A wide variation in height gain rate is observed in children small for gestational age (SGA) treated with growth hormone (GH). The aim of this study was to evaluate prepubertal and pubertal growth, height gain attained at adult age and to assess potential predictive factors in catch-up growth. Changes in metabolic profile were also analyzed.
Patients and methodsSeventy-eight children born SGA were treated with a GH median dose of 33.0±2.8mcg/kg/day at a mean age of 7.3±2.0 (boys) and 6.0±1.8 (girls).
ResultsMean height (SDS) at GH onset was −3.31±0.7 for boys and −3.48±0.7 for girls. According to age at pubertal growth spurt onset patients were classified in their pubertal maturity group. Adult height attained expressed in SDS was −1.75±0.7 for boys and −1.69±1.0 for girls, both below the range of their mid-parental height. The greatest height gain occurred during the prepubertal period. Patients with greater height gain were lighter (p<0.001), shorter (p=0.005), and younger (p=0.02) at the start of GH, and also showed a greater increase in growth velocity during the first year on GH (p<0.001). SGA children started puberty at the same age and with the same distribution into pubertal maturity group as the reference population. No relevant GH-related adverse events were reported, including in the insulin resistance parameters evaluated. Differences were found in fasting plasma glucose values, but were without clinical relevance. IGF-I plasma values remained within the safety range.
ConclusionsGH therapy is safe and beneficial for SGA children. The response to GH therapy is widely heterogeneous, suggesting that GH should be started at a young age and the GH dose prescribed should be individualized. SGA children started puberty at the same age as the reference population. The only factor that predicts greater adult height is growth velocity during the first year of therapy.
Existe una gran variabilidad en la ganancia de talla en los pacientes pequeños para edad gestacional (PEG) tratados con hormona de crecimiento (GH). El objetivo de nuestro trabajo fue evaluar el crecimiento prepuberal y puberal, la ganancia de talla alcanzada a la edad adulta, analizar los factores predictivos de mejor ganancia estatural y evaluar los cambios en el perfil metabólico.
Pacientes y métodosSetenta y ocho niños PEG iniciaron tratamiento con GH a una edad media de 7,3±2,0 (niños) y de 6,0±1,8 (niñas), con una talla media (DE) de –3,31±0,7 (niños) y de –3,48±0,7 (niñas). Dosis de GH: 33,0±2,8mcg/kg/día. De acuerdo con la edad al inicio del brote puberal de crecimiento, los pacientes se clasificaron de acuerdo a su grupo madurador.
ResultadosLa talla alcanzada a la edad adulta fue de –1,75±0,7 (niños) y de –1,69±1,0 (niñas), y estaba situada por debajo de su talla media parental. Los pacientes que mostraron una mayor ganancia de talla fueron los de menor peso (p<0,001), menor talla (p=0,005) y menor edad (p=0,02) al inicio de la GH, y también los que mostraron un mayor incremento de la velocidad de crecimiento durante el primer año de tratamiento con GH (p<0,001). Los pacientes PEG iniciaron la pubertad a la misma edad y con una distribución en los grupos maduradores similar a la población de referencia. No se observaron eventos adversos relacionados con la GH ni en los índices de resistencia a la insulina. Tan solo se hallaron diferencias significativas sin relevancia clínica en los valores de glucosa plasmática en ayunas. Los valores plasmáticos de IGF-I se mantuvieron dentro del rango de seguridad.
ConclusionesEl tratamiento con GH es seguro y beneficioso para los niños PEG. La mayor ganancia de talla ocurre durante el período prepuberal y es heterogénea, lo que sugiere que la GH debe iniciarse a una edad temprana y que es preciso individualizar su dosis. La pubertad se inicia a una edad similar a la población general. El único factor que predice una mayor ganancia de talla adulta es la velocidad de crecimiento durante el primer año de tratamiento.
Small for Gestational Age (SGA) is defined as a birth weight and/or length values less than −2 standard deviation score (SDS) of those of a normal reference population.1
The causes of insufficient growth during the fetal period are multiple and varied. Fetal, maternal and placental factors can be involved, whereas in most cases the etiology remains unknown.1,2
Most SGA children (either preterm or at term) experience catch-up growth during the first months of life, but by the age of 4 years, 10–15% remain below −2 SDS of height.3,4 Growth hormone (GH) therapy is approved in SGA children to improve growth in order to attain adult height (AH) above −2 SDS and normalize body composition. Clinical studies have found that age and height at the start of GH therapy and parental height are predictive factors of height gain.5–9 Despite these variables, a variation in height gain rate is observed among SGA children.
Studies investigating puberty in SGA children have shown that, although puberty begins at an appropriate time (based on chronological age and actual height) the total pubertal height gain is lower than for the reference population, resulting in a lower adult height (AH) than expected.10,11 Growth studies in reference populations show a wide range in pubertal height gain according to age at puberal growth spurt onset (PGSO),12 however few data have been published on SGA.9
The aim of this study was to evaluate prepubertal and pubertal growth gain in 78 SGA children treated with GH from prepubertal age to adult height age (AHA) and to elucidate which factors could predict a greater response to GH therapy. Changes in metabolic profile were also analyzed.
Patients and methodsThis was a retrospective, observational and descriptive study. From an initial sample of 110 SGA patients, 78 reached adult height. Seventeen were not included due to not having reached adult height at the time of inclusion and 15 due to a lack of complete data regarding their follow-up. Seventy-eight SGA children (51 boys and 27 girls) were longitudinally followed and retrospectively evaluated from 1 year before the onset of GH therapy until AHA, which corresponds to the age of 18 years (boys) and 16 years (girls). The GH median dose was 33.0±2.8 mcg/kg/day
Inclusion criteria were: (a) a birth length and/or birth weight<−2 SDS according to the Spanish reference for newborns13; (b) not having had catch-up growth at 4 years of age; (c) a height less than −2.5 SDS below the mean for age and sex and less than −1 SDS adjusted to genetic height; (d) GH started two or more years before puberty onset, in order to minimize interference of the pubertal growth spurt with the effect of GH; (e) normal gastrointestinal, liver, kidney, metabolic, pulmonary and thyroid functions; (f) normal body proportion; (g) never having been treated with GH, anabolic agents or gonadotropin releasing hormone analog therapy (GnRHa).
Exclusion criteria were: (a) GH deficiency of known etiology, (b) neonatal brain injury, (c) chromosome disorders, (d) malformation syndromes, (e) chronic diseases, (f) steroid therapy.
According to birth anthropometric parameters SGA children were classified as light (birth weight<2SD) (n=9), short (birth length<2SD) (n=34) or both short and light (n=35). Twenty-one percent (n=14) were born before 37 weeks of gestation.
Before starting GH therapy all patients underwent two different GH-release stimuli between January 1989 and December 2009. According to the GH peaks after the two stimuli, children were classified by: GH peaks<10ng/ml after both stimuli (n=40); GH peaks<10ng/ml after the first stimulus and >10ng/ml after the second (n=26); GH peaks 10–20ng/ml after both stimuli (n=12). The median and interquartile range of GH values with the stimulation tests were: baseline 1.4ng/dl (0.5–3.6) and post stimuli 6.7ng/dl (3.9–10.1). GH was measured with the Immulite® assay calibrated against the first IS 80/505, in which a GH concentration of 10ng/ml is equivalent to a GH concentration of 7.4ng/dl in the more recent calibrated assay IS 98/574 (Technical Bulletin 2323/2112, 2009).
Weight, the body mass index (BMI), height and cumulative height gain were evaluated one year before GH therapy, at the start of GH therapy, at the end of the first and second year on GH therapy, at PGSO, at the end of GH therapy (Bone Age [BA] of 15 years for boys and 13 years for girls) and at AHA. PGSO coincided with a testicular volume of 4ml in boys and the presence of breast bud in girls. According to age at PGSO boys and girls were classified in each pubertal maturity group.12 Pubertal height gain was defined as AHA minus height (cm) at puberty onset. AH attainment was considered when there was no change in height or a change<0.5cm in two consecutive measures at 6–12 month intervals and BA was between 15 and 16 years for boys and between 13.5 and 14 years for girls. Anthropometric values were transformed into SD scores according to age, sex and pubertal stage-matched control values recently reported in the Barcelona longitudinal growth study 1995–2017.12 Paternal and maternal height were recorded and transformed into SDS, and mid-parental height (SDS) (mean value of paternal and maternal height) was also calculated. BA was evaluated according to the Greulich and Pyle atlas.
Hormone and biochemical measurementsGH (ng/ml) and IGF-I (ng/ml) were measured with the commercial immunoassays (Immulite®; Diagnostic Products Corp., Los Angeles, Calif., USA). GH was measured with the Immulite® assay calibrated against the first IS 80/505.
IGF-I values were expressed as the percentage of the mean according to age, sex and pubertal stage-matched control values.14 Serum thyrotropin (TSH), free thyroxine (fT4), glucose, insulin (measured with Siemens Centaur Equip), the HOMA index (calculated as glucose[mg/dl]×Insulin[mU/L]/405), total cholesterol, and triglyceride values were measured with validated methods. All parameters were evaluated at the start of GH therapy, at the first and second year on GH therapy, PGSO, at the end of GH therapy and at AHA.
Statistical analysisResults are expressed as mean±SD. Differences for the variables evaluated were calculated using parametric (Student's T Test or corrected ANOVA/Bonferroni) or nonparametric (Mann–Whitney U and Kruskal–Wallis) tests; the Kolmogorov–Smirnov test was applied to test for normal distribution.
The Pearson test was used in the correlation analysis. The analysis of measures of efficacy and safety was performed with the nonparametric tests of Wilcoxon and Friedman. Multivariate stepwise regression analysis was performed with the variables that showed p<0.001 in the univariate analysis. Adult height gain (SDS) was considered the outcome variable, and height (SDS) at the start of GH therapy and height (SDS) at puberty onset were considered the predictor variables.
Statistical analysis was performed using the IBM SPSS software version 20. The level of statistical significance was set at p<0.05.
ResultsA cohort of 78 SGA children (51 boys and 27 girls) was followed up for 11.6±2.1 years (boys) and 11.3±2.1 years (girls).
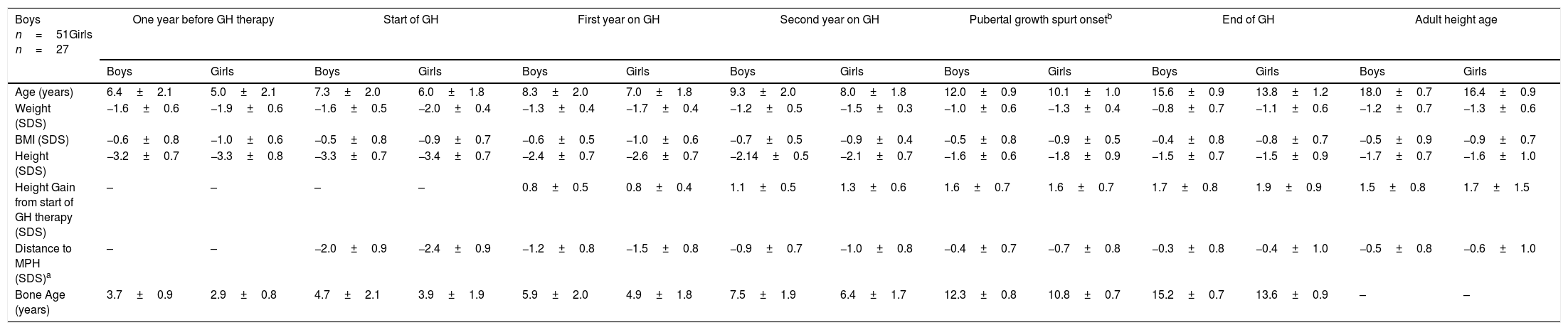
Patients started GH therapy at a mean age of 7.3±2.0 (boys) and 6.0±1.8 (girls) with a mean height (SDS) of −3.3±0.7 (boys) and −3.4±0.7 (girls), and they attained an adult height of −1.7±0.7 SDS, corresponding to 165.8±4.4cm (boys) and −1.6±1.0 SDS, corresponding to 153.5±5.3cm (girls). A progressive increase in height was observed from the start to the end of GH therapy. The largest increase was observed during the first two years on GH therapy and was maintained during the prepubertal and pubertal period. At PGSO, height gain (SDS) was the same for boys and girls (+1.6±0.7) and these values were similar to those found at AHA. A progressive reduction in distance to mid-parental height (MPH) from the start of GH therapy to AHA was observed, although adult height was below their MPH. Weight (SDS) and the BMI (SDS) increased throughout the study. Patients started GH therapy with a delayed BA of about 2 years. This delay was maintained during the prepubertal period. At the start of PGSO, BA was according to its initial pubertal stage. At the end of GH therapy patients showed a BA according to their chronological age. The results are shown in Table 1.
Age and anthropometric parameters of SGA children during GH therapy. Distance to MPH-SDS and bone age (years) are also shown (mean±SD).
| Boys n=51Girls n=27 | One year before GH therapy | Start of GH | First year on GH | Second year on GH | Pubertal growth spurt onsetb | End of GH | Adult height age | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boys | Girls | Boys | Girls | Boys | Girls | Boys | Girls | Boys | Girls | Boys | Girls | Boys | Girls | |
| Age (years) | 6.4±2.1 | 5.0±2.1 | 7.3±2.0 | 6.0±1.8 | 8.3±2.0 | 7.0±1.8 | 9.3±2.0 | 8.0±1.8 | 12.0±0.9 | 10.1±1.0 | 15.6±0.9 | 13.8±1.2 | 18.0±0.7 | 16.4±0.9 |
| Weight (SDS) | −1.6±0.6 | −1.9±0.6 | −1.6±0.5 | −2.0±0.4 | −1.3±0.4 | −1.7±0.4 | −1.2±0.5 | −1.5±0.3 | −1.0±0.6 | −1.3±0.4 | −0.8±0.7 | −1.1±0.6 | −1.2±0.7 | −1.3±0.6 |
| BMI (SDS) | −0.6±0.8 | −1.0±0.6 | −0.5±0.8 | −0.9±0.7 | −0.6±0.5 | −1.0±0.6 | −0.7±0.5 | −0.9±0.4 | −0.5±0.8 | −0.9±0.5 | −0.4±0.8 | −0.8±0.7 | −0.5±0.9 | −0.9±0.7 |
| Height (SDS) | −3.2±0.7 | −3.3±0.8 | −3.3±0.7 | −3.4±0.7 | −2.4±0.7 | −2.6±0.7 | −2.14±0.5 | −2.1±0.7 | −1.6±0.6 | −1.8±0.9 | −1.5±0.7 | −1.5±0.9 | −1.7±0.7 | −1.6±1.0 |
| Height Gain from start of GH therapy (SDS) | – | – | – | – | 0.8±0.5 | 0.8±0.4 | 1.1±0.5 | 1.3±0.6 | 1.6±0.7 | 1.6±0.7 | 1.7±0.8 | 1.9±0.9 | 1.5±0.8 | 1.7±1.5 |
| Distance to MPH (SDS)a | – | – | −2.0±0.9 | −2.4±0.9 | −1.2±0.8 | −1.5±0.8 | −0.9±0.7 | −1.0±0.8 | −0.4±0.7 | −0.7±0.8 | −0.3±0.8 | −0.4±1.0 | −0.5±0.8 | −0.6±1.0 |
| Bone Age (years) | 3.7±0.9 | 2.9±0.8 | 4.7±2.1 | 3.9±1.9 | 5.9±2.0 | 4.9±1.8 | 7.5±1.9 | 6.4±1.7 | 12.3±0.8 | 10.8±0.7 | 15.2±0.7 | 13.6±0.9 | – | – |
GH: growth hormone.
According to age at pubertal onset, boys and girls were classified as very early (n=4), early (n=20), intermediate (n=27), late (n=21) and very late (n=6) maturers.17,18
No statistically significant differences, except for age when GH was initiated, were found between boys and girls for any of these values.
Height gain did not differ (p=0.34) between those born preterm (+1.8±1.0 SDS) or at term (1.6±0.9 SDS), and also did not differ (p=0.43) between those born light (1.7±0.8 SDS), short (1.6±0.8 SDS) or both light and short (1.6±1.0 SDS).
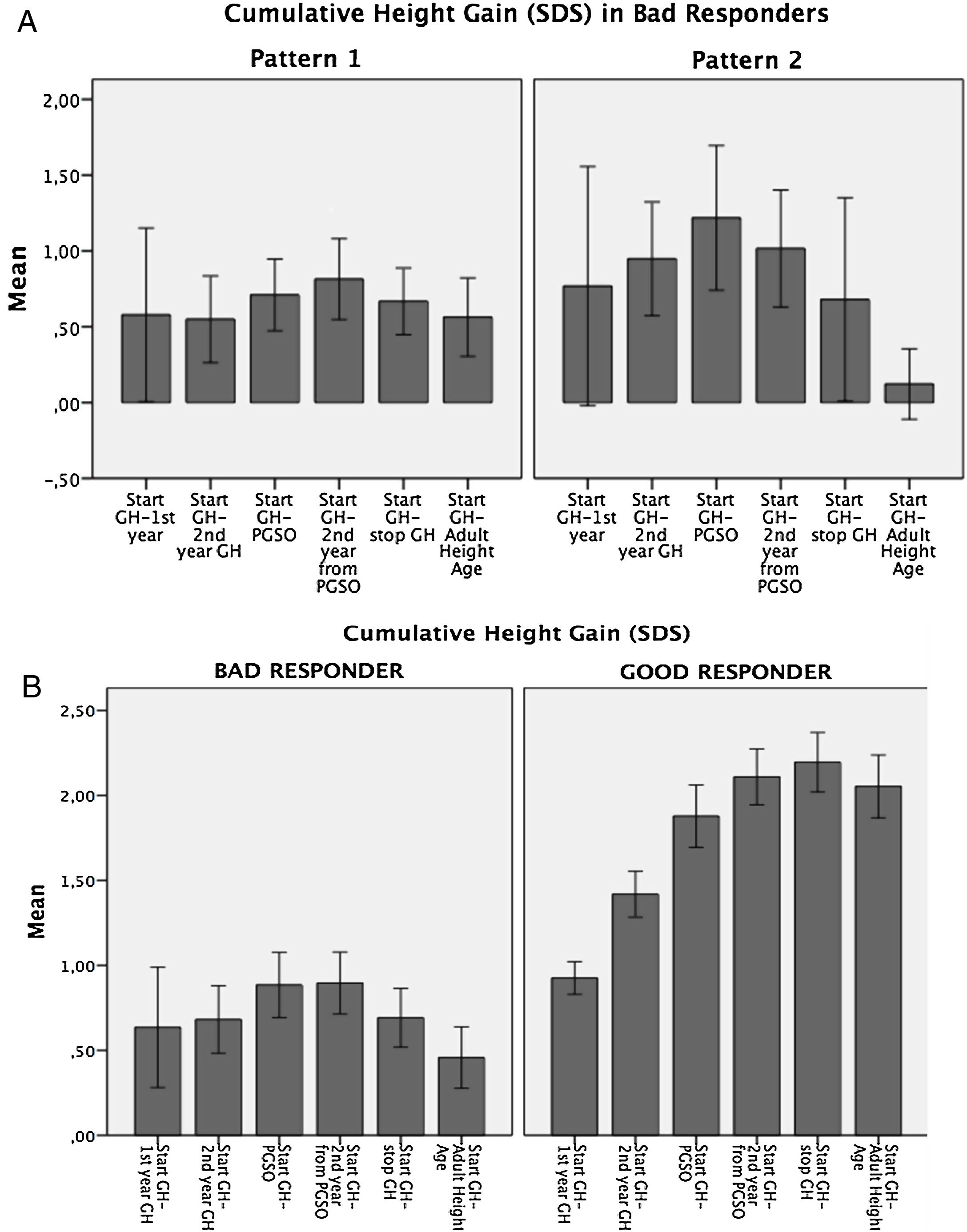
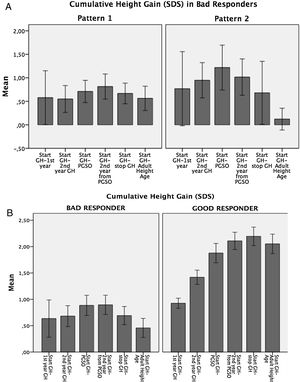
Variability in the auxological response to GH therapyAccording to cumulative height gain from GH therapy start to AHA, patients were distributed into two groups: Bad Responders: height gain equal/lower than 1 SDS (n=20; 25%), and Good Responders: height gain higher than 1 SDS (n=58; 75%). (Fig. 1a).
In the Bad Responders group, two growth patterns were identified: 1) poor response from the start of GH therapy (n=12) and 2) height loss greater than 0.5 SDS during puberty (n=5) (Fig. 1b). The 3 remaining patients showed height loss during puberty but did not reach 0.5 SDS. In the Good Responders group a height loss greater than 0.5 SDS during puberty was observed in 5 patients.
Univariate analysis showed that Good Responders were lighter (p<0.001), shorter (p=0.005), younger (p=0.02) and with a lower BMI (SDS) (p=0.003) at the start of GH therapy and showed more growth velocity increase during the first year on GH therapy (p<0.001) than Bad Responders. No statistically significant differences were found for weight (SDS) and height (SDS) at birth, MPH (SDS), height (SDS) at PGSO, prepubertal and pubertal years on GH therapy and years at the end of GH therapy. Moreover, no statistically significant differences were found among patients classified by gender, by being preterm or at term, being short, light or both at birth, by their response to two GH release stimuli or by the pubertal maturity group (data not shown)
Predictors of height gain with GH therapyAdult height gain (SDS) correlated negatively at the start of GH therapy with: weight (SDS) (r−0.4, p<0.001), height (SDS) (r−0.5, p<0.001), the BMI (SDS) (r−0.3, p=0.003) and IGF-I values (r−0.2, p=0.034) but positively with growth velocity during the first year on GH therapy (r+0.4, p<0.001), height at PGSO (r+0.2, p=0.038) and the duration of GH therapy (years) (r+0.2, p=0.049).
No correlation was found with weight and length (SDS) at birth, MPH, age at start and at the end of GH therapy and IGF-I values at the first and second year on GH therapy. In the multivariate analysis, adult height gain (SDS) was negatively dependent on height (SDS) at the start of GH therapy (B=−1.01, p<0.000) and positively dependent on height (SDS) at puberty onset (B=0.75, p<0.000).
PubertySGA boys started puberty at 12.0±0.9 and girls at 10.1±1.0 years of age. Puberty lasted 3.6±0.8 and 3.7±0.7 years for boys and girls, respectively. None of them presented precocious puberty or rapidly progressive puberty. According to age at PGSO, children were classified as very early (n=4;5%), early (n=20;25%), intermediate (n=27,36%), late (n=21;26%) or very late (n=6;8%) maturers. This distribution showed no statistically significant differences with the reference population.12
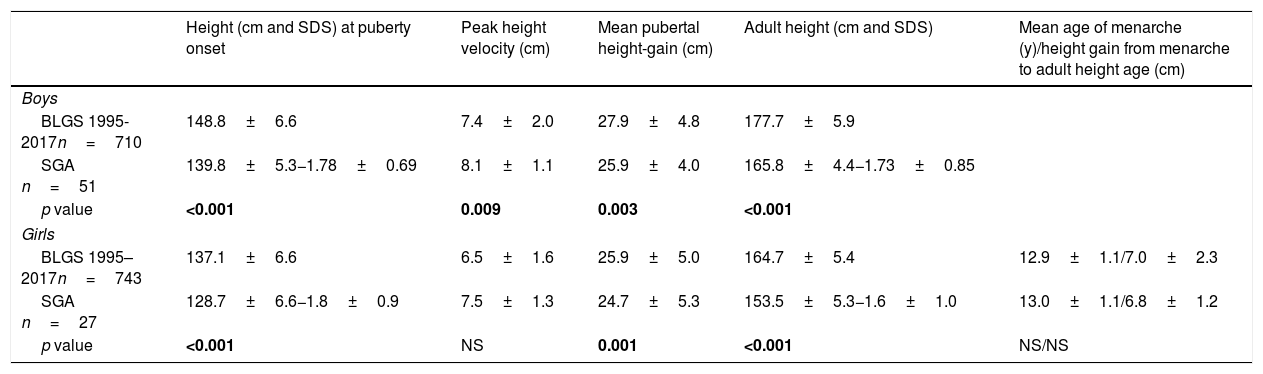
At PGSO and at AHA, SGA patients were shorter than the reference population (p<0.001). Slight differences for mean pubertal height gain were observed for SGA children. In SGA girls, the mean age of menarche and height gain from menarche to adult height showed no statistically significant differences to the reference population (Table 2). At PGSO, the BMI (SDS) was below zero and BA showed no delay in respect of chronological age for either gender (Table 1). In analyzing girls classified as being short, light or both at birth, no statistically significant differences were found between the mean age at PGSO and the mean age of menarche (data not shown).
Pubertal growth of SGA children.
| Height (cm and SDS) at puberty onset | Peak height velocity (cm) | Mean pubertal height-gain (cm) | Adult height (cm and SDS) | Mean age of menarche (y)/height gain from menarche to adult height age (cm) | |
|---|---|---|---|---|---|
| Boys | |||||
| BLGS 1995-2017n=710 | 148.8±6.6 | 7.4±2.0 | 27.9±4.8 | 177.7±5.9 | |
| SGA n=51 | 139.8±5.3−1.78±0.69 | 8.1±1.1 | 25.9±4.0 | 165.8±4.4−1.73±0.85 | |
| p value | <0.001 | 0.009 | 0.003 | <0.001 | |
| Girls | |||||
| BLGS 1995–2017n=743 | 137.1±6.6 | 6.5±1.6 | 25.9±5.0 | 164.7±5.4 | 12.9±1.1/7.0±2.3 |
| SGA n=27 | 128.7±6.6−1.8±0.9 | 7.5±1.3 | 24.7±5.3 | 153.5±5.3−1.6±1.0 | 13.0±1.1/6.8±1.2 |
| p value | <0.001 | NS | 0.001 | <0.001 | NS/NS |
BLGS 1995–2017: Barcelona Longitudinal Growth Study 1995–2017.
GH therapy was well tolerated and no remarkable GH-related adverse events were reported.
Mean IGF-I plasma values were similar between the genders at the start, during GH therapy and at AHA. At the start of GH therapy IGF-I plasma values were approximately 40% of the mean according to age, sex and pubertal stage-matched control values. On GH therapy, these values increased progressively and ranged between 120% and 180% of the reference values, being higher at the second year on GH therapy and during puberty. Lower values were observed at AHA, when GH therapy had been withdrawn (85–119%). Statistically significant differences (p<0.05) were observed between IGF-I plasma values at the start of GH therapy and those during GH therapy (Data not shown).
Mean fasting plasma glucose values at the start of GH therapy significantly differed from those observed at puberty (80±10mg/dl vs. 88.4±7mg/dl; p<0.05) and those at the end of GH therapy (80±10mg/dl vs. 90.8±7mg/dl; p<0.05). However, no statistical significance was found for plasma insulin and HOMA index values at these ages. At AHA, fasting plasma glucose, plasma insulin and HOMA index values were in the normal range for all patients (Data not shown).
Mean plasma total-cholesterol values at the start of GH therapy significantly differed from those observed at puberty (176.3±24 vs. 158.8±24mg/dl; p<0.05). Mean plasma triglycerides and the thyroid hormone profile were in the normal range at the start, during GH therapy and at AHA (Data not shown).
DiscussionIn this cohort of 78 SGA children, GH therapy improved adult height (SDS) and 64% of them reached an adult height within the normal range (>−2SDS). The most important height gain was observed during the prepubertal period and height gain attained at PGSO was similar to that attained at AHA. This suggests that SGA children should start GH therapy at a young age in order to attain a greater adult height, as has been published.6,15–17 General pediatricians should monitor the growth pattern of these patients and refer them to specialists in pediatric endocrinology if they do not catch up growth at 4 years of age. We agree with de Kort et al that preterm children born SGA also show a good response to GH therapy.18
A wide range in height gain was observed. In Bad Responders, two growth patterns were identified: the first, in which patients showed poor height increase from the start of GH therapy to PGSO suggested poor chronic compliance with GH therapy or some degree of GH resistance, although lack of compliance was denied by parents and no baseline GH value before the stimulus suggested GH resistance. As a first option, an increase in GH doses should be considered in these patients and subsequently GH therapy should be discontinued if the growth rate cannot be increased by at least+0.5 SDS.1 The second pattern, in which patients showed a height loss greater than 0.5 SDS during puberty, was observed in 5 patients in the Bad Responders group and in 5 patients in the Good Responders group, and this was also observed by other authors,19 suggesting a height loss gain in puberty inherent in SGA children and maybe also poor compliance with GH therapy during this period, so it is especially important to monitor adherence.
Good Responders (height gain>1 SDS) were lighter, shorter, younger at the start of GH therapy and had more growth velocity during the first year on GH therapy than Bad Responders (height gain<1 SDS). These results are in agreement with those published by Dahlgren et al.9 On the other hand, variables such as weight (SDS) and height (SDS) at birth, mid-parental height (SDS), height (SDS) at PGSO, and patients classified by being preterm or at term, being short, light or both at birth, their response to two GH release stimuli and their pubertal maturity group were not able to differentiate responsiveness to GH therapy, as has also been published by other authors.9
As there is a wide variation in the response to GH therapy in SGA children, several studies have looked for predictors of growth response to GH therapy. Most studies report correlations with age at the start of GH therapy,20,21 others with height (SDS) at the start,6,19 others with prepubertal GH dose20,21 and others with mid-parental height and IGFBP3 values.7,8 Our study showed that height (SDS) gain correlated negatively with weight (SDS), height (SDS) and IGF-I values at the start of GH therapy and positively with growth velocity during the first year on GH therapy, height at PGSO and the duration of GH therapy.
Given the variability of response to GH treatment, the heterogeneity of SGA patients, and that growth velocity during the first year on GH therapy is the only reliable factor for greater height gain, GH therapy in SGA children should be individualized.
In the present study we reported pubertal growth of SGA children according to PGSO. SGA children started puberty at the same age and had the same distribution into pubertal maturity groups as the reference population.6,15,16,22 Likewise, the mean age of menarche showed no statistically significant difference. However, other studies showed an earlier PGSO and an earlier age of menarche in girls born light for gestational age.10,23 Our SGA children were shorter at PGSO and at AHA and their pubertal height gain was slightly lower compared to the Spanish reference population. This lower pubertal height gain has also been reported by other authors10,11 and the possible explanation given for this phenomenon was an early onset of puberty and a premature fusion of growth cartilage in SGA children. With our data we cannot confirm these hypotheses. Puberty started at the same age as the reference population and a premature closure of the growth cartilages was not observed. In our study, the use of GnRHa to suppress puberty was not assessed, as it was one of the exclusion criteria. Studies using the combination regimen showed controversial results, with minimal benefit or even a reduction of growth outcomes, so cannot be generally recommended.24
GH therapy involves changes in auxological, metabolic and body composition parameters. Pharmacovigilance studies have shown their safety in the short term, with little known long-term effects. To date, no specific side effects of GH therapy have been observed in SGA. It is increasingly recognized that being born SGA carries an elevated risk of developing metabolic disease in later life, particularly obesity, insulin resistance, carbohydrate intolerance, and dyslipidemia.25 GH therapy itself has also been associated with an increase in insulin values, metabolic disorders and alterations in the lipid profile. Thus, the effect of GH on metabolism in children born SGA has been a potential concern. Although children born SGA tend to develop higher fasting insulin values and relative insulin resistance during GH therapy, but without developing glucose intolerance or diabetes mellitus type 2, reassuringly these changes are reversible when treatment is stopped.16,26 We found statistically significant differences in fasting plasma glucose values with GH therapy, although they were not clinically relevant. No differences were found in plasma insulin values and the HOMA index. It is important to mention that we did not have the same number of patients for all the variables of carbohydrate metabolism at each period of the study and this difference could explain why we did not obtain statistically significant results in insulinemia and the HOMA index, as found in other studies.16,17
IGF-I plasma values increased significantly during the first year of treatment, but remained within the safety range, as has been published.9,21,27
Focusing on the lipid profile during GH therapy, a decrease in total cholesterol values was observed. An improvement in the lipid profile has been reported with GH therapy in SGA patients. This fact is relevant given the inherent risk in the SGA population of cardiovascular disease and dyslipidemia.5,27
The main limitation of our study was not having a control group of SGA patients without GH therapy to make comparisons. However, since SGA patients are indicated for GH treatment, it would have been unethical to have a control group. An old study that evaluated growth in 486 Spanish SGA children without catch up growth and who had not been treated with GH therapy, showed that these children starting from a height of -3.51 SDS attained an adult height of -2.78 SDS.4
We would also like to have had a larger number of patients to confirm our results regarding puberty and metabolic profile changes.
ConclusionIn summary, growth hormone therapy is safe in the short term and beneficial for SGA children who do not present catch-up growth at four years of age. The most important height gain occurs during the prepubertal period, suggesting that GH therapy should be started at a young age. SGA children start puberty at the same age and have the same distribution into pubertal maturity groups as the reference population, although pubertal height gain is slightly lower.
Height increase during GH therapy in SGA children is heterogeneous and the GH dose prescribed should be individualized. The only factor that seems to predict a greater adult height is growth velocity during the first year of treatment. Other factors, such as the GH response to stimuli or the anthropometrics at birth, are not predictive factors.
Authors’ contributionMFC contributed to data collection. MCL, EMV, AFM contributed to the revision of the manuscript. ACL and DYF contributed to the writing of the manuscript.
Compliance with ethical statementsEthical approvalThis article does not contain any studies with human participants or animals performed by any of the authors.
Informed consentInformed consent was obtained from all individual participants included in the study.
FundingThere is no funding source.
Conflict of interestThe authors declare that they have no conflict of interest.
Fidelma Greaves is acknowledged for useful help with the English version of the manuscript and Dolors Giralt for the statistical analysis.