Increased visceral adipose tissue mass is strongly associated to metabolic disorders. Visfatin is a visceral fat adipocytokine. There is epidemiological evidence of a link between a suboptimal gestational environment and a greater propensity to develop metabolic disease in adult life. The objective of this study was to establish whether visfatin concentrations in umbilical cord blood are different in newborns small for gestational age (SGA), appropriate for gestational age (AGA), and large for gestational age (LGA).
Subjects and methodsTerm newborns from an university medical center were included in the study. A blood sample was taken from the umbilical cord vein of each baby immediately after birth. Visfatin was measured using an enzyme immunoassay in the study population, consisting of 35 subjects in the SGA group, 58 in the AGA group, and 35 in the LGA group.
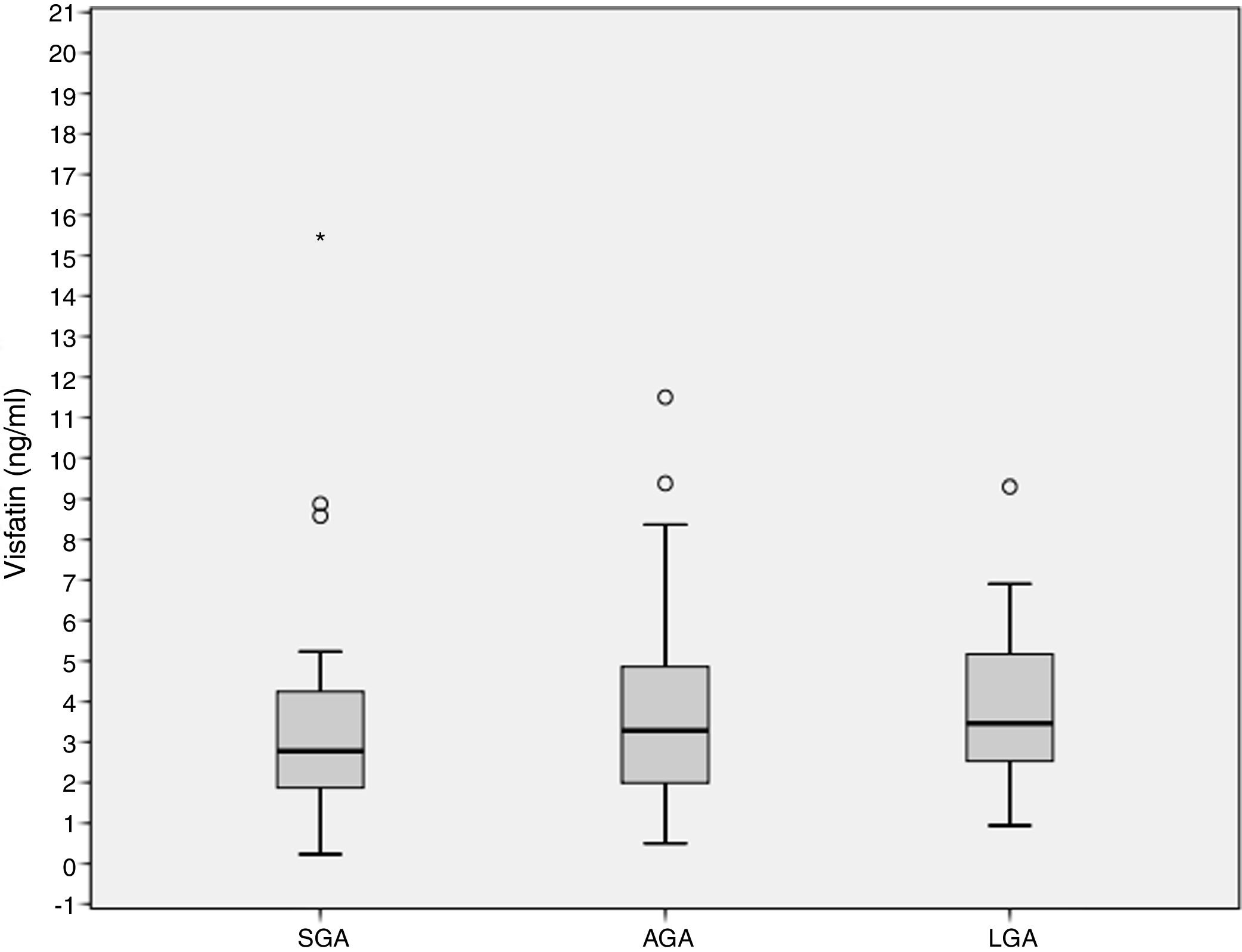
ResultsCord blood visfatin concentrations were not different in the three groups, with respective values of 2.78 (1.86–4.49) ng/mL, 3.28 (1.98–4.97) ng/mL, and 3.46 (2.48–5.38) ng/mL in the SGA, AGA and LGA groups (p=0.141). Gestational weight gain (GWG) (14.09±6.37kg) was negatively associated to visfatin levels (r=−0.218, p=0.036). GWG is an independent predictor of visfatin concentrations (r2=−0.067, p=0.027).
ConclusionsThere were no differences in cord blood visfatin concentrations depending on birth weight. GWG is an independent predictor of visfatin levels in the cord blood of term newborns.
El aumento de la masa de tejido adiposo visceral está fuertemente asociado con trastornos metabólicos. La visfatina es una adipocitoquina de la grasa visceral. Existe evidencia epidemiológica de un vínculo entre un entorno gestacional subóptimo y una mayor propensión a desarrollar enfermedades metabólicas en la vida adulta. El objetivo de este estudio es determinar si las concentraciones de visfatina en la sangre del cordón umbilical son diferentes entre recién nacidos pequeños para edad gestacional (PEG), apropiados para edad gestacional (AEG) y grandes para edad gestacional (GEG).
Materiales y métodosSe incluyeron los recién nacidos a término de un centro médico universitario. Se tomó una muestra de sangre de la vena del cordón umbilical de cada niño inmediatamente después del nacimiento. La visfatina se midió mediante inmunoensayo enzimático en la población de estudio, que incluyó 35 sujetos en el grupo PEG, 58 en el AEG y 35 en el GEG.
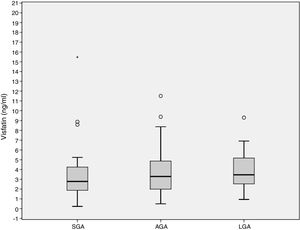
ResultadosLas concentraciones de visfatina en sangre del cordón umbilical no fueron diferentes entre los 3 grupos de estudio, 2,78 (1,86–4,49) ng/ml, 3,28 (1,98–4,97) ng/ml, 3,46 (2,48–5,38) ng/ml para el PEG, AEG y GEG, respectivamente (p=0,141). El aumento de peso gestacional (GWG) (14,09±6,37kg) se asoció negativamente con los niveles de visfatina (r=−0,218, p=0,036). El GWG es un predictor independiente de los niveles de visfatina (r2=−0,067, p=0,027).
ConclusionesLos niveles de visfatina en sangre del cordón umbilical no tienen un comportamiento diferenciado según el peso al nacer. El GWG es un predictor independiente de los niveles de visfatina en la sangre del cordón umbilical de recién nacidos a término.
Increased visceral adipose tissue mass is strongly associated with metabolic disorders related to insulin resistance.1,2 Adipose tissue is recognized as an active endocrine organ that produces biologically active substances.3 Visfatin is a visceral-fat adipocytokine with proinflammatory effects.4 It probably links the expansion of the adipose depot to insulin resistance. The insulin-mimetic activity of visfatin has been demonstrated by binding to the insulin receptor at a distinct site as compared to the insulin molecule.5
There is sufficient epidemiological evidence of a link between a suboptimal gestational environment and an increased propensity for developing metabolic disease in adult life.6 This supports the idea that small for gestational age (SGA) newborns have an increased risk of morbidity and mortality in later life due to diseases such as type 2 diabetes and hypertension.7 In large for gestational age (LGA) newborns, exposure to the maternal intrauterine environment of diabetes and obesity increases the risk of developing metabolic syndrome.8
Previous findings on the association cord visfatin levels and newborn anthropometrics9–13 have found inconsistent results. Additionally, the SGA and LGA operational definitions have been challenged regarding their ability to distinguish those subjects with metabolic and health risk both at short- and long-term.14 Accordingly, the objective of this study is to determine if the cord blood concentrations of visfatin of term newborns differs between SGA, appropriate for gestational age (AGA), and LGA newborns.
Material and methodsThe study took place from December 2012 to January 2015 at the Universidad Autonoma de Nuevo Leon Medical School and the “Dr. Jose E. Gonzalez” University Hospital in Monterrey, Mexico. The study was approved by the Ethics and Research Committee of the University Hospital. The mother provided written informed consent before data collection and cord blood sampling.
The study population included term newborns classified according to birth weight for gestational age by the intrauterine growth curves of Olsen et al.14 as: SGA (less than 10th percentile); AGA (percentile between 10 and 90) or LGA (greater than 90th percentile). Newborns who in their immediate neonatal period did not have any disease associated with their birth weight (hypoglycemia, hypocalcemia, polycythemia) were included. Subjects were not eligible if any disease that required inpatient management; newborns of mothers with gestational diabetes, pregestational diabetes mellitus, pre-eclampsia, hypertension, or thyroid disease were excluded.
The blood sample was taken from the umbilical cord vein from each child immediately after birth and centrifuged at 1600×g to 4°C. Aliquots of serum were separated, frozen, and stored at −70°C for later analysis of visfatin. Birth weight was documented prospectively using a Torrey scale (Torrey, S.A. de C.V., Monterrey, Mexico) and length was obtained using a SECA 210 infantometer (SECA North America, Chino, CA) at birth. Maternal anthropometric data were obtained from clinical charts, if the mother's pregnancy surveillance was done at another medical facility, every effort was made to obtain the data from her health care provider, if data were not available from a medical source, patient interview and self reported data were considered the primary data source.
Methodology for the determination of visfatinVisfatin was measured by enzyme immunoassay (EIA) using a commercial kit (Phoenix Pharmaceuticals, Inc., Burlingame, CA) according to the manufacturer's instructions. Sensitivity of the assay is 1.85ng/mL with a range of 0.1–1000ng/mL, the intra-assay and interassay coefficient of variation in our study was 9.2% and 17%, respectively.
Statistical analysisThe parametric data were expressed as means±standard deviation (SD) and nonparametric data as medians (25–75 percentiles). Variable distribution was evaluated using the Shapiro–Wilk Test. Fisher's exact test was used to compare proportions. For comparison of dimensional continuous variables, a non-parametric Kruskall–Wallis test was performed. One-way ANOVA was used for normally distributed data. Pearson correlation coefficient was used to detect any significant correlations. For multiple regression analysis, the stepwise forward model was employed. A p value≤0.05 was considered statistically significant.
To calculate the sample size for comparison between groups, we used the previously reported difference of 10ng/mL assuming a standard deviation of 7 with an alpha of 0.05 and a power of 80% resulting in 31 patients per group.
Descriptive analyses and variable distribution were performed in MedCalc® Software (Ostend, Belgium). Fisher's exact test and median tests were performed using SPSS Statistics for Mac v.22.0 (IBM Corp., Armonk, NY).
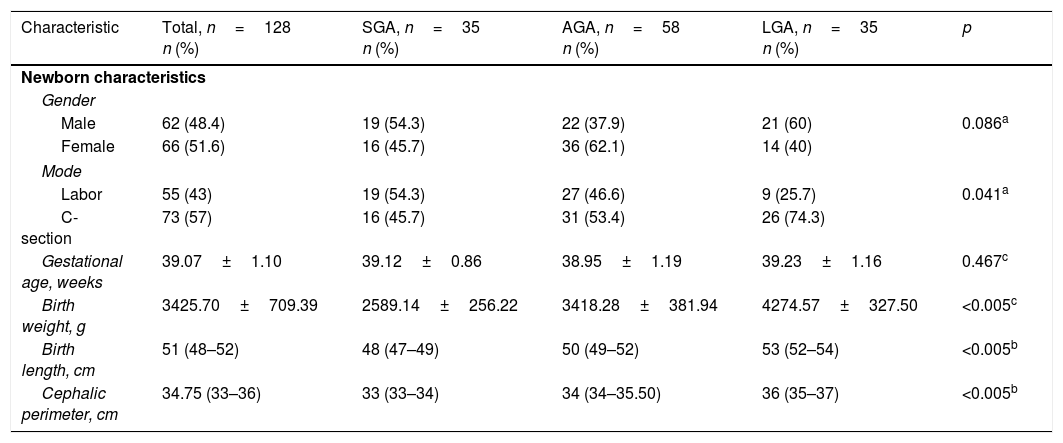
ResultsThe study population (n=128) included 35 subjects in the SGA group, 58 in the AGA group, and 35 in the LGA group. No differences were found in maternal sociodemographic characteristics. Of the total mothers, 96 (75%) reported having had prenatal care (≥5 consultations and 1 ultrasound), and 32 (25%) reported not having had prenatal care. Demographic and anthropometric characteristics are presented in Table 1. A higher proportion of LGA newborns were delivered by C-section than in the AGA or SGA groups (p=0.041). A non-Gaussian distribution was shown for visfatin in the whole study population as well as in the SGA and AGA groups.
Clinical characteristics of newborns, total population and study groups.
| Characteristic | Total, n=128 n (%) | SGA, n=35 n (%) | AGA, n=58 n (%) | LGA, n=35 n (%) | p |
|---|---|---|---|---|---|
| Newborn characteristics | |||||
| Gender | |||||
| Male | 62 (48.4) | 19 (54.3) | 22 (37.9) | 21 (60) | 0.086a |
| Female | 66 (51.6) | 16 (45.7) | 36 (62.1) | 14 (40) | |
| Mode | |||||
| Labor | 55 (43) | 19 (54.3) | 27 (46.6) | 9 (25.7) | 0.041a |
| C-section | 73 (57) | 16 (45.7) | 31 (53.4) | 26 (74.3) | |
| Gestational age, weeks | 39.07±1.10 | 39.12±0.86 | 38.95±1.19 | 39.23±1.16 | 0.467c |
| Birth weight, g | 3425.70±709.39 | 2589.14±256.22 | 3418.28±381.94 | 4274.57±327.50 | <0.005c |
| Birth length, cm | 51 (48–52) | 48 (47–49) | 50 (49–52) | 53 (52–54) | <0.005b |
| Cephalic perimeter, cm | 34.75 (33–36) | 33 (33–34) | 34 (34–35.50) | 36 (35–37) | <0.005b |
SGA: small for gestational age; AGA: appropriate for gestational age; LGA: large for gestational age.
Data are given as means±SD, and as medians (25–75 percentiles).
Cord blood visfatin concentrations were not different among the 3 groups, 2.78 (1.86–4.49) ng/mL, 3.28 (1.98–4.97) ng/mL, 3.46 (2.48–5.38) ng/mL for the SGA, AGA and LGA, respectively (p=0.141) (Fig. 1). Visfatin concentrations were not different in any of the three groups or in the total population when comparing the labor vs. the C-section newborn population. A positive correlation was found between gestational weight gain (GWG) and birth weight (r=0.266, p=0.010).
A statistically significant correlation was found between the age of the mother at the time of birth [24 (20–28) years] and visfatin levels (r=−0.209, p=0.018). Likewise, a negative correlation was found between GWG (14.09±6.37kg) and visfatin levels (r=−0.218, p=0.036). No significant correlation between visfatin concentration and prepregnancy maternal body mass index (BMI) was found.
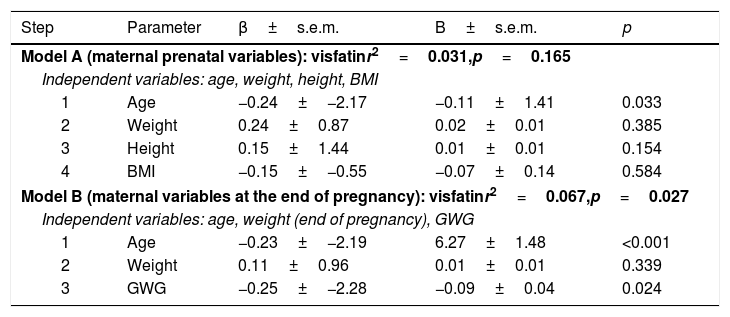
Multiple regression analysis was performed using two models with covariables that modify fetal growth, reporting the age of the mother, and the GWG as the independent predictors of visfatin levels in umbilical cord blood (Table 2).
Multiple regression analyses for independent associations of visfatin.
| Step | Parameter | β±s.e.m. | B±s.e.m. | p |
|---|---|---|---|---|
| Model A (maternal prenatal variables): visfatinr2=0.031,p=0.165 | ||||
| Independent variables: age, weight, height, BMI | ||||
| 1 | Age | −0.24±−2.17 | −0.11±1.41 | 0.033 |
| 2 | Weight | 0.24±0.87 | 0.02±0.01 | 0.385 |
| 3 | Height | 0.15±1.44 | 0.01±0.01 | 0.154 |
| 4 | BMI | −0.15±−0.55 | −0.07±0.14 | 0.584 |
| Model B (maternal variables at the end of pregnancy): visfatinr2=0.067,p=0.027 | ||||
| Independent variables: age, weight (end of pregnancy), GWG | ||||
| 1 | Age | −0.23±−2.19 | 6.27±1.48 | <0.001 |
| 2 | Weight | 0.11±0.96 | 0.01±0.01 | 0.339 |
| 3 | GWG | −0.25±−2.28 | −0.09±0.04 | 0.024 |
BMI: body mass index; GWG: gestational weight gain.
This study includes the determination of visfatin levels in cord blood of three study groups (SGA, AGA and LGA). The total sample includes term newborns of mothers with no morbidity and in spite of the above, a sample of the three study groups was obtained which had no added metabolic and cardiovascular risk. Characterization of the traditional and novel adipokines in these populations may help to build a high risk profile for both weight gain and metabolic comorbidities, we acknowledge that this cross sectional study will not address the full question on the role visfatin measurement in the long-term metabolic risk; however, finding or not differences between these well described populations open new hypothesis and warrant the inclusion of the visfatin in the long term studies aiming to evaluate the metabolic risk.
Measures of cord blood performed at birth are a reflection of the intrauterine environment, in contrast to measures performed during the neonatal period, as in the case of the study by Malamistsi-Puchner9 who studied two term newborn groups with AGA and asymmetric intrauterine growth restriction (IUGR), including newborns of mothers with preeclampsia, gestational diabetes, hypothyroidism and the habit of smoking, reported that there was no significant difference in visfatin levels between the two study groups in cord blood; however, they reported higher circulating visfatin levels in the group with IUGR on day one and four, with this not being a reflection of the intrauterine environment. Likewise, the study by Mazaki-Tovi et al.10 included term AGA and SGA newborns of normotensive mothers and reported that there were no differences in cord blood visfatin concentrations.
Meral et al.11 included three study groups classified according to birth weight (SGA, AGA, and LGA). Children of mothers without morbidities were included. The researchers reported that LGA newborns had higher concentrations of visfatin followed by SGA, and finally, by AGA. Moreover, Shang et al.12 reported increased visfatin levels in newborns with IUGR compared to a control group of newborns with macrosomy. Cekmez et al.13 reported visfatin cord levels slightly higher in the SGA group compared to the AGA group suggest that these adipocytokines can be used as a marker to search for disturbances in the glucose metabolism and insulin resistance.
In our study we report none differences on levels of visfatin between three study groups. One possible explanation for this behavior may be differences in the definition of a newborn with SGA weight and the use of different growth curves, such as those of Lubchenco, which seem to underestimate the percentage of infants born SGA and overestimating the percentage of infants born LGA14 in certain cases with the undifferentiated use of the term SGA and IUGR, and ethnicity, since the referenced studies were conducted in populations from Turkey and China.
Meanwhile, El Shemi et al.15 included three study groups: the IUGR group (included newborns of mothers with pre-eclampsia with a diagnosis of autoimmune diseases or chronic hypertension); AGA and the LGA group of newborns, (included mothers with gestational or pre gestational diabetes) in which they reported visfatin cord blood concentrations increased in IUGR and LGA groups, nevertheless, visfatin was measured by enzyme-linked immunoabsorbent assay (ELISA), one of the lesser-used techniques for measuring visfatin in this population, limiting the generalizability of their results. Our population included newborns of mothers without comorbidities and used EIA to measure visfatin concentrations.
It has been previously suggested that having SGA and LGA increases the risk of developing insulin resistance.8,16 One question that remains to be answered in future studies is the interaction of visfatin-insulin and its relationship with adiposity and inflammation.
Regarding visfatin levels according to type of birth, we did not find differences in neonates born vaginally compared with those born via cesarean section in the population in general or by study groups; however, El Shemi et al.15 reported in the group IUGR increased concentrations of visfatin in neonates born vaginally compared with those born via cesarean section. This could be because, despite documenting the type of birth, the time of labor before birth was not registered in our study, since a possible association of increased visfatin during labor is known.17
Previously, it has been found that advanced maternal age and pregnancy in adolescents are associated with an increased risk of adverse pregnancy outcomes. The prevalence of diabetes, hypertension, overweight and obesity increase with age,18 and younger adolescents have an increased risk of maternal anemia, preterm delivery, postpartum hemorrhage, preeclampsia, increased liver enzyme levels, and low platelet syndrome.19 Although age does not significantly influence birth weight,20 in this study, we found a negative correlation between maternal age and visfatin levels. More studies that include target population (adolescents) are necessary to determine the association between maternal age and visfatin.
GWG is one of the most important predictors of birth weight.21–23 We found an association between GWG and birth weight. We also found GWG as a predictor of visfatin cord blood levels, with a negative correlation between GWG and visfatin levels, which could reflect alterations in the insulin-glucose metabolism in newborns whose fetal growth occurred under suboptimal conditions resulting in a lower birth weight.
Visfatin interacts in a wide neuroendocrine network and this study is focused on finding differences according to the birth weight. Future studies addressing the various components of this network will allow a more profound understanding about the role of visfatin in the neonatal period.
In this study population, no difference in visfatin cord blood levels among the three study groups was found. C-section and vaginal delivery did not influence visfatin cord blood levels either. GWG is an independent predictor of visfatin levels in the cord blood of term newborns.
Additional characterization of other adipokines and their interactions is needed to understand the metabolic impact, if any, of visfatin on birth weight and the risk of developing future diseases.
Conflict of interestThe authors declare no conflict of interest.
This work was supported by grants from the Universidad Autónoma de Nuevo León through the Scientific and Technological Research Support Program (PAICYT).
We thank Sergio Lozano-Rodriguez, MD, Scientific Publications Support Coordinator of the “Dr. Jose E. Gonzalez” University Hospital, for his help in reviewing the manuscript.