The need for parathyroidectomy to treat asymptomatic patients with primary hyperparathyroidism is controversial. The aim of this study was to assess the impact of parathyroidectomy vs. surveillance on skeletal outcomes such as bone mineral density (BMD) and incident fractures.
MethodsThis was a retrospective cohort study including 170 patients (112 treated with surgery and 58 subject to active surveillance) between 1991 and 2014. Changes in BMD in lumbar spine, femoral neck, total hip, and radius, and incidence of fractures, were monitored for 2–6 years.
ResultsPatients treated with surgery had BMD gains at 2 years of 4.37%, as compared to 1.59% in non-operated patients (p<0.05) in the lumbar spine, 3.90% vs. 0.19% (p<0.05) in the femoral neck, and 2.70% vs. 0.14% (p<0.05) in total hip. Gain in BMD in the lumbar spine and femoral neck remained significant in operated patients at 4 and 6 years. No improvement was seen in the radius in operated patients. No significant difference was seen in fracture occurrence between operated and non-operated patients.
ConclusionPatients with primary hyperparathyroidism treated with surgery experience greater BMD gains than non-operated patients, especially in the lumbar spine and femoral neck. The risk of fracture does not decrease in the group of operated patients.
Existe cierta controversia sobre la indicación quirúrgica del hiperparatiroidismo primario, sobre todo en pacientes asintomáticos. El objetivo de este estudio es valorar la evolución de la densidad mineral ósea (DMO) y la aparición de fracturas en pacientes operados vs. pacientes seguidos sin cirugía.
MétodosSe trata de un estudio retrospectivo de cohortes en el que se incluyó a 170 pacientes (112 tratados con cirugía y 58 seguidos sin cirugía) entre los años 1991 y 2014. Se analizó la evolución de la DMO en columna lumbar, cuello femoral, cadera total y radio en 2-6 años de seguimiento, así como la aparición de fracturas.
ResultadosLos pacientes tratados con cirugía experimentaron una ganancia de DMO a los 2 años en columna lumbar del 4,37% vs. el 1,59% en no operados (p<0,05); en cuello femoral del 3,90% vs. el 0,19% (p<0,05) y en cadera total del 2,70% vs. el 0,14% (p<0,05). La ganancia de DMO continuó siendo significativa en pacientes operados a los 4 y 6 años, en columna lumbar y cuello femoral. No se observó mejoría en radio distal en los pacientes tratados quirúrgicamente. La aparición de fracturas durante el tiempo de seguimiento no mostró diferencia significativa entre ambos grupos.
ConclusionesLos pacientes con hiperparatiroidismo primario tratados con cirugía experimentan una ganancia de DMO superior a los pacientes no operados, tanto en columna lumbar como en cuello femoral. El riesgo de fractura no desciende en el grupo de pacientes tratados con cirugía.
Primary hyperparathyroidism (PHP) is an endocrine disease characterized by the excessive or inappropriate production of parathyroid hormone (PTH) by one or more of the parathyroid glands. The clinical spectrum of PHP changed drastically in the early 1970s with the introduction of calcium in multichannel biochemical analyzers, which resulted in the detection of a significant number of patients with previously unsuspected asymptomatic disease.
Bone is a classic target organ in PHP, and it is well known that bone turnover is reversibly increased in these patients, leading to a decrease in bone mineral density (BMD)1 and a potential increased risk of fracture. The surgical treatment of PHP is associated with an increase in BMD, particularly at sites rich in trabecular bone,2,3 while locations predominantly characterized by cortical bone such as the distal third of the radius experience substantially irreversible losses.4 The existing information on fracture risk reduction after surgery is contradictory. Some studies report a decreased risk of hip and forearm fracture, but no decrease in vertebral fracture risk.5 Other studies have observed no increased risk of fractures as compared to controls after surgery, and some have reported an increased risk of distal radius fracture consistent with the low bone mass gain commented on above.6
The management of asymptomatic forms of PHP remains the subject of debate. No studies on the course of this disease have been made in Spain. An analysis of what is happening in daily clinical practice in terms of BMD evolution and fracture risk in patients subjected to surgery and in patients managed on a conservative basis is therefore of considerable relevance.
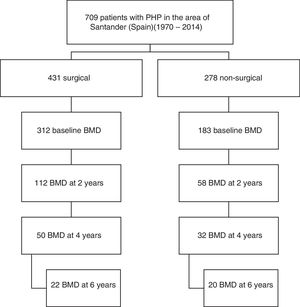
Material and methodsA retrospective cohort study was carried out, with a total of 709 cases of PHP in the healthcare area of Santander (Spain) during the period 1970–2014 being identified. Cases of PHP were defined as hypercalcemia together with high or inappropriately normal PTH levels on at least two occasions. We excluded cases of normocalcemic PHP, familial hypocalciuric hypercalcemia, cases of PHP in the context of multiple endocrine neoplasms, and transient calcium or PTH elevations after drug treatments such as vitamin D supplements or bisphosphonates had been started. We also excluded hypercalcemia associated with advanced chronic renal failure or kidney transplantation. All patients were over 18 years of age and of Caucasian origin.
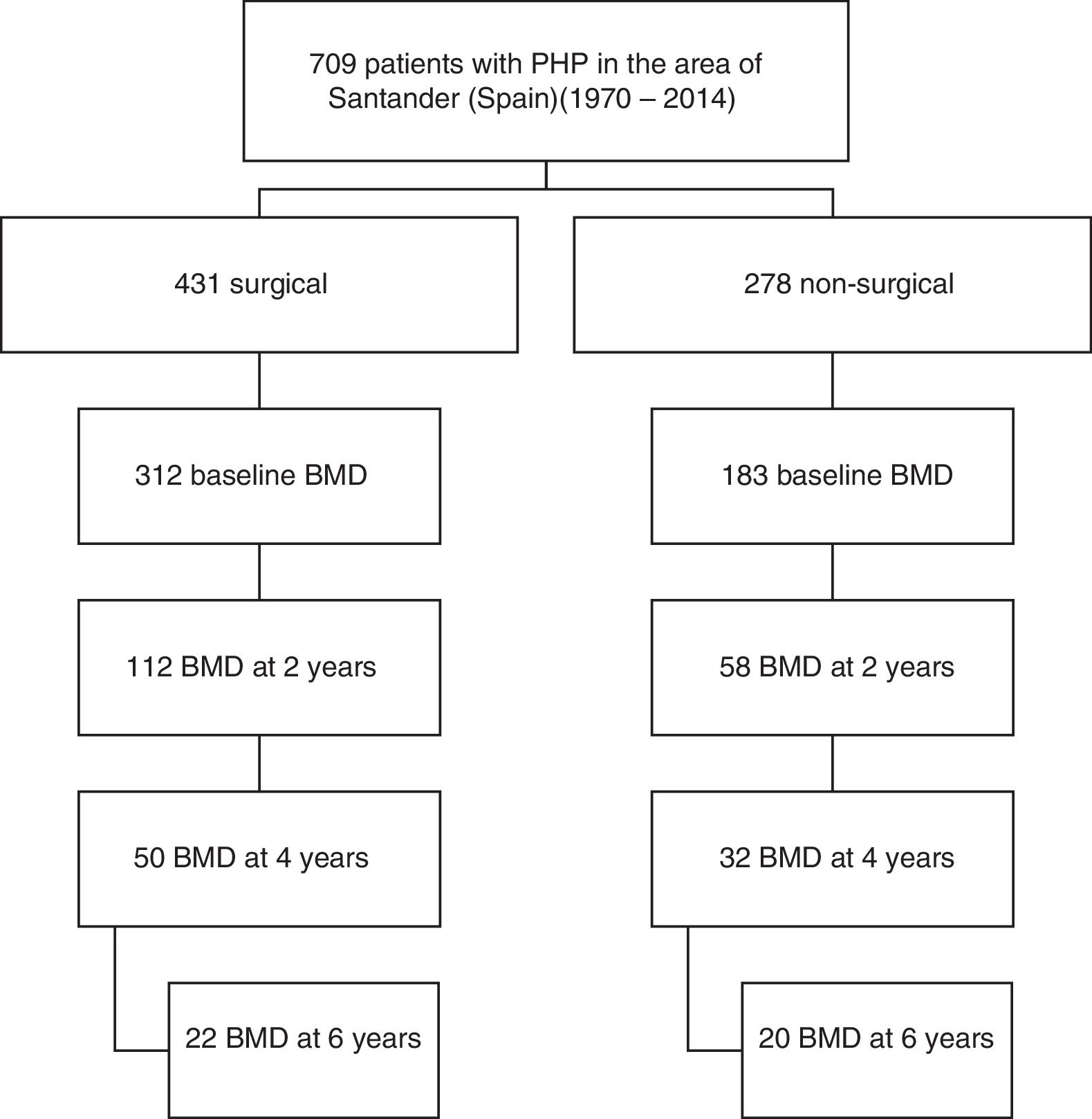
Of the total cases, 170 patients (92% women) from the period 1991–2014 who met the requirement of having a bone densitometry study at baseline and at least another study after two years of follow-up were selected. Symptomatic patients were defined as those with symptoms or complications directly attributable to hypercalcemia or excess PTH, such as lithiasis and osteitis fibrosa cystica. A total of 112 patients had surgery, while another 58 were managed on a conservative basis. Data referring to the subsequent evolution of BMD up to 6 years were available in part for both study arms (Fig. 1).
The analysis of fracture incidence and BMD changes was carried out in the 170 patients until the end of 2016, with follow-up periods of 6 years (4–9) (median and interquartile range [IQR]), from parathyroid surgery in the surgical patients, and 8 years (6–11) from diagnosis in the non-surgical patients. Spontaneous or traumatic fractures of vertebra, hip or radius were recorded. Patients receiving treatment for osteoporosis were not excluded from the fracture analysis. For the analysis of bone remodeling markers, we analyzed a subgroup of 56 patients meeting a number of criteria that make these markers valid: the absence of renal failure and of recent infections or inflammatory conditions, and the absence of treatment with potentially interfering drugs such as bisphosphonates, strontium ranelate, corticosteroids, anticonvulsants, estrogens or thiazides. Of the 56 patients, 42 corresponded to surgical cases and 14 to non-surgical cases.
Biochemical analysesTotal calcium (serum and urine), phosphorus and creatinine were determined using automated methods (ADVIA 2000, Siemens Corp., Tarrytown, NY, USA). Total serum calcium was corrected for albumin. Ionized calcium was measured using automated selective calcium electrodes on a Ciba Corning 634 Ca++ pH analyzer (Ciba Corning Diag. Corp., Medfield, MA, USA). The measurement of intact PTH has changed over the years. From 1991 to 2001 it was assayed by immunoradiometric assay (IRMA) (Nichols Institute, San Juan Capistrano, CA, USA); from 2002 to 2005 with Duo PTH IRMA (Scantibodies Laboratory, USA); and from 2006 to 2012 using Liason automated chemiluminescent assay (DiaSorin, Stillwater, MN, USA). Lastly, from 2012 to date, PTH measurement has been based on Ysis automated chemiluminescent assay (IDS-iSYS, Immunodiagnostic Systems Ltd., Great Britain). In turn, from 1993 to 2005, 25-OH-vitamin D3 (25-OH-vitamin D) was measured by radioimmunoassay (RIA) (DiaSorin, Stillwater, MN, USA), while from 2006 to 2010 use was made of Liaison chemiluminescent assay (DiaSorin), and from 2011 to 2015 the Ysis automated chemiluminescent assay (IDS-iSYS) was employed. Bone alkaline phosphatase was measured by immunoassay (Alkphase B kit, Metra Biosystems, Mountain View, CA, USA). Serum osteocalcin was measured by immunoradiometric analysis (OSTEO-RIACT kit, CIS Bio international, Gif-sur-Yvette, France). The levels of amino-terminal propeptide of type I collagen (P1NP) were measured by RIA (Orion Diagnostica, Espoo, Finland). Crosslaps were measured using ELISA (Nordic Bioscience Diagnostics, Herlev Hovedgade, Denmark).
Evaluation of bone mineral densityBone mass was evaluated by X-ray absorptiometry at three different levels: the lumbar spine (L2–L4), the hip (femoral neck and total projection), and the distal radius. Changes in BMD values were expressed as the percentage variation from baseline. A Hologic QDR 4500 densitometer (DXA, Hologic, Waltham, MA, USA) with a precision error in the lumbar spine of 1.08% and in the femoral neck of 1.50% was used in over 80% of the patients. In the remaining cases, we used a Lunar DPX-L densitometer (Lunar Corp., Madison, WI, USA), with a precision error in the lumbar spine of 1.22% and in the femoral neck of 1.97%. Each patient was always followed-up on with the same equipment being used as in the first exploration.
MedicationTreatment with vitamin D supplements or drugs for osteoporosis was not considered an exclusion criterion, but was taken into account when we were analyzing the data.
Statistical analysisThe data were processed using the SPSS version 15.0 statistical package. In relation to the statistical analysis, the Kolmogorov–Smirnov test was used first to determine whether the data exhibited a normal distribution or not. Continuous variables were expressed as the mean±standard deviation (SD) in the presence of a normal distribution, or as the median and interquartile range (IQR) if otherwise. Categorical variables were given as percentages. The Student t-test was used to compare the surgical and non-surgical groups in the case of variables with a normal distribution, while the Mann–Whitney U-test was applied to variables with a non-normal distribution. Categorical data were analyzed using the chi-squared test. A logistic regression model was used to identify what in the existing literature are considered risk factors for hip fracture: age, gender, previous fractures, treatment for osteoporosis, and surgical treatment for PHP. The Spearman correlation coefficient (rs) was applied to establish correlations between variables exhibiting a non-normal distribution. Statistical significance was considered for p<0.05.
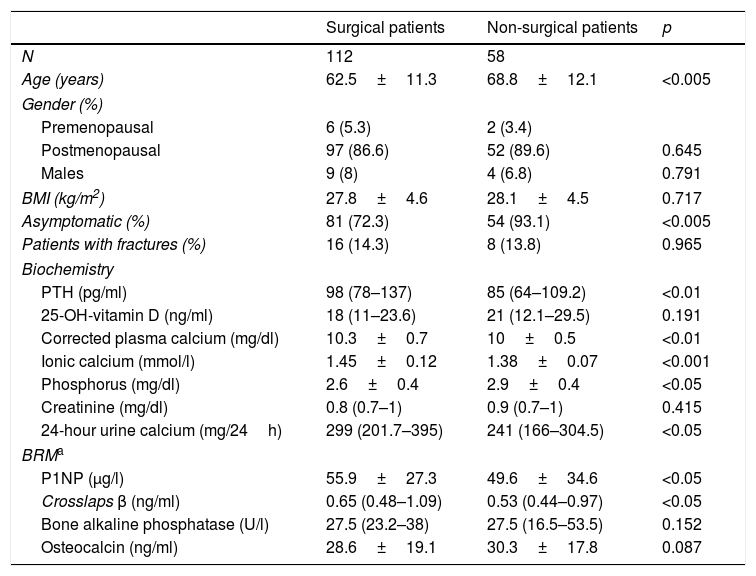
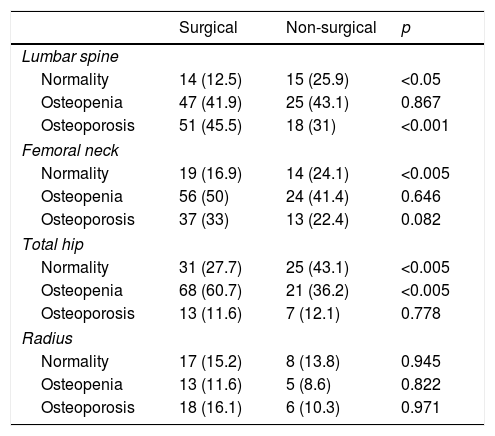
ResultsAt baseline, the patients subjected to surgery were younger and had a higher prevalence of symptoms such as lithiasis than those who did not undergo surgery (21.4% vs. 6.9%; p<0.05). They also had higher blood and urine calcium levels, and higher PTH levels. The surgical patients also had a greater percentage of osteoporosis in the lumbar spine and higher remodeling marker values (Tables 1 and 2).
Baseline characteristics of the study population.
| Surgical patients | Non-surgical patients | p | |
|---|---|---|---|
| N | 112 | 58 | |
| Age (years) | 62.5±11.3 | 68.8±12.1 | <0.005 |
| Gender (%) | |||
| Premenopausal | 6 (5.3) | 2 (3.4) | |
| Postmenopausal | 97 (86.6) | 52 (89.6) | 0.645 |
| Males | 9 (8) | 4 (6.8) | 0.791 |
| BMI (kg/m2) | 27.8±4.6 | 28.1±4.5 | 0.717 |
| Asymptomatic (%) | 81 (72.3) | 54 (93.1) | <0.005 |
| Patients with fractures (%) | 16 (14.3) | 8 (13.8) | 0.965 |
| Biochemistry | |||
| PTH (pg/ml) | 98 (78–137) | 85 (64–109.2) | <0.01 |
| 25-OH-vitamin D (ng/ml) | 18 (11–23.6) | 21 (12.1–29.5) | 0.191 |
| Corrected plasma calcium (mg/dl) | 10.3±0.7 | 10±0.5 | <0.01 |
| Ionic calcium (mmol/l) | 1.45±0.12 | 1.38±0.07 | <0.001 |
| Phosphorus (mg/dl) | 2.6±0.4 | 2.9±0.4 | <0.05 |
| Creatinine (mg/dl) | 0.8 (0.7–1) | 0.9 (0.7–1) | 0.415 |
| 24-hour urine calcium (mg/24h) | 299 (201.7–395) | 241 (166–304.5) | <0.05 |
| BRMa | |||
| P1NP (μg/l) | 55.9±27.3 | 49.6±34.6 | <0.05 |
| Crosslaps β (ng/ml) | 0.65 (0.48–1.09) | 0.53 (0.44–0.97) | <0.05 |
| Bone alkaline phosphatase (U/l) | 27.5 (23.2–38) | 27.5 (16.5–53.5) | 0.152 |
| Osteocalcin (ng/ml) | 28.6±19.1 | 30.3±17.8 | 0.087 |
Variables with a normal distribution are given as the mean±standard deviation, while variables with a non-normal distribution are reported as the median and interquartile range.
BMI: body mass index; BRM: bone remodeling markers.
Reference values:
PTH: 10–45pg/ml; 25-OH-vitamin D: insufficiency <20ng/ml; plasma calcium: 8.1–10.4mg/dl; ionic calcium: 1.16–1.30mmol/l; 24-hour urine calcium: males <300mg/24h, females <250mg/24h.
Bone mineral density: baseline status.
| Surgical | Non-surgical | p | |
|---|---|---|---|
| Lumbar spine | |||
| Normality | 14 (12.5) | 15 (25.9) | <0.05 |
| Osteopenia | 47 (41.9) | 25 (43.1) | 0.867 |
| Osteoporosis | 51 (45.5) | 18 (31) | <0.001 |
| Femoral neck | |||
| Normality | 19 (16.9) | 14 (24.1) | <0.005 |
| Osteopenia | 56 (50) | 24 (41.4) | 0.646 |
| Osteoporosis | 37 (33) | 13 (22.4) | 0.082 |
| Total hip | |||
| Normality | 31 (27.7) | 25 (43.1) | <0.005 |
| Osteopenia | 68 (60.7) | 21 (36.2) | <0.005 |
| Osteoporosis | 13 (11.6) | 7 (12.1) | 0.778 |
| Radius | |||
| Normality | 17 (15.2) | 8 (13.8) | 0.945 |
| Osteopenia | 13 (11.6) | 5 (8.6) | 0.822 |
| Osteoporosis | 18 (16.1) | 6 (10.3) | 0.971 |
Data expressed as the number of patients and the percentage with respect to the total patients in each group. Normality was defined as: a T score greater than −1 SD; osteopenia: a T score between −1 and −2.5 SD; osteoporosis: a T score equal to or less than −2.5 SD.
Thirty-three of the non-surgical patients (57%) received vitamin D supplements, vs. 65 of the surgical patients (58%). With regard to treatment for osteoporosis, 58% of the surgical patients and 63.8% of the non-surgical patients received some type of treatment, fundamentally bisphosphonates (51.8% of the surgical patients and 56.9% of the non-surgical patients), at some point during follow-up.
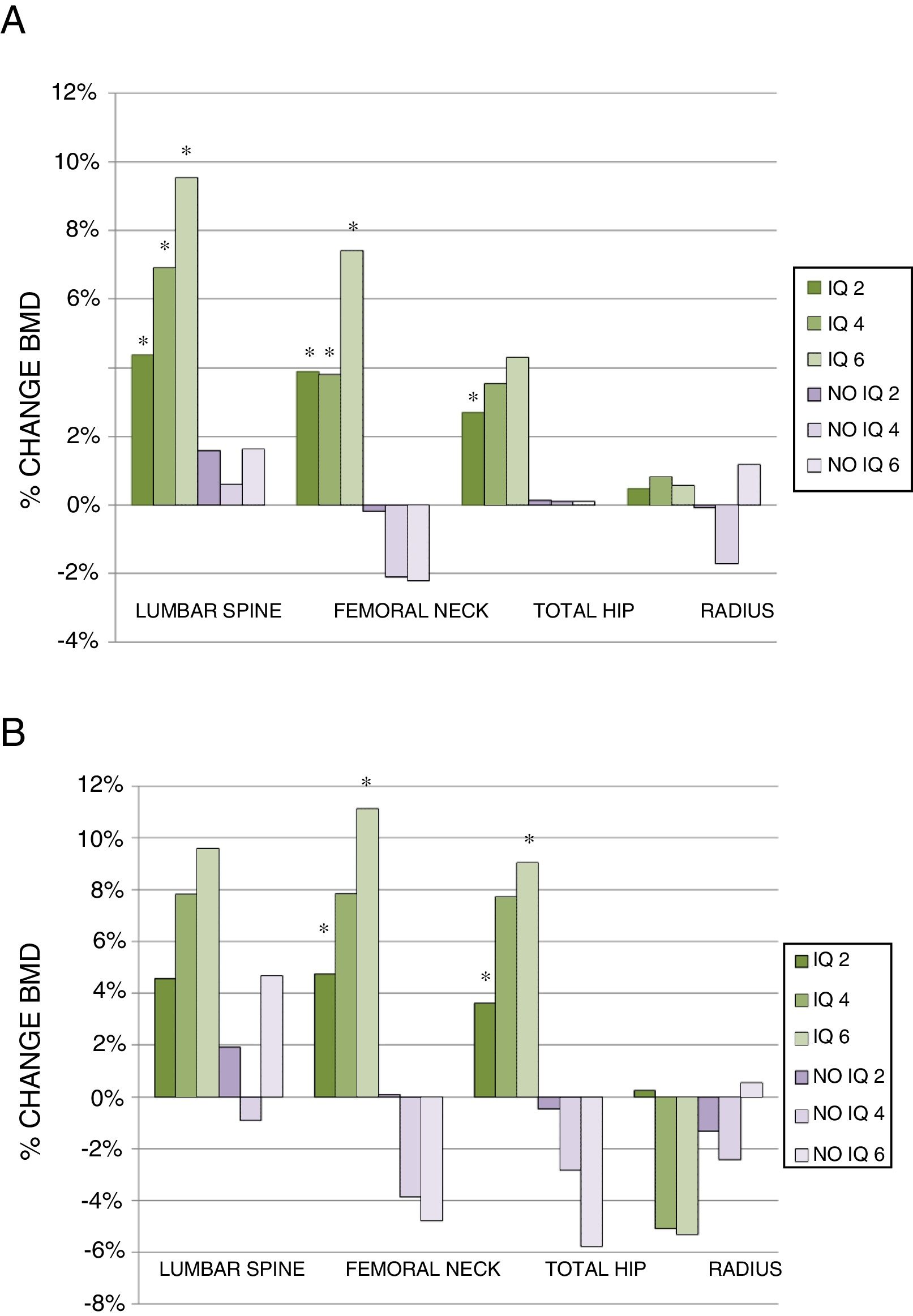
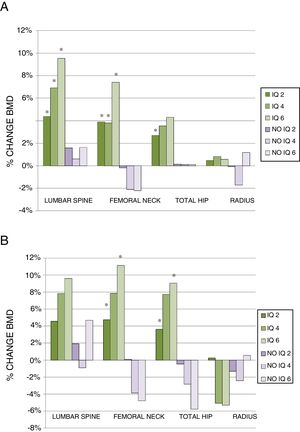
Two years after surgery, the surgical patients showed a BMD gain of 4.37% (0.25–10.27) (median and interquartile range) in the lumbar spine, while the non-surgical patients presented a gain of 1.59% (1.94–5.04)(p<0.005). In turn, the surgical patients showed a BMD gain of 3.90% (0.79–8.28) in the femoral neck, while the non-surgical patients experienced a decrease of −0.19% (−2.84–1.79) (p<0.001). At hip level the variation was 2.70% (−0.17–5.77) vs. 0.14% (−3.11–2.83), respectively (p<0.005). By contrast, no improvement was observed in the surgical patients in the distal third of the radius (Fig. 2A).
Percentage change in BMD in the total patients (A) and in the group of patients without treatment for osteoporosis (B), at 2, 4 and 6 years of follow-up. Data presented as median percentage change.
(A) IQ 2: CL (112), CF (98), CT (103), Radius (38); IQ 4: CL (50), CF (43), CT (45), Radius (17); IQ 6: CL (22), CF (21), CT (21), Radius (9).
No IQ 2: CL (58), CF (50), CT (51), Radius (17); No IQ 4: CL (32), CF (28), CT (31), Radius (8); No IQ 6: CL (20), CF (20), CT (21), Radius (6).
(B) IQ 2: CL (38), CF (37), CT (38), Radius (17); IQ 4: CL (6), CF (7), CT (7), Radius (3); IQ 6: CL (6), CF (5), CT (5), Radius (2).
No IQ 2: CL (21), CF (18), CT (18), Radius (7); No IQ 4: CL (4), CF (4), CT (4), Radius (2); No IQ 6: CL (8), CF (8), CT (8), Radius (1).
*p<0.05.
CF: femoral neck; CL: lumbar spine; CT: total hip; IQ 2: surgical patients at 2 years; IQ 4: surgical patients at 4 years; IQ 6: surgical patients at 6 years; No IQ 2: non-surgical patients at 2 years; No IQ 4: non-surgical patients at 4 years; No IQ 6: non-surgical patients at 6 years; Radius: distal third of radius.
On analyzing the data at four years, we found the percentage change to remain higher in the surgical patients both in the lumbar spine (6.89% [0.66–16.68] vs. 0.62% [−3.88–7.74]) and in the femoral neck (3.84% [−4.01–10.97] vs. −2.09% [−6.07–2.46]; p<0.005). More discrete changes in BMD were seen in the hip and distal third of the radius, with no significant differences between the two groups (Fig. 2A).
At 6 years, the above trends persisted, with a greater magnitude of change. In this regard, BMD improved in the lumbar spine (9.54% [2.64–18.59] vs. 1.63% [−4.22–10.54]) and femoral neck (7.42% [−2.20–11.13] vs. −2.20% [−6.52–5.73]) in the surgical patients, the differences being statistically significant between the groups in both cases. Bone mineral density increased in total hip and in the radius, though without reaching statistical significance (Fig. 2A).
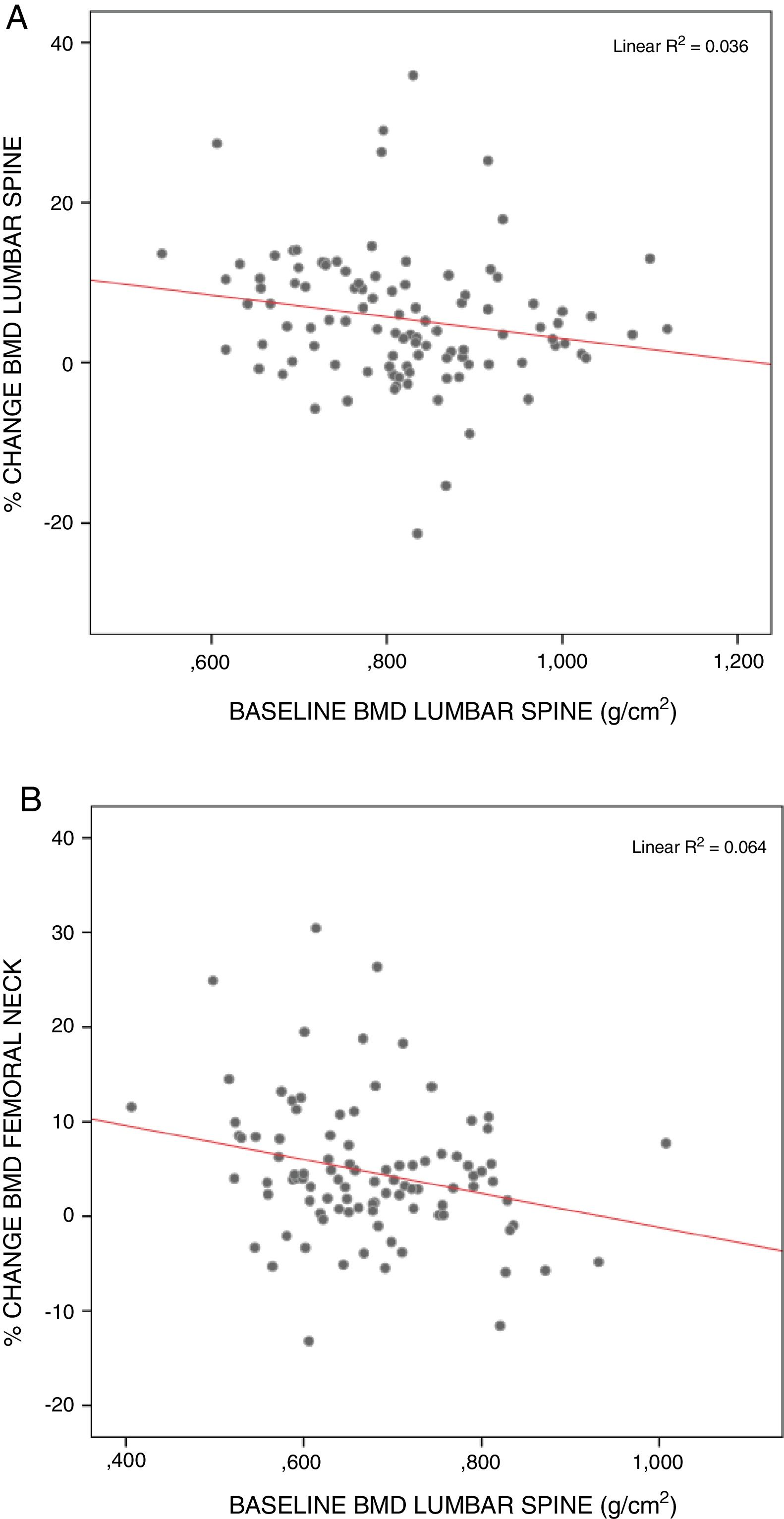
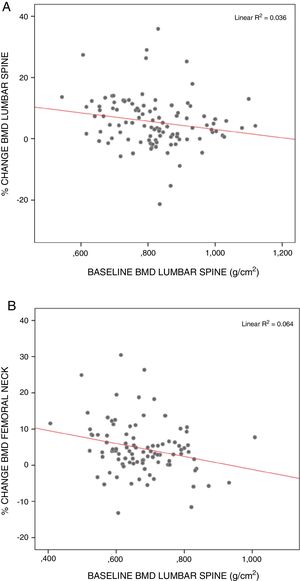
The surgical patients starting from the lowest BMD values showed the greatest recoveries, particularly in the lumbar spine (rs=−0.249; p<0.05) and femoral neck (rs=−0.250; p<0.05) (Fig. 3).
After excluding the patients who had received treatment for osteoporosis, the results showed similar trends, though with a smaller sample size. Densitometry at two years revealed a greater percentage change among the surgical patients in all locations, with the difference being statistically significant in the femoral neck and total hip. At four years, the BMD findings worsened in the non-surgical patients in all locations except in the lumbar spine, where BMD was maintained. The differences vs. the surgical patients failed to reach statistical significance, however. At 6 years, an important decrease in BMD was recorded in the femoral neck and total hip in the non-surgical patients, while the BMD values were seen to improve significantly in these same locations among the surgical patients. As mentioned above, no major changes in BMD were seen in the lumbar spine in the non-surgical patients, and no statistically significant difference was noted in the distal third of the radius (Fig. 2B).
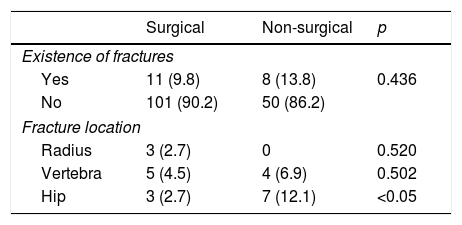
There were no statistically significant differences in the percentage of total fractures between the surgical and non-surgical groups. By contrast, there was a statistically significant difference in the percentage of hip fractures, which proved more common among the non-surgical patients (p<0.05) (Table 3). The logistic regression analysis only identified age as a risk factor for hip fracture, with an odds ratio (OR) of 1.12 (1.02–1.24).
Fractures over follow-up.
| Surgical | Non-surgical | p | |
|---|---|---|---|
| Existence of fractures | |||
| Yes | 11 (9.8) | 8 (13.8) | 0.436 |
| No | 101 (90.2) | 50 (86.2) | |
| Fracture location | |||
| Radius | 3 (2.7) | 0 | 0.520 |
| Vertebra | 5 (4.5) | 4 (6.9) | 0.502 |
| Hip | 3 (2.7) | 7 (12.1) | <0.05 |
Data expressed as the number of patients and the percentage with respect to the total patients in each group.
Surgery is the only curative treatment for primary hyperparathyroidism (PHP), and should be recommended in all symptomatic patients. There is some controversy regarding the indication of surgery in asymptomatic patients. The present study retrospectively analyzed the changes in BMD, as well as the occurrence of fractures in surgical and non-surgical patients in standard clinical practice, where over 80% of all patients with PHP are asymptomatic.7 This is the first study of this kind conducted in Spain in patients with PHP.
In our series, the increase in BMD in the lumbar spine proved statistically significant at 2, 4 and 6 years after surgery. The gain in BMD after surgery is greater in areas rich in trabecular bone, and occurs early in the first months after surgery. This rapid increase after surgery is due to a reduction in bone resorption and an expanded bone remodeling space filling effect.8 An increase in BMD in the lumbar spine has also been reported in studies conducted in Nordic populations.3,9,10 In the study conducted by Ambrogini, comparing asymptomatic PHP patients randomized to surgery or to follow-up, the BMD values in the lumbar spine improved one year after surgery (4.16% vs. −1.12%, respectively).11 The improvement in lumbar BMD found in our series four years after surgery (6.89%) is clearly lower than that reported by Silverberg after the same period of time (12.8%).12 Although age-related loss of BMD in the lumbar spine is to be expected, this was not seen in the nonsurgical patients of our series, where a gain in BMD was recorded at two years, regardless of whether patients receiving treatment for osteoporosis were included in the analysis or not. These results are consistent with previous observations in which the BMD of the lumbar spine was relatively well preserved against the loss of bone mass in PHP.13
The percentage changes in femoral neck and total hip were higher than those reported by Rao et al.14 and lower than those of Ambrogini et al.11 and Silverberg et al.12 The improvement in femoral neck BMD was significantly maintained at 4 and 6 years, and cortical bone loss therefore may be partially reversible over the long term after surgery. An annual decrease in BMD of the femoral neck of 0.6% is expected in the general female population.15 The non-surgical patients showed a lesser loss at two years, regardless of whether we considered the total group of non-surgical patients or only those not receiving treatment for osteoporosis. However, at 4 and 6 years, the non-surgical patients not receiving other treatments showed a BMD loss in the femoral neck of −3.85% and −4.77%, respectively, these figures being higher than would normally be expected according to age.
Surgery has a lesser impact upon cortical bone than on trabecular bone. In our study, BMD did not improve in locations characterized by a predominance of cortical bone, such as the distal third of the radius. This is consistent with the findings of other studies.11,16–19
Patients starting with lower BMD values were correlated to greater percentage change in the densitometry values at two years in the lumbar spine and femoral neck. According to the observations of other authors, the patients exhibiting the greatest recovery are those starting from poorer BMD values.20
In our series, no statistically significant differences were seen between baseline BMD and the BMD values after 2, 4 and 6 years of follow-up in any location in the non-surgical patients. These findings are in line with those reported in the meta-analysis published by Sankaran: untreated patients did not experience rapid BMD loss.21 By contrast, a worsening of the BMD values was noted in locations with more cortical bone such as the femoral neck and radius at 2 and 4 years, though statistical significance was not reached. These data agree with those reported by Rubin, who noted a decrease in BMD in the femoral neck and radius before 10 years of follow-up, though the lumbar spine was not affected over the 15 years of follow-up.13 Other studies have also reported a decrease in BMD in the femoral neck and radius in non-surgical patients.10,16 The loss of BMD in the radius was not confirmed by either Rao or Ambrogini, who measured BMD over a period of 1–2 years. This suggests that the mentioned time interval may be too short to reveal differences.11,14
It should be taken into account that one of the limitations of our study is the use of drugs with actions upon bone, a circumstance also found in other published studies.9,10 A total of 58% of the surgical patients and 63.8% of the non-surgical patients received treatment for osteoporosis. This is a retrospective study conducted in the context of standard clinical practice. As a result, there is a bias, since patients monitored for several years in the clinic with densitometric evaluations every two years are usually those initially presenting with diminished BMD, while the remainder are either discharged or undergo biochemical controls with only occasional densitometries. Although this should be taken into account, it minimizes the differences between the two groups and does not explain an increase in BMD. In fact, after excluding the patients who have received treatment for osteoporosis, we continued to observe an important gain in BMD in femoral neck and total hip two years after surgery, and this gain moreover persisted at 4 and 6 years.
Despite the differences in the evolution of BMD after surgery vs. conservative management (patient observation), it is unclear whether BMD improvement reduces fracture risk. In our study, the small number of fractures observed over time does not allow us to draw firm conclusions. Nevertheless, we observed a higher percentage of fractures in the non-surgical group, though statistical significance was not reached. By contrast, significant differences were observed in hip fractures, though on adjusting for other factors, only age was identified as a significant risk factor, in concordance with the findings of other studies.22 The decrease in fracture risk among surgical patients has been reported in a number of studies.23 However, just as in our series, the meta-analysis published by Singh Ospina recorded no differences in fracture risk reduction between surgical and non-surgical patients.24 With regard to fracture location, a greater number of radius fractures were seen in the surgical group, though statistical significance was not reached. This has also been reported by other authors,6 and is consistent with the mentioned absence of BMD improvement in the radius after surgery. A number of studies have shown that factors such as bone microarchitecture may be of some relevance in bone resistance to fracture. A useful tool for evaluating this possibility is the Trabecular Bone Score, which is strongly correlated to the number of trabeculae and their connectivity.25 Another very useful tool for estimating fracture risk in daily clinical practice is the FRAX, which takes into account other risk factors, some of which are dependent upon BMD (female gender, early menopause, prolonged immobilization), while others are not (age, low body weight, smoking).26
Most studies do not take into consideration low calcium intake and vitamin D deficiency associated with PHP,17 and in this regard it would be interesting to evaluate the influence of calcium and vitamin D supplementation upon postoperative BMD changes.
The main limitation of our study is its retrospective design, which means that a number of potential biases were beyond our control. Data were collected from patients with PHP over a period of 23 years, but during these years there were changes not only in the analytical methods used, but also in the surgical criteria applied. The surgical indication was decided by the physician in charge of the patient in each case. This explains why the surgical patients were those with clinical symptoms (lithiasis and osteitis fibrosa cystica), of younger age, and with fewer comorbidities, lower BMD and increased bone remodeling. All of this accounts for the baseline differences found between the groups. Despite the above, we consider the results obtained to be relevant, as they allow for an analysis of what is done in standard daily clinical practice.
It may be concluded that in our series patients with PHP subjected to surgery experienced a greater BMD gain in the lumbar spine compared with those not subjected to surgery, in concordance with the observations of previous studies. A significant BMD gain was also seen in the femoral neck, with no significant changes in BMD in the distal third of the radius. However, we were unable to demonstrate a decrease in fracture risk in surgical patients, with patient age being identified as the main risk factor for fractures.
Conflicts of interestThe authors state that they have no conflicts of interest in relation to this article.
Please cite this article as: Ramos L, Piedra M, Muñoz P, Vázquez LA, García-Unzueta MT, Montalbán C, et al. Evolución de la densidad mineral ósea y aparición de fracturas en una cohorte de pacientes con hiperparatiroidismo primario tratados con cirugía paratiroidea vs. vigilancia activa sin cirugía en 6 años de seguimiento. Endocrinol Diabetes Nutr. 2019;66:41–48.