Growth hormone (rhGH) is used in children with intrauterine growth retardation without catch-up growth. The Advisory Committee of Castilla y León was implemented in 2010 to watch for consistent application of the criteria for using rhGH. The aim is to assess anthropometric and clinical changes in children treated with growth hormone.
Patients and methodsA retrospective, longitudinal study of patients diagnosed with intrauterine growth retardation without catch-up growth in Castilla y León since 2010 who have received treatment for at least 3 years. Changes in anthropometric, clinical, and laboratory parameters were assessed.
ResultsForty-three children with a mean age of 6.06 years (58.14%<5 years) were enrolled and treated with a mean dose of 0.038mg/kg/day. A significant increase was seen in height (−3.05 to −1.58SD). Both weight and BMI (14.51–15.80kg/m2) increased throughout the study. Growth rate peaked during the first year of treatment (0.74SD). IGF-1 levels increased throughout the study (99.96–392.88ng/mL). There were significant increases in glycosylated hemoglobin levels in the first year, and in basal blood glucose and insulin levels during the second year. The LDL/HDL ratio decreased during the study period (1.70–1.50).
ConclusionTreatment with rhGH promotes growth in children with intrauterine growth retardation. Peak effect occurs in the first 12 months of treatment, and is greater when growth hormone is started before the age of 5 years.
La hormona de crecimiento recombinante humana (rhGH) se utiliza en niños con crecimiento intrauterino retardado sin recuperación posnatal de talla. En 2010 se inició el Comité Asesor de Castilla y León para aplicar de forma homogénea los criterios de utilización de la rhGH. El objetivo es valorar la evolución antropométrica y clínica de niños sometidos a tratamiento.
Pacientes y métodosEstudio retrospectivo, longitudinal, con pacientes de Castilla y León diagnosticados de crecimiento intrauterino retardado sin crecimiento recuperador a partir del año 2010, con al menos 3 años de tratamiento. Se evaluaron las modificaciones en diferentes parámetros antropométricos, clínicos y analíticos.
ResultadosSe incluyeron 43 niños con una edad media de 6,06 años (58,14%<5 años), tratados con una dosis media de 0,038mg/kg/día. Se observó un aumento significativo en la talla (−3,05 hasta −1,58 E). El peso se incrementó durante todo el estudio, así como el IMC (14,51 hasta 15,80kg/m2). La velocidad de crecimiento alcanzó su pico máximo durante el primer año de tratamiento (0,74DE). Los valores de IGF-1 se incrementaron durante todo el estudio (99,96 hasta 392,88ng/ml). Se observaron incrementos significativos de la glucohemoglobina en el primer año y de la glucemia y de la insulinemia basal durante el segundo año, así como un descenso en el cociente LDL/HDL (1,70 hasta 1,50).
ConclusiónEl tratamiento con hormona de crecimiento favorece el crecimiento de niños con crecimiento intrauterino retardado, observándose su máximo efecto en los primeros 12 meses, siendo mayor si la edad de comienzo es anterior a los 5 años de edad.
The term “small for gestational age” (SGA) was defined in 2001 by the International Small for Gestational Age Advisory Board Consensus Development Conference as a newborn infant (whether at term or preterm) with a body weight and/or length at least two standard deviations (SD) below average for the gestational age according to the statistics of the corresponding reference population.1
The origins underlying SGA or intrauterine growth retardation (IUGR) are varied: fetal, maternal, placental or (in at least 40% of the cases) idiopathic.2
It is estimated that approximately 85–90% of these children spontaneously experience catch-up growth, reaching normal height and weight values during the first 2–3 years of life.1 The reasons why the remaining 10–15% do not experience such spontaneous growth are not known, and catch-up growth is very unlikely to occur beyond four years of age.3,4
In this latter group of children, recombinant human growth hormone (rhGH) was approved for this therapeutic indication in 2003 by the United States Food and Drug Administration (FDA) and the European Medicines Evaluation Agency (EMEA).5
The studies show that 85% of all cases achieve normal adult height in excess of −2SD, and that 98% achieve heights within the range corresponding to their genetic height. In addition to its impact on linear growth, rhGH exerts a normalizing effect upon the body mass index (BMI), and is associated with lowered blood pressure and an improved lipid profile secondary to a reduction in total cholesterol and LDL-cholesterol levels.6,7
Different post-marketing studies have demonstrated the efficacy and safety of rhGH when used for indications approved by the different regulatory agencies.8
In order to ensure a consistent application of the criteria for the diagnosis of diseases amenable to rhGH treatment and for the selection of patients amenable to therapy, it is useful to have an Advisory Committee for the use of rhGH. Such a committee can also serve to ensure equity of access to rhGH and related substances, as well as the rational and efficient use of National Health System resources. In the Spanish Autonomous Community of Castilla y León, the Advisory Committee for the therapeutic use of growth hormone and related substances was established in December 2007,9 and its effective implementation took place in January 2010.
The objectives of the present study are:
- •
To establish the number of children with IUGR in Castilla y León treated for at least three years with rhGH, and to analyze the anthropometric and laboratory test changes occurring during this period.
- •
To assess the influence of different variables (prematurity, gender and age at the start of treatment) upon height gain in the patients treated with rhGH.
This is a retrospective longitudinal study of patients from the Autonomous Community of Castilla y León diagnosed with IUGR since 2010 and with at least three years of continuous treatment with rhGH. All information was extracted from the documents requesting the start and renewal of treatment with rhGH, submitted to the Growth Hormone Advisory Committee of Castilla y León. The patients included in the study were required to meet the following criteria established by the mentioned Advisory Committee for children with IUGR:
- •
Body length and/or weight at birth under −2SD, based on the Spanish reference growth Tables.10
- •
No catch-up growth by four years of age.
- •
Body length under −2.5SD (Spanish reference growth Tables)10 and under −1SD adjusted to mean parental height (with exceptions) at the time of the request for rhGH therapy.
The exclusion criteria for this study were:
- •
Onset of puberty
- •
Carbohydrate intolerance
- •
Diabetes mellitus
The evolution of different clinical, auxologic and laboratory parameters was documented and analyzed:
Clinical and auxologic:
- •
At birth: weight, length, height of parents and gestational age. The genetic height of each child was calculated as an additional parameter using the following formulas:
- o
Boys: (father's height+mother's height+13)/2
- o
Girls: (father's height+mother's height−13)/2
- •
Before the start of therapy and at each annual review: chronological age, bone age, weight, height, growth rate according to chronological age, systolic and diastolic blood pressure, and target height. The chronological age/bone age ratio and the BMI were calculated as additional parameters.
Laboratory test data:
Before the start of therapy and at each annual review, we analyzed the following laboratory test parameters under fasting conditions: IGF-1 (insulin-like growth factor-1), IGFBP3 (insulin-like growth factor binding protein 3), basal glycemia, basal insulin, glycosylated hemoglobin (Hb1Ac), total cholesterol, HDL-cholesterol, LDL-cholesterol and triglycerides. The ratios IGF-1/IGFBP3, total cholesterol/HDL-cholesterol and LDL-cholesterol/HDL-cholesterol were calculated as additional parameters.
In addition, the rhGH doses prescribed in each of the study years were monitored.
Statistical analysisTo describe the different baseline characteristics of the sample, we calculated the mean, standard deviation (SD), median, minimum, maximum, and interquartile range (IQR) of the quantitative variables.
All hypothesis tests were performed using parametric techniques, since the sample met the Kolmogorov–Smirnov and Shapiro–Wilk normal data distribution criteria. The effectiveness of rhGH treatment was determined by comparing mean body height in each year of treatment versus the previous measurement, using the Student t-test for related samples.
The Student t-test for independent samples was used to compare the mean values of the different variables among prematurity, gender and age groups at the start of rhGH treatment. The Pearson chi-squared test was used for categorical variables. As regards age at the start of treatment, two different groups were established: under 5 years of age and ≥5 years of age.
Statistical significance was considered for p<0.05. The SPSS® version 20 statistical package was used throughout.
ResultsBaseline characteristicsThe study involved 43 patients: 25 boys (58.14%) and 18 girls (41.86%). Of the total patients, 7% corresponded to multiple pregnancies, while 51.16% were born prematurely. There were more premature boys than girls (68.18% versus 31.82%, respectively), though the difference was not statistically significant.
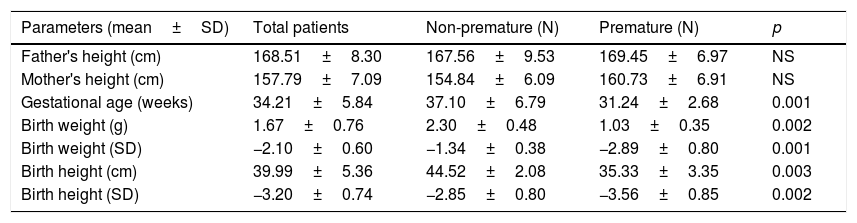
At birth (Table 1), the mean gestational age was 34.21±5.84 weeks (37.1±6.79 in non-premature and 31.24±2.68 in premature infants; p<0.05), with a mean length of 39.99±5.36cm (−3.2±0.7SD). Length at this point was greater in non-premature infants: 44.52±2.08cm (−2.85±0.8SD) vs. 35.33±3.35cm (−3.56±0.85SD) in premature infants (p<0.05). Mean weight in turn was 1.67±0.76kg (−2.1±0.6SD), and was greater in non-premature infants: 2.30±0.48kg (−1.34±0.38SD) vs. 1.03±0.35kg (−2.89±0.8SD) in premature infants (p<0.05). No significant gender differences were observed for any of these variables. The target height for males was 169.4±5.6cm (−1.26±0.35SD) vs. 156.4±3.8cm (−1.33±0.42SD) for females, with no differences in height in relation to SD.
Birth data according to whether the patients were premature or otherwise.
| Parameters (mean±SD) | Total patients | Non-premature (N) | Premature (N) | p |
|---|---|---|---|---|
| Father's height (cm) | 168.51±8.30 | 167.56±9.53 | 169.45±6.97 | NS |
| Mother's height (cm) | 157.79±7.09 | 154.84±6.09 | 160.73±6.91 | NS |
| Gestational age (weeks) | 34.21±5.84 | 37.10±6.79 | 31.24±2.68 | 0.001 |
| Birth weight (g) | 1.67±0.76 | 2.30±0.48 | 1.03±0.35 | 0.002 |
| Birth weight (SD) | −2.10±0.60 | −1.34±0.38 | −2.89±0.80 | 0.001 |
| Birth height (cm) | 39.99±5.36 | 44.52±2.08 | 35.33±3.35 | 0.003 |
| Birth height (SD) | −3.20±0.74 | −2.85±0.80 | −3.56±0.85 | 0.002 |
SD: standard deviation.
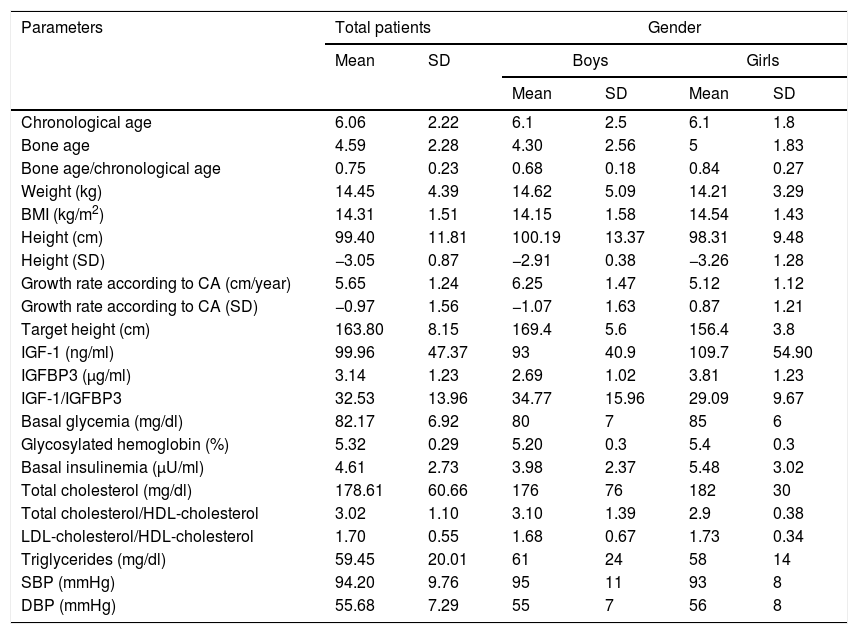
The mean age at the start of treatment was 6.06±2.22 years (Table 2). With regard to age distribution, 25 patients (58.14%)(60% boys and 40% girls) were under 5 years of age, while 18 (41.86%)(55.56% boys and 44.44% girls) were ≥5 years of age. Mean height at the start of treatment was 99.40±11.81cm, which is equivalent to −3.05±0.87SD.
Laboratory test and auxologic data at the start of treatment.
| Parameters | Total patients | Gender | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Boys | Girls | |||
| Mean | SD | Mean | SD | |||
| Chronological age | 6.06 | 2.22 | 6.1 | 2.5 | 6.1 | 1.8 |
| Bone age | 4.59 | 2.28 | 4.30 | 2.56 | 5 | 1.83 |
| Bone age/chronological age | 0.75 | 0.23 | 0.68 | 0.18 | 0.84 | 0.27 |
| Weight (kg) | 14.45 | 4.39 | 14.62 | 5.09 | 14.21 | 3.29 |
| BMI (kg/m2) | 14.31 | 1.51 | 14.15 | 1.58 | 14.54 | 1.43 |
| Height (cm) | 99.40 | 11.81 | 100.19 | 13.37 | 98.31 | 9.48 |
| Height (SD) | −3.05 | 0.87 | −2.91 | 0.38 | −3.26 | 1.28 |
| Growth rate according to CA (cm/year) | 5.65 | 1.24 | 6.25 | 1.47 | 5.12 | 1.12 |
| Growth rate according to CA (SD) | −0.97 | 1.56 | −1.07 | 1.63 | 0.87 | 1.21 |
| Target height (cm) | 163.80 | 8.15 | 169.4 | 5.6 | 156.4 | 3.8 |
| IGF-1 (ng/ml) | 99.96 | 47.37 | 93 | 40.9 | 109.7 | 54.90 |
| IGFBP3 (μg/ml) | 3.14 | 1.23 | 2.69 | 1.02 | 3.81 | 1.23 |
| IGF-1/IGFBP3 | 32.53 | 13.96 | 34.77 | 15.96 | 29.09 | 9.67 |
| Basal glycemia (mg/dl) | 82.17 | 6.92 | 80 | 7 | 85 | 6 |
| Glycosylated hemoglobin (%) | 5.32 | 0.29 | 5.20 | 0.3 | 5.4 | 0.3 |
| Basal insulinemia (μU/ml) | 4.61 | 2.73 | 3.98 | 2.37 | 5.48 | 3.02 |
| Total cholesterol (mg/dl) | 178.61 | 60.66 | 176 | 76 | 182 | 30 |
| Total cholesterol/HDL-cholesterol | 3.02 | 1.10 | 3.10 | 1.39 | 2.9 | 0.38 |
| LDL-cholesterol/HDL-cholesterol | 1.70 | 0.55 | 1.68 | 0.67 | 1.73 | 0.34 |
| Triglycerides (mg/dl) | 59.45 | 20.01 | 61 | 24 | 58 | 14 |
| SBP (mmHg) | 94.20 | 9.76 | 95 | 11 | 93 | 8 |
| DBP (mmHg) | 55.68 | 7.29 | 55 | 7 | 56 | 8 |
HDL-cholesterol: high density lipoprotein-bound cholesterol; LDL-cholesterol: low density lipoprotein-bound cholesterol; SD: standard deviation; CA: chronological age; IGF-1: insulin-like growth factor 1; IGFBP3: insulin-like growth factor binding protein 3; BMI: body mass index; DBP: diastolic blood pressure; SBP: systolic blood pressure.
Both the clinical blood pressure data and the laboratory test findings were within normal ranges before treatment. In comparison with the boys, the girls were seen to have higher values regarding IGFBP3 (3.81±1.23μg/ml vs. 2.69±1.02μg/ml; p<0.05), basal glycemia (84.94±6.16mg/dl vs. 80.28±6.89mg/dl; p<0.05) and glycosylated hemoglobin (5.45±0.30 vs. 5.24±0.26%; p<0.05). There were no differences according to preterm status.
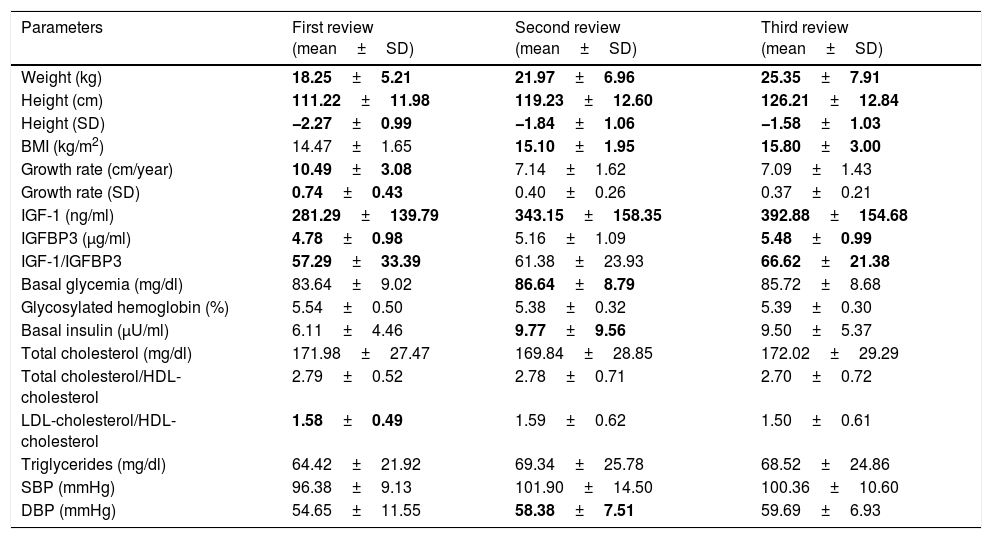
Evolution of the auxologic dataTable 3 shows the evolution of the auxologic data throughout the study period.
Evolution of the laboratory test and auxologic parameters during the study.
| Parameters | First review (mean±SD) | Second review (mean±SD) | Third review (mean±SD) |
|---|---|---|---|
| Weight (kg) | 18.25±5.21 | 21.97±6.96 | 25.35±7.91 |
| Height (cm) | 111.22±11.98 | 119.23±12.60 | 126.21±12.84 |
| Height (SD) | −2.27±0.99 | −1.84±1.06 | −1.58±1.03 |
| BMI (kg/m2) | 14.47±1.65 | 15.10±1.95 | 15.80±3.00 |
| Growth rate (cm/year) | 10.49±3.08 | 7.14±1.62 | 7.09±1.43 |
| Growth rate (SD) | 0.74±0.43 | 0.40±0.26 | 0.37±0.21 |
| IGF-1 (ng/ml) | 281.29±139.79 | 343.15±158.35 | 392.88±154.68 |
| IGFBP3 (μg/ml) | 4.78±0.98 | 5.16±1.09 | 5.48±0.99 |
| IGF-1/IGFBP3 | 57.29±33.39 | 61.38±23.93 | 66.62±21.38 |
| Basal glycemia (mg/dl) | 83.64±9.02 | 86.64±8.79 | 85.72±8.68 |
| Glycosylated hemoglobin (%) | 5.54±0.50 | 5.38±0.32 | 5.39±0.30 |
| Basal insulin (μU/ml) | 6.11±4.46 | 9.77±9.56 | 9.50±5.37 |
| Total cholesterol (mg/dl) | 171.98±27.47 | 169.84±28.85 | 172.02±29.29 |
| Total cholesterol/HDL-cholesterol | 2.79±0.52 | 2.78±0.71 | 2.70±0.72 |
| LDL-cholesterol/HDL-cholesterol | 1.58±0.49 | 1.59±0.62 | 1.50±0.61 |
| Triglycerides (mg/dl) | 64.42±21.92 | 69.34±25.78 | 68.52±24.86 |
| SBP (mmHg) | 96.38±9.13 | 101.90±14.50 | 100.36±10.60 |
| DBP (mmHg) | 54.65±11.55 | 58.38±7.51 | 59.69±6.93 |
HDL-cholesterol: high density lipoprotein-bound cholesterol; LDL-cholesterol: low density lipoprotein-bound cholesterol; SD: standard deviation; CA: chronological age; IGF-1: insulin-like growth factor 1; IGFBP3: insulin-like growth factor binding protein 3; BMI: body mass index; DBP: diastolic blood pressure; SBP: systolic blood pressure.
p<0.05.
Anthropometric data: The height values increased from −3.05±0.87 to −1.58±1.03SD (p<0.05), with the observation of differences in the three years, the maximum increase occurring during the first year of rhGH treatment. The mean height increment in the course of the study was 1.47±0.67SD. A greater height increase was seen in the patients under 5 years at the start of treatment: 1.50±0.73 vs. 1.43±0.6SD (p<0.05) in the case of those ≥5 years of age. No differences were seen in terms of prematurity status or patient gender.
Body weight increased from 14.45±4.39 to 25.35±7.91kg (p<0.05), with differences being observed in the three years of the study. Likewise, the BMI increased during the study period, with differences being observed during the second and third years of treatment (p<0.05), progressing from 14.51±1.31 at baseline to 15.80±3 in the third year.
The growth rate observed during treatment was higher than during the period prior to drug use. The growth rate exhibited a maximum peak in the first year of treatment: 0.74±0.43SD (p<0.05) in the first year, 0.40±0.26SD in the second year, and 0.37±0.21SD in the third year. No differences in these parameters were observed in terms of prematurity, gender or age at the start of treatment.
Clinical data: Patient blood pressure remained stable and within normal limits throughout the study period. However, an increase in diastolic blood pressure was detected between the first and second year of treatment (54.65±11.55 vs. 58.38±7.51mmHg; p<0.05). No differences in these parameters were observed in terms of prematurity, gender or age at the start of treatment.
Laboratory test data: The IGF-1 levels increased significantly (p<0.05) during the three years of the study, while IGFBP3 and the IGF-1/IGFBP3 ratio increased in the first and third year of treatment (p<0.05).
The glycosylated hemoglobin levels increased during the first year. In addition, both glycemia and basal insulin increased during the second year of treatment (p<0.05). With regard to the lipid profile, the only significant change during the study period was a decrease in the LDL-cholesterol/HDL-cholesterol ratio in the first year of treatment. No differences in these parameters were observed in terms of prematurity, gender or age at the start of treatment.
The mean rhGH dose prescribed at the start of treatment was 0.038±0.005mg/kg/day, and no significant changes were seen throughout the study period. Likewise, no differences in dose were observed in terms of prematurity, gender or age at the start of treatment.
DiscussionThis study describes the results obtained over three years of treatment with rhGH in children diagnosed with intrauterine growth retardation (IUGR) and who met all the requirements established by the Advisory Committee of Castilla y León for the therapeutic use of rhGH and related substances. The results of the study indicate a gradual height increment in the course of three years of rhGH treatment. The mean rhGH dose used was 0.038±0.005mg/kg/day, and the observed total growth was 1.47±0.67SD. This finding is in agreement with the data of other published studies.1,5
Considering the results obtained in our study, the different factors influencing the initial patient response to rhGH were age at the start of treatment and the baseline height of the patients, as previously described by other authors.11,12 The results of this study indicate a greater gain in height in those patients who started treatment before 5 years of age. This finding is consistent with the results of other studies.5,13
Several authors have reported a total increase in height during rhGH treatment equivalent to +2SD when therapy is started before 8 years of age, though this figure may decrease to 0.6SD if the start of rhGH treatment is delayed until the patient is about 11 years old.7,14 These data, along with our own findings, appear to indicate the importance of starting treatment at an early age.
In the present study, 51.16% of the patients were born prematurely, the frequency being twice as high in boys as in girls. This is a very high proportion, since the prematurity rates published in Europe are around 5–9% of all births.15 The number of preterm infants was higher than in other similar studies conducted in Spain, with prematurity rates in the order of 20%.16,17 Significant improvements in height and weight were also seen in these premature infants, with figures similar to those of full-term infants.18
The patient age at the start of rhGH treatment authorized by the different regulatory agencies is four years. However, the mean age at the start of treatment in our study was approximately 6.06 years. Nevertheless, this age at the start of therapy is consistent with the data from other similar studies conducted in Spain.13,16,17
Similarly as reported by other authors,19,20 the growth rate increased significantly during the study period, with a peak occurring during the first year. This resulted in an increase in final height that was close to the genetic height of the patients. During the second and third year of follow-up, the growth rate was lower than in the first year, but higher than in the pre-treatment stage, implying a less pronounced gain in height.
At the start of our study, the patients with IUGR in Castilla y León presented BMI values at the lower limit of normal for their age. After the start of rhGH treatment, these values increased and normalized in the course of the study period, as reported elsewhere.1,5
The mean rhGH dose received by the patients throughout the study period was 0.038±0.005mg/kg/day, which is at the lower limit of the therapeutic range recommended for patients with IUGR (0.035–0.060mg/kg/day). The dosing range of the treatments prescribed in this study is in line with that described in the literature.5,21,22
With regard to the different laboratory test parameters, the literature7 reports that rhGH treatment induces a dose-dependent response on the part of the IGF-1 and IGFBP3 levels. The results of our study show that the IGF-1 levels increased significantly during the three years of follow-up, while the IGFBP3 levels increased significantly in the first and third year of the study. The greatest increase in these two parameters occurred during the first year of treatment. As a result of the above, this same significant increase was observed in the IGF-1/IGFBP3 ratio during the first and the third year of treatment. However, the IGF-1 levels remained within normal ranges throughout the study, without exceeding 1.5 and 2SD.23 The literature reports that patients with IUGR treated at doses from 0.066 to 0.1mg/kg/day may experience increases in IGF-1 levels to above those considered normal for their age.24
The increase in height of our patients was accompanied by a sustained increase in glycemia and basal insulin, which proved significant during the second year of the study, as well as by a rise in glycosylated hemoglobin that was significant in the first year of treatment. This increase does not appear to be of clinical relevance, however. In other studies, increases in these values were seen particularly during the first year of treatment, indicating a degree of insulin resistance.25,26 These values tend to normalize once rhGH treatment has been suspended.7,26
The lipid profile of the patients was not modified during the follow-up period, with the exception of a statistically significant decrease in the LDL-cholesterol/HDL-cholesterol ratio during the first year, which may imply a possible improvement in the atherogenic profile of these patients.
Finally, as regards the clinical parameters, blood pressure remained stable during treatment, with only a statistically significant increase in diastolic blood pressure being recorded between the first and the second year of the study.
Although adult height at the end of treatment was not available in our study, it may be assumed that treatment with rhGH at the doses used is effective and safe in children with IUGR without postnatal catch-up growth, affording an increase in height, as has also been reported in other publications.27–30
With regard to the inclusion criteria, an exception was made in our study, since we included a patient aged 3.9 years at the start of treatment and with a height at that time of −2.11SD. This was done because the clinical characteristics of the patient recommended therapy, which in turn was approved by the Advisory Committee.
One of the most serious limitations of our study has to do with the data source used, which did not allow us to collect information that would have been useful for the study, such as side effects or patient adherence to treatment. The collection and analysis of such data could be a good starting point for continuing the study in the future.
In conclusion, the results of our study show that rhGH treatment favors growth in children with IUGR, with maximum effect being observed in the first 12 months. Likewise, the effect is greater when treatment is started before the patient reaches 5 years of age.
AuthorshipAll the authors have contributed intellectually to the work, meet the conditions for authorship, and have approved the final version of the article.
Study design and data collection, analysis and interpretation: Eduardo Gutiérrez Abejón, Eva Pilar Campo Ortega and Pablo Prieto Matos.
The drafting of the article: Eduardo Gutiérrez Abejón, María Pilar Bahillo Curieses and María Teresa Breñas Villalón.
The review of the manuscript: Eva Pilar Campo Ortega, Pablo Prieto Matos, María Teresa Breñas Villalón and Nieves Martín-Sobrino.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors form part of the Castilla and Leon Evaluation Committee for the therapeutic use of growth hormone and related substances.
Please cite this article as: Gutiérrez-Abejón E, Campo-Ortega EP, Prieto-Matos P, Bahíllo-Curieses MP, Breñas-Villalón MT, Martín-Sobrino N. Respuesta clínica al tratamiento con hormona de crecimiento en niños con retraso de crecimiento intrauterino sin recuperación posnatal de talla en Castilla y León. Endocrinol Diabetes Nutr. 2018;65:584–591.