a nonparametric distribution, and Student's t test for parametric samples. Spearman's nonparametric correlation test (r) was performed on the ordinal parameters survival time (months) and age (years). (Table 7). Qualitative nominal sample data such as anatomic location and type of treatment were presented in descriptive tables, with no statistical analysis (tables 8 and 9; figures 7 and 8). A 5% significance level (a 0.05) and 2-tailed tests were adopted. Results were rounded according to scientific criteria. For the statistical analysis we used the 2002 Microsoft Excel software (Microsoft Corporation) and the 1996 GraphPad Prism V.2.01 (GraphPad Software Inc.).
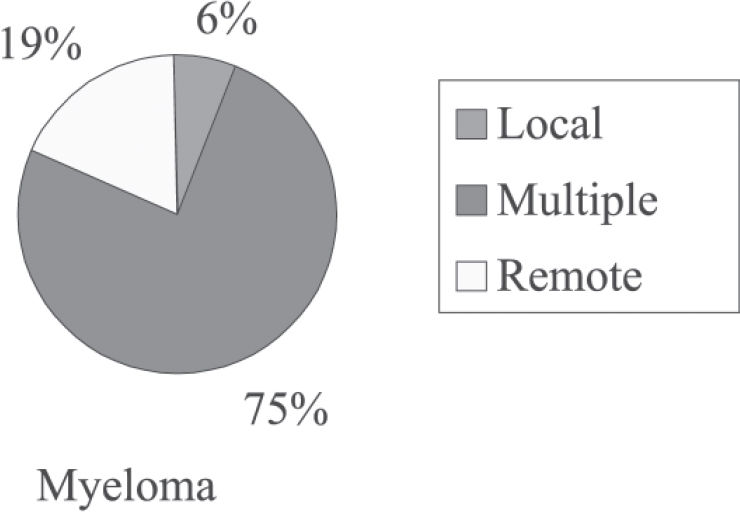
Type of recurrence of primary tumors in patients with plasmacytoma that progressed to myeloma (absolute and relative (%) frequency distribution)
| Type of Recurrence | Myeloma | |
|---|---|---|
| n | % | |
| Local | 1 | 6.2 |
| Multiple | 12 | 75 |
| Remote | 3 | 18.8 |
| TOTAL | 161 | 100 |
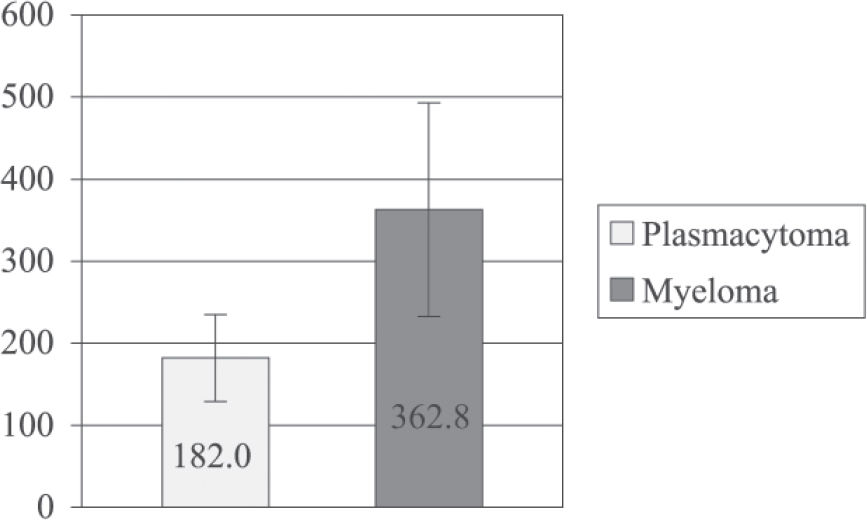
Time (days) from symptom onset until diagnosis in patients with plasmacytoma vs patients with plasmacytoma that progressed to multiple myeloma
| PERIOD OF DISEASE (days) | ||
|---|---|---|
| PLASMACYTOMA | MYELOMA | |
| MEAN | 182.0 | 362.8 |
| SD | 191.8 | 469.5 |
| SEM | 53.2 | 130.2 |
| MAX | 735.0 | 1835.0 |
| MIN | 31.0 | 8.0 |
| N | 13 | 131 |
Age (in years) in patients with plasmacytoma at the time of the first visit vs patients with plasmacytoma that progressed to multiple myeloma
| Age (years) | ||
| Plasmacytoma | Myeloma | |
| Mean | 62.6 | 52.3 |
| SD | 12.2 | 10.9 |
| SEM | 3.4 | 2.6 |
| MAX | 85 | 69 |
| MIN | 47 | 27 |
| N | 13 | 17 |
Student's t test t = 2.4 P = 0.02*
SD = standard deviation. SEM = standard error of the mean. N = number of patients
Progression time (months) in patients with plasmacytoma that progressed to multiple myeloma (descriptive statistics)
| Plasmacytoma to Multiple Myeloma | |
|---|---|
| Mean | 41 |
| SD | 38.8 |
| SEM | 10 |
| MAX | 120 |
| MIN | 1.5 |
| N | 151 |
Anatomic location of primary tumors in patients with plasmacytoma and in patients with plasmacytoma that progressed to myeloma (absolute and relative (%) frequency distribution)
| Anatomic | Plasmacytoma | Myeloma | Total | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Spinal Column | 8 | 26.7 | 8 | 26.7 | 16 | 53.3 |
| Clavicula | 0 | 0 | 1 | 3.3 | 1 | 3.3 |
| Humerus | 1 | 3.3 | 3 | 10 | 4 | 13.3 |
| Ilium | 2 | 6.7 | 2 | 6.7 | 4 | 13.3 |
| Femur | 2 | 6.7 | 3 | 10 | 5 | 16.7 |
| TOTAL | 13 | 43.3 | 17 | 56.7 | 30 | 100 |
n = number of patients
Type of treatment received by patients with plasmacytoma and by patients with plasmacytoma that progressed to myeloma (absolute and relative (%) frequency distribution)
| Type of treatment | Plasmacytoma | Myeloma | Total | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Surgery only | 3 | 10.7 | 1 | 3.6 | 4 | 14.3 |
| Chemotherapy only | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Radiotherapy only | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| CT/RDT | 3 | 10.7 | 2 | 7.1 | 5 | 17,9 |
| RDT/Surgery | 1 | 3.6 | 0 | 0.0 | 1 | 3,6 |
| CT/Surgery | 2 | 7.1 | 7 | 25.0 | 9 | 32.1 |
| CT/RDT/Surgery | 3 | 10.7 | 6 | 21.4 | 9 | 32.1 |
| TOTAL | 121 | 42.9 | 161 | 57.1 | 281 | 100.0 |
The average age at diagnosis of patients who progressed to multiple myeloma was less than that of patients who did not progress (52.3 ± 2.6 vs 62.6 ± 3.4 years (mean ± SEM), respectively, P = 0.02).
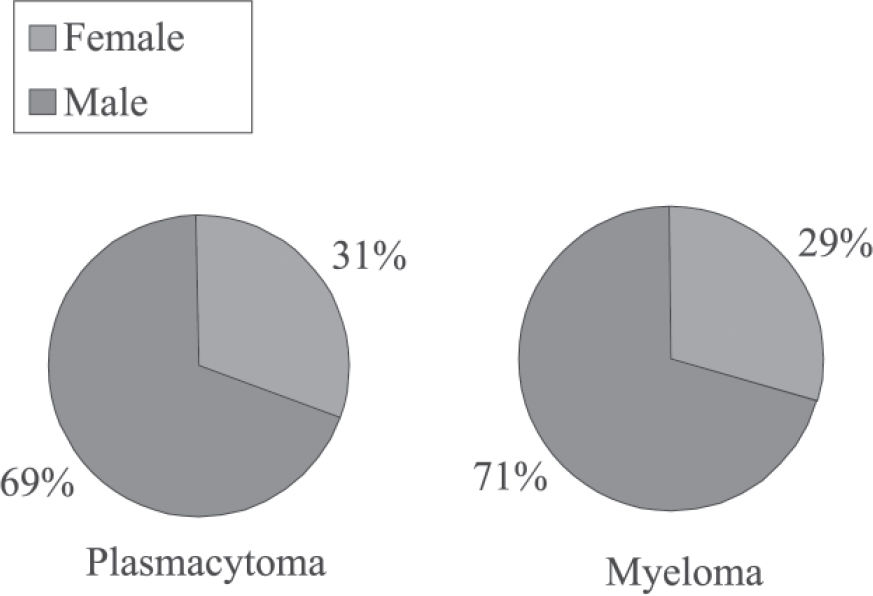
There was a prevalence of the male sex in both groups (patients who progressed to myeloma, 71%; patients who did not progress, 69%; patients overall, 70%). No significant difference was found between groups (P = 0.99)
There was a higher incidence of multiple recurrence than of single recurrence of primary tumors in patients with plasmacytoma that progressed to multiple myeloma (multiple recurrence, 75%; local recurrence, 6.2%; distant recurrence, 19%; P = 0.049).
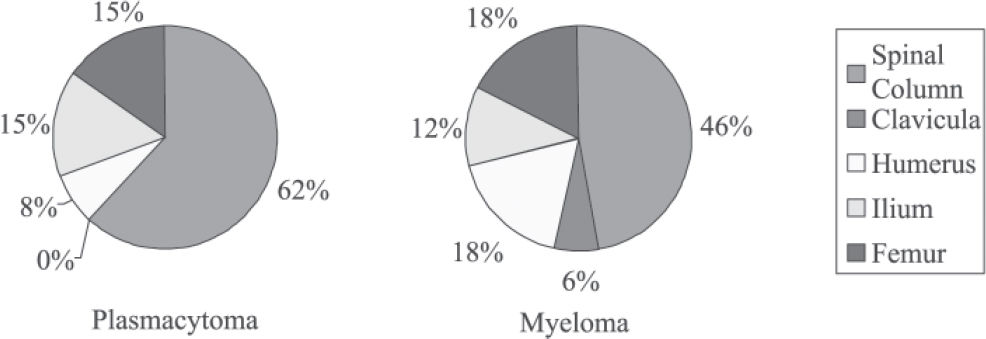
Regarding anatomical location, the vertebral column was the most frequent location in both groups (61% and 47% in the plasmacytoma and multiple myeloma groups, respectively).
Regarding the time from the onset of symptoms until diagnosis, there was no significant difference between groups (182 ± 53.2 vs 362.8 ± 130.2 days (mean ± SEM), P = 0.34, for patients who did not progress vs patients who did progress to multiple myeloma, respectively).
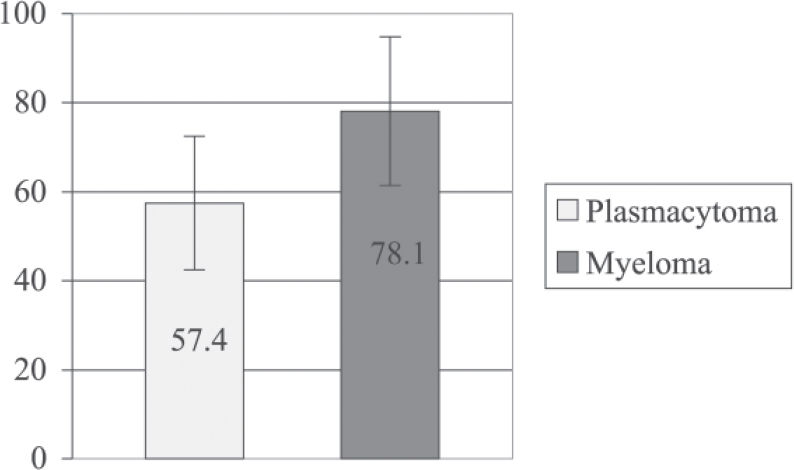
Regarding survival time, there was no difference between groups (57.4 ± 15 vs 78 ± 16.71 months (mean ± SEM), P = 0.34, for patients who did not progress vs patients who did progress to multiple myeloma, respectively).
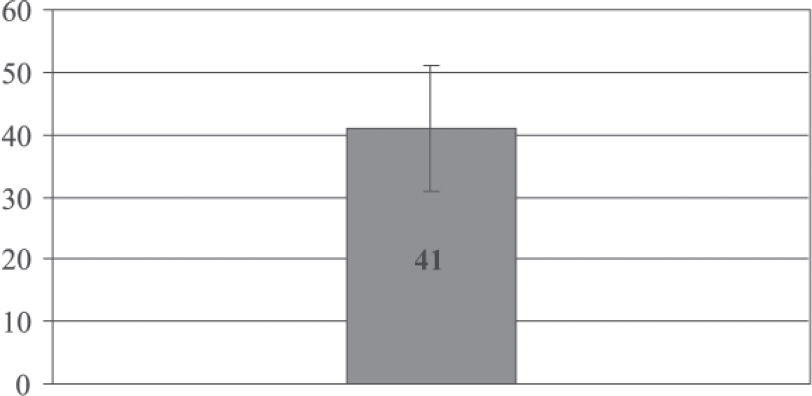
Regarding time to progression, the average was 41 ± 10 months (mean ± SEM). No correlation was found between the time to progression and age (P = 0.13). The progression rate of plasmacytoma to multiple myeloma was 57% (17 of 30 patients).
Regarding anatomic location, in the group with plasmacytoma, we found the following: vertebral column, 62%; humerus, 8%; pelvis, 15%; and femur, 15%. In the group with plasmacytoma that progressed to multiple myeloma, the values were as follows: vertebral column, 47%; clavicula, 6%; humerus, 18%; pelvis, 12%; and femur, 18%.
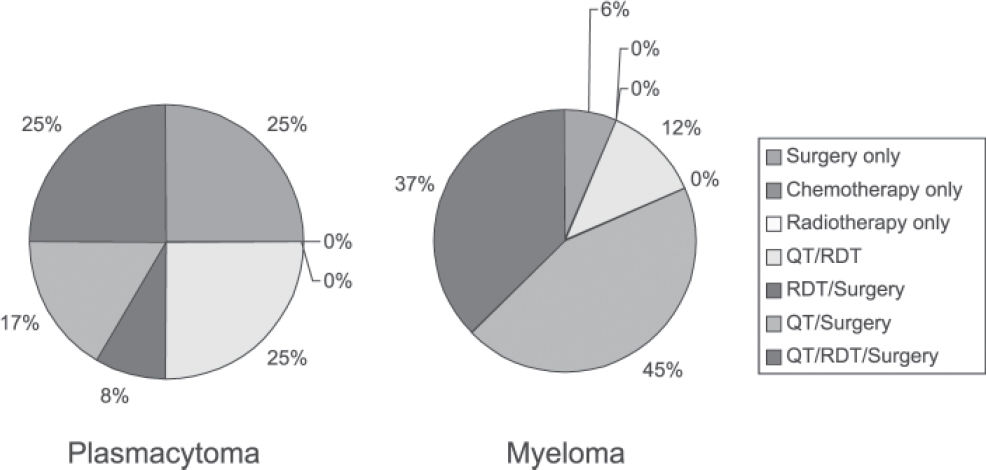
Treatments administered to patients with plasmacytoma were as follows: 25% surgery only; 25% chemotherapy and radiotherapy; 8% radiotherapy and surgery; 17% chemotherapy and surgery; and 25% chemotherapy, radiotherapy, and surgery. Treatments administered to patients with plasmacytoma that progressed to multiple myeloma were as follows: 6.25% surgery only; 12.5% chemotherapy and radiotherapy; 43.75% chemotherapy and surgery; and 37.5% chemotherapy, radiotherapy, and surgery.
Regarding survival time, there was no significant difference between groups (57.4 ± 15 vs 78.1± 16.7 months (mean ± SEM), P = 0.34, for patients with plasmacytoma vs patients who progressed to multiple myeloma, respectively).
DISCUSSIONThis study is part of an institutional effort to retrospectively summarize some of our most significant patient series.29–31
Holland et al16 found a mean survival time of 10.7 years for patients with plasmacytoma, whereas we found the survival time to average only 4.8 years. Olmo et al12 and Schajowicz et al16 observed a mean survival time of 2 years for patients who progressed to multiple myeloma, whereas for our series, the mean survival time was 6.5 years. Therefore, our results are different from those reported in literature, which might be explained by the fact that a small sample was used or because it was difficult to obtain data about the progression of patients. In the study by Bataille et al.13 the authors mentioned that it was difficult to find a relationship between plasmacytoma and multiple myeloma because these are rare bone tumors.
From the statistical analysis performed we concluded that patients who did not progress to multiple myeloma were older than patients who did. In contrast to our findings, in the study by Bataille et al,13 patients with plasmacytoma were younger than those who had plasmacytoma that progressed to myeloma (45.7 ± 9.5 vs 51.1 ± 12 years (mean ± SD), respectively, P < 0.1). Additionally, Dimopoulos et al11 report that patients with plasmacytoma were approximately 7 years younger than those with multiple myeloma
Regarding the frequency distribution of patients’ sex, no statistically significant difference was found between groups (plasmacytoma and plasmacytoma that progressed to multiple myeloma), although in both groups the male sex was more affected. Seventy percent of all patients were men, which is in agreement with the data of Dimopoulos et al11 and Holland et al.16
After analyzing the frequency distribution of the type of recurrence of primary tumors in patients with plasmacytoma that progressed to multiple myeloma, we observed a higher incidence (78%) of multiple recurrence, than that (58%) found by Bataille and Sany.13 These authors observed that 85% of patients showed progression to multiple myeloma, while in our study only 57% of patients showed this progression, which is similar to the 53% found by Holland et al,16 and to the 54% found by Frassica et al.27
We found the prevalence of vertebral column injuries of 53%, a value higher than the 33.3% found by Dimopoulos et al11 and the 34% found by Holland et al.16 According to Bataille and Sany,13 in patients with plasmacytoma, vertebral column injuries were found in 26.7% of cases, while in patients with plasmacytoma who progressed to multiple myeloma this percentage was 61.8% (P < 0.01), which differs from the present study, where patients with plasmacytoma that did not progress to multiple myeloma presented more injuries in their vertebrae.
Regarding the time to progression, the average was 41 months compared to 31 months reported by Bataille and Sany.13
CONCLUSIONPatients who progressed to multiple myeloma were younger than those who did not; more than half of our patients progressed to multiple myeloma. There are no significant differences between both groups in terms of sex, time from symptom onset to diagnosis, or survival time. The most affected anatomic location in both groups was the vertebral column, and most patients were males. The mean time to progression to multiple myeloma was 41 months, which was somewhat higher than previously reported. Multiple recurrences were significantly more frequent than single recurrences.