To assess the control of cLDL in diabetic patients, to measure the impact on such control of inertia with lipid-lowering agents and to explore factors that allow for predicting this inertia.
MethodsStudy of historical cohorts of diabetic patients. The proportion of patients who achieved the target cLDL levels was estimated. Therapeutic inertia was considered when the dose of the lipid-lowering agents was not adjusted, or a lipid-lowering agent was not changed or added in patients with initial cLDL outside the target. Change in cLDL from the first to the last visit and inertia with lipid-lowering drugs were analyzed according to comorbidities, cardiovascular risk factors and treatments used.
ResultsThe study simple consisted of 639 patients (mean follow-up time 11.1±11.2 months), of whom 27.5% achieved target cLDL levels. Inertia occurred in 43.6% of patients with initial cLDL outside the target. Independent predictors of inertia were the initial cLDL (p<0.001), polyneuropathy (p=0.014), adjustment of antihypertensive agents (p=0.002), adequacy of lipid-lowering agents (p<0.001), use of ezetimibe (p=0.001) and adherence to lipid-lowering drugs (p=0.015).
ConclusionsInertia with lipid-lowering agents in a diabetic patient is less frequent in the presence of higher cLDL values, in cases of polyneuropathy, when antihypertensive agents are adjusted or changed, and when non-adherence is detected. The adequate initial prescription of statins and the association with ezetimibe decrease the likelihood of committing inertia.
Valorar el control del cLDL de pacientes con diabetes, medir la influencia en este control de la inercia con los hipolipidemiantes y explorar sus factores predictores.
MétodosEstudio de cohortes históricas de pacientes con diabetes. Se midió el porcentaje que alcanzó un cLDL dentro de objetivo. Se consideró inercia terapéutica cuando no se ajustó la dosis de los hipolipidemiantes, ni se cambió ni añadió ningún nuevo hipolipidemiante en los pacientes con cLDL inicial fuera de objetivo. Se estudiaron el cambio experimentado en el cLDL entre la primera y la última visita y la inercia con los hipolipidemiantes en función de las comorbilidades, factores de riesgo cardiovascular asociados y tratamientos utilizados.
ResultadosSe incluyó a 639 pacientes (tiempo medio de seguimiento 11,1±11,2 meses). El 27,5% alcanzó un cLDL dentro de objetivo. Se produjo inercia en el 43,6% de los pacientes con un cLDL inicial fuera de objetivo. Resultaron predictores independientes de la inercia el cLDL inicial (p<0,001), la polineuropatía (p=0,014), el ajuste de los antihipertensivos (p=0,002), la adecuación de los hipolipidemiantes (p<0,001), el uso de ezetimiba (p=0,001) y la adherencia a los hipolipidemiantes (p=0,015).
ConclusionesLa inercia en el tratamiento hipolipidemiante de un paciente con diabetes es menos frecuente ante valores iniciales de cLDL más altos, en los casos de polineuropatía, cuando se ajustan o cambian los antihipertensivos y cuando se detecta falta de adherencia. La prescripción inicial adecuada de estatinas y la asociación con ezetimiba disminuyen la probabilidad de caer en la inercia.
The Di@bet.es study showed that type 2 diabetes mellitus (DM2) affects 13.8% of the Spanish population,1 and that the LDL-cholesterol control rate among these patients is 45.3% for an LDL-cholesterol target<100mg/dl versus only 11.3% for an LDL-cholesterol target<70mg/dl.2 The Hispanic Community Health Study/Study of Latinos analyzed the degree of control regarding glycosylated hemoglobin (HbA1c), blood pressure (BP) and LDL-cholesterol in 2148 Hispanic patients with diabetes residing in the United States, and found that 36.6% of them achieved LDL-cholesterol<100mg/dl, with a prevalence of statin use of 38.2%.3 Statins, regarded as the first-line treatment for dyslipidemia in patients with diabetes,4 were found in a meta-analysis of 18,686 of such patients followed-up on for an average of 4.3 years to be associated with a 13.9% decrease in cardiovascular mortality for every 39mg/dl reduction in LDL-cholesterol.5 It is therefore of interest to investigate the causes underlying the limited percentages of statin use and LDL-cholesterol control documented in real-world studies of patients with diabetes.
Therapeutic inertia, defined as physician failure to initiate or intensify treatment when indicated, is common in diabetes care,6 and has important implications for public health and healthcare costs.7 The INERCIA study observed this phenomenon regarding the management of dyslipidemia in 23.2% of patients with hypertensive diabetes or smokers with associated ischemic heart disease.8 Therapeutic inertia in high-risk dyslipidemic patients is associated with a 2.18-fold increase in the risk of a first ischemic event in just 18 months.9 Understanding the factors underlying such therapeutic inertia could help in the design of strategies to reduce the problem10 and thus improve dyslipidemia control.
The present study was carried out to assess current LDL-cholesterol control in patients with DM2 followed-up on in an Endocrinology clinic; to measure the influence of lipid-lowering drug treatment inertia upon the degree of control; and to explore the factors allowing us to predict such inertia.
MethodsStudy design. Inclusion and exclusion criteriaA historical cohort study was carried out. The inclusion criteria were patients over 18 years of age, of either gender, diagnosed with DM2 and treated at the Endocrinology clinic of Hospital Dr. José Molina Orosa (Lanzarote, Canary Islands, Spain) between September 2011 and July 2016. Patients receiving concomitant antiretroviral therapy were excluded. The patients followed-up on at the clinic gave informed consent to their participation in the study. The study protocol was approved by the local Clinical Research Ethics Committee.
Data collectionThe medical records of patients meeting the inclusion criteria were reviewed. The following variables were compiled:
- -
Gender, age, duration of diabetes, comorbidities (diabetic retinopathy, cataracts, chronic kidney disease, diabetic polyneuropathy, ischemic heart disease, arrhythmias, stroke, peripheral artery disease, mental illness, hypothyroidism). The diagnosis of diabetic polyneuropathy was established by asking the patient on each visit about symptoms consistent with neuropathic pain, and exploring diabetic foot.
- -
Metabolic parameters and cardiovascular risk factors. The values recorded at the first and the last visit was collected.
- ∘
Total cholesterol, LDL-cholesterol, HDL-cholesterol and triglycerides. LDL-cholesterol was calculated using the Friedewald formula.11 We measured the percentage of patients reaching LDL-cholesterol on target at the last visit (<70mg/dl for those with cardiovascular disease, a glomerular filtration rate [GFR]<60ml/min, age>40 years with arterial hypertension, smokers or subjects with albuminuria>30mg/g and <100mg/dl for the remaining patients).12
- ∘
Glycosylated hemoglobin (HbA1c), the presence of hypoglycemic episodes.
- ∘
The body mass index (BMI), waist circumference, blood pressure. The BMI was calculated by dividing the weight in kg by the height in meters squared.
- ∘
Smoking and smoking cessation.
- ∘
- -
Follow-up indicators. Follow-up time and number of visits.
- -
Lipid-lowering drugs. Statins, ezetimibe, fibrates, omega-3 fatty acids. Adequate statin prescription was documented, adequate being regarded as the prescription of high intensity statins (40–80mg atorvastatin, 20–40mg rosuvastatin) in patients with cardiovascular disease and moderate intensity statins (the rest of the statins) in patients without cardiovascular disease but ≥40 years of age.13 Adherence to lipid-lowering therapy was assessed by asking each patient the Haynes–Sackett question: “Most patients have difficulty taking all their tablets. Do you have difficulty taking yours?”.14
- -
Other treatments used. Therapeutic education, physical exercise, the number of daily drugs. Dietary measures were explained in the course of ongoing therapeutic education. We assessed whether the glucose-lowering, antihypertensive and lipid-lowering treatment had been adjusted during follow-up, and the patients were classified into one of the following three categories for each drug group: no change, dose adjustment, change or addition of drugs. Therapeutic inertia was identified when the dose of lipid-lowering medication was not adjusted, and no lipid-lowering drug was either changed or added in patients with initial LDL-cholesterol off-target.
The change in LDL-cholesterol between the first and the last visit, and therapeutic inertia with lipid-lowering drugs, were studied according to the demographic characteristics, comorbidities, associated cardiovascular risk factors, follow-up indicators and treatments used. Quantitative variables were reported as the mean and standard deviation, and categorical variables as percentages. Continuous variables were compared with the Student's t-test, while analysis of variance (ANOVA) was used for the comparison of more than two groups in the presence of normal data distribution. In the absence of normal data distribution, we used the Mann–Whitney U-test, or the Kruskal–Wallis test when comparing more than two groups. The chi-squared was used for the contrasting of proportions. Correlations between quantitative variables were assessed using the Pearson or Spearman coefficients, depending on whether the data exhibited a normal distribution or not. After the comparisons had been established, the results were subjected to the corresponding multivariate analyses: changes in LDL-cholesterol were assessed using a linear regression technique, while therapeutic inertia with lipid-lowering drugs was assessed by means of binary regression analysis. The SPSS version 21.0 statistical package (Chicago, IL, USA) was used throughout. Statistical significance was considered for p<0.05.
ResultsPatients included. Baseline characteristicsThe study comprised a total of 639 patients treated by a single endocrinologist. The mean follow-up time in the clinic was 11.1±11.2 months, the average number of visits was 3±2.4, and the time dedicated to each consultation was 30min on the first visit and 15min in the case of subsequent visits. Fifty patients died in the course of follow-up (7.8%). A total of 89 patients missed their follow-up visits (13.9%).
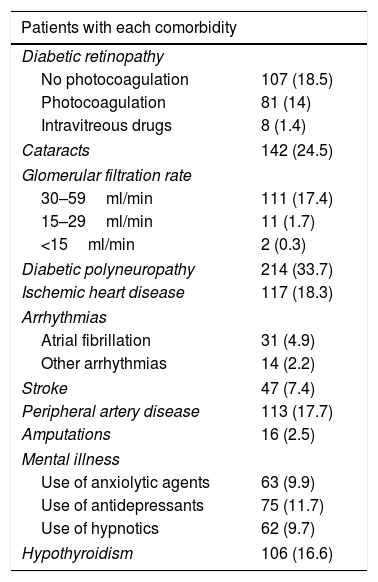
The baseline characteristics were: mean age 62±11.5 years; mean duration of diabetes 11.7±9.5 years; female proportion 52.6%; the BMI 32.3±6.9kg/m2; waist circumference 105.9±13.2cm; HbA1c 8.6±1.7%; systolic BP 140.9±21.1mmHg; diastolic BP 80.7±12.6mmHg; total cholesterol 192.8±45.1mg/dl; LDL-cholesterol 113±37.9mg/dl; HDL-cholesterol 47.1±14.5mg/dl; triglycerides 178.1±136mg/dl and the proportion of smokers 15.4%. The prevalences of the different comorbidities are reported in Table 1.
Prevalence of comorbidities.
| Patients with each comorbidity | |
|---|---|
| Diabetic retinopathy | |
| No photocoagulation | 107 (18.5) |
| Photocoagulation | 81 (14) |
| Intravitreous drugs | 8 (1.4) |
| Cataracts | 142 (24.5) |
| Glomerular filtration rate | |
| 30–59ml/min | 111 (17.4) |
| 15–29ml/min | 11 (1.7) |
| <15ml/min | 2 (0.3) |
| Diabetic polyneuropathy | 214 (33.7) |
| Ischemic heart disease | 117 (18.3) |
| Arrhythmias | |
| Atrial fibrillation | 31 (4.9) |
| Other arrhythmias | 14 (2.2) |
| Stroke | 47 (7.4) |
| Peripheral artery disease | 113 (17.7) |
| Amputations | 16 (2.5) |
| Mental illness | |
| Use of anxiolytic agents | 63 (9.9) |
| Use of antidepressants | 75 (11.7) |
| Use of hypnotics | 62 (9.7) |
| Hypothyroidism | 106 (16.6) |
Data reported as n (%).
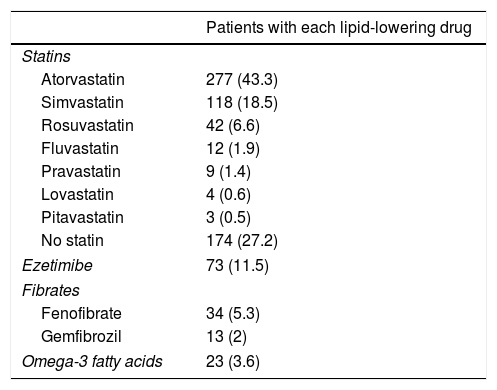
A total of 62.9% of the patients received therapeutic education and 54.7% performed physical exercise. Smokers were given smoking cessation advice, and 25% were able to abandon the habit. The mean daily number of drugs per patient was 8.6±4.1. Table 2 details the lipid-lowering medication used. High-intensity statin therapy was prescribed in 18.9% of the patients and moderate-intensity statins in 53.7%. Adequate statin prescription was noted in 56.9% of the patients. The dose of lipid-lowering drugs was adjusted during follow-up in 5.8% of the patients, and lipid-lowering treatments were added or replaced by others in 31.6%.
Lipid-lowering drugs used.
| Patients with each lipid-lowering drug | |
|---|---|
| Statins | |
| Atorvastatin | 277 (43.3) |
| Simvastatin | 118 (18.5) |
| Rosuvastatin | 42 (6.6) |
| Fluvastatin | 12 (1.9) |
| Pravastatin | 9 (1.4) |
| Lovastatin | 4 (0.6) |
| Pitavastatin | 3 (0.5) |
| No statin | 174 (27.2) |
| Ezetimibe | 73 (11.5) |
| Fibrates | |
| Fenofibrate | 34 (5.3) |
| Gemfibrozil | 13 (2) |
| Omega-3 fatty acids | 23 (3.6) |
Data reported as n (%).
Poor adherence to lipid-lowering therapy was detected in 58 patients (10.1%). There were no significant differences in poor adherence between moderate-intensity statins (8.4%) and high-intensity statin therapy (8.9%; p=0.1). A lack of adhesion was recorded in 10 patients in the case of ezetimibe (15.2%; p=0.016), four patients in the case of fenofibrate (13.3%; p=0.325), four patients in the case of gemfibrozil (36.4%; p=0.001) and four patients in the case of omega-3 fatty acids (22.2%; p=0.04).
Evolution of LDL-cholesterol controlThe initial LDL-cholesterol levels were off-target in most of the patients (86.7%). The percentage of patients with on-target LDL-cholesterol increased from 13.3% at baseline to 27.5% at the end of follow-up (p<0.001). The following mean changes in lipid concentration were recorded between the first and the last visit: total cholesterol −23.8±45mg/dl; LDL-cholesterol −19.5±38.7mg/dl; HDL-cholesterol 0.07±8.6mg/dl, and triglycerides −34±126.7mg/dl. No comorbidities were associated with a more significant decrease in LDL-cholesterol. LDL-cholesterol levels decreased by −17.6±40.6mg/dl in females and by −21.8±36.2mg/dl in males (p=0.27). The change in LDL-cholesterol concentration was correlated to initial systolic BP (rho=−0.167; p=0.004); diastolic BP (rho=−0.204; p<0.001); LDL-cholesterol (rho=−0.602; p<0.001); and HDL-cholesterol (rho=−0.111; p=0.048). A correlation was found between the variation in LDL-cholesterol and the change in body weight (rho=0.148; p=0.011), systolic BP (rho=0.137; p=0.018) and triglycerides (rho=0.251; p<0.001). No correlation was observed between the variation in LDL-cholesterol and patient age, diabetes duration, the initial BMI, waist circumference, initial HbA1c, the initial triglyceride concentration, albuminuria, change in HbA1c, change in diastolic BP, change in HDL-cholesterol, polypharmacy, the follow-up time at the clinic or the number of visits.
The decrease in LDL-cholesterol did not vary significantly between the patients receiving therapeutic education (−18.5±38 vs. −21.7±44.4mg/dl in those without; p=0.995), those performing physical exercise (−18.2±38.5 vs. −21.5±42.3mg/dl; p=0.918), those treated with GLP-1 receptor agonists (−20±36.2 vs. −19.3±39.3mg/dl; p=0.718), or those treated with insulin (−18.2±39.2 vs. −21.1±38.1mg/dl; p=0.601). The decrease in LDL-cholesterol was −14.5±30.4mg/dl without adjusting the lipid-lowering drugs, −9.6±31.8mg/dl on adjusting the dose, and −22.8±41.2mg/dl on replacing or adding lipid-lowering treatment (p=0.156). A decrease in LDL-cholesterol of −15.3±34.6mg/dl was recorded without adjusting antihypertensive medication, versus −27.1±27.3mg/dl on adjusting the dose, and −27.2±46.7mg/dl on replacing or adding antihypertensive treatment (p=0.032). Those patients that stopped smoking showed a decrease in LDL-cholesterol of −31±29.8mg/dl, versus −23.1±40.3mg/dl in those that did not (p=0.03).
The improvements in LDL-cholesterol concentration according to the type of lipid-lowering drug used were as follows: rosuvastatin −38±46.8mg/dl, atorvastatin −24.5±36.3mg/d, simvastatin −18.6±47.1mg/dl, pravastatin −13.6±38.4mg/dl, lovastatin −11.3±29.3mg/dl, fluvastatin −9.8±29.6mg/dl, no statins −0.4±21.4mg/dl (p<0.001), ezetimibe −31.2±54.3mg/dl, no ezetimibe −17.1±34.3mg/dl (p=0.075), fenofibrate −1.7±17.3mg/dl, gemfibrozil −3.3±24.2mg/dl, no fibrates −20.3±39.1mg/dl (p=0.140), omega-3 fatty acids −6.8±47.3mg/dl and no omega-3 fatty acids −19.6±38.3mg/dl (p=0.265). Patients presenting criteria of adequate lipid-lowering drug prescription showed a decrease in LDL-cholesterol of −25±40.8mg/dl, while those without such criteria showed a decrease of −9.1±32.1mg/dl (p<0.001). A decrease in LDL-cholesterol of −0.1±21.6mg/dl was observed without lipid-lowering drug adjustment, versus −13.4±36.2mg/dl on adjusting the dose and −42.1±41.9mg/dl on replacing or adding lipid-lowering treatment (p<0.001). The decrease in LDL-cholesterol was −2.4±21.7mg/dl in the case of therapeutic inertia with lipid-lowering drugs and −40.1±42.7mg/dl in the absence of such inertia (p<0.001). The patients that adhered to lipid-lowering drug treatment showed a decrease in LDL-cholesterol of −27.5±39.2mg/dl, while those failing to adhere showed an increase of 10.3±29.3mg/dl (p<0.001).
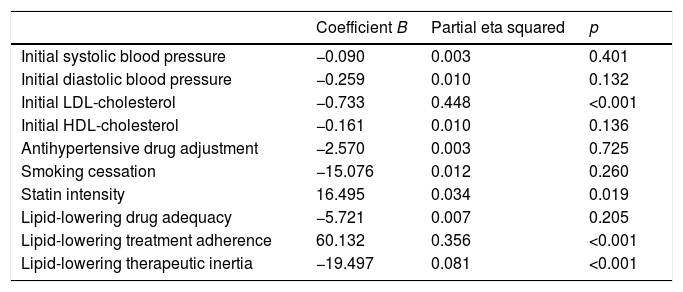
The change in the LDL-cholesterol concentration between the first and the last visit was assessed by linear regression analysis (Table 3). The variables that retained their significance in explaining the change in LDL-cholesterol in this model were the prescribed statin treatment intensity, therapeutic inertia with lipid-lowering drugs, adherence and the initial LDL-cholesterol concentration. These explained 44.8% of the variation in LDL-cholesterol in the course of follow-up (p<0.001).
Predictors of the change in LDL-cholesterol according to the multivariate analysis.
| Coefficient B | Partial eta squared | p | |
|---|---|---|---|
| Initial systolic blood pressure | −0.090 | 0.003 | 0.401 |
| Initial diastolic blood pressure | −0.259 | 0.010 | 0.132 |
| Initial LDL-cholesterol | −0.733 | 0.448 | <0.001 |
| Initial HDL-cholesterol | −0.161 | 0.010 | 0.136 |
| Antihypertensive drug adjustment | −2.570 | 0.003 | 0.725 |
| Smoking cessation | −15.076 | 0.012 | 0.260 |
| Statin intensity | 16.495 | 0.034 | 0.019 |
| Lipid-lowering drug adequacy | −5.721 | 0.007 | 0.205 |
| Lipid-lowering treatment adherence | 60.132 | 0.356 | <0.001 |
| Lipid-lowering therapeutic inertia | −19.497 | 0.081 | <0.001 |
R2=0.681 (R2 corrected=0.663).
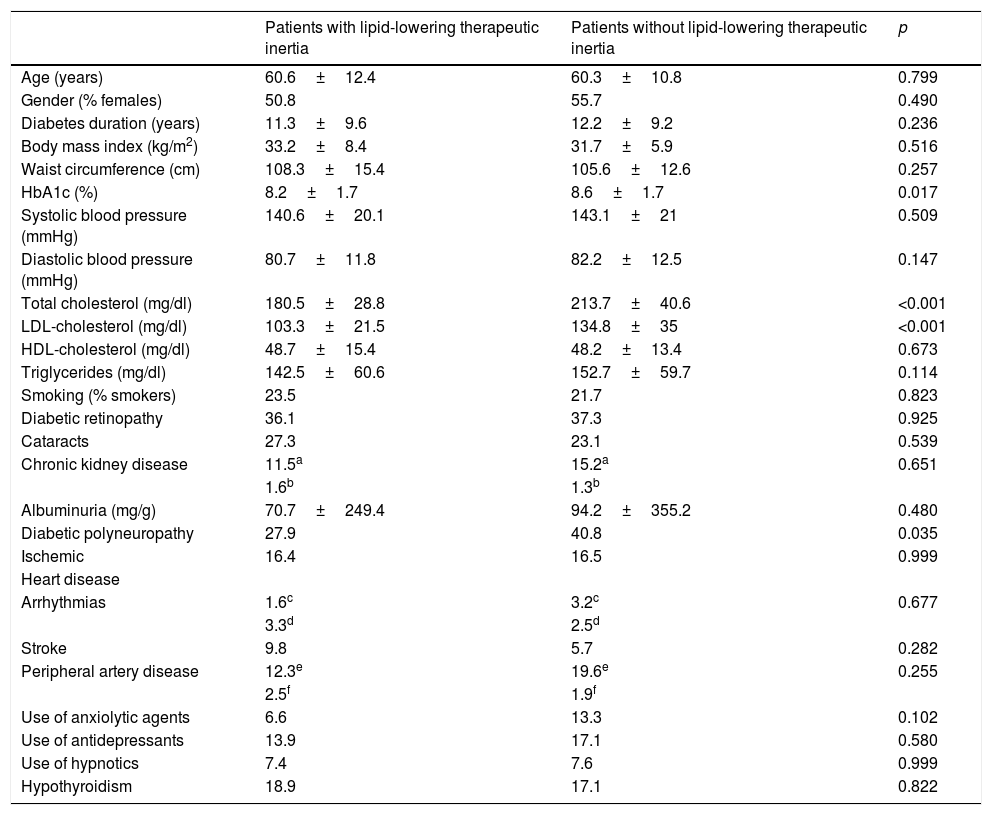
Therapeutic inertia with lipid-lowering drugs accounted for 8.1% of the variation in LDL-cholesterol during follow-up (p<0.001). Therapeutic inertia was recorded in 43.6% of the patients with an initial LDL-cholesterol concentration off-target, and was moreover associated with a lesser decrease in total cholesterol (−5.5±26.3mg/dl with inertia vs. −45.4±47.6mg/dl without inertia; p<0.001), LDL-cholesterol (−2.4±21.7mg/dl with inertia vs. −39.4±42.2mg/dl without inertia; p<0.001) and triglycerides (−11.7±55.2mg/dl with inertia vs. −22.6±59.1mg/dl without inertia; p=0.048), and a lesser final HbA1c concentration (7±1.2 with inertia vs. 7.5±1.5 without inertia; p=0.01). The patients with therapeutic inertia with regard to lipid-lowering drugs presented an initial total cholesterol level of 180.5±28.8mg/dl and an LDL-cholesterol concentration of 103.3±21.5mg/dl, while those without such inertia presented an initial total cholesterol level of 213.7±40.6mg/dl (p<0.001) and an LDL-cholesterol concentration of 134.8±35mg/dl (p<0.001). Therapeutic inertia was recorded in 34.7% of the patients with diabetic polyneuropathy and in 48.6% of the patients without polyneuropathy (p=0.035). The patients exposed to therapeutic inertia did not differ significantly from those without inertia in terms of age, gender, diabetes duration, the initial BMI, waist circumference, BP, HDL-cholesterol, triglycerides, smoking, albuminuria or the prevalence of comorbidities other than polyneuropathy (Table 4).
Differences in baseline characteristics according to lipid-lowering therapeutic inertia.
| Patients with lipid-lowering therapeutic inertia | Patients without lipid-lowering therapeutic inertia | p | |
|---|---|---|---|
| Age (years) | 60.6±12.4 | 60.3±10.8 | 0.799 |
| Gender (% females) | 50.8 | 55.7 | 0.490 |
| Diabetes duration (years) | 11.3±9.6 | 12.2±9.2 | 0.236 |
| Body mass index (kg/m2) | 33.2±8.4 | 31.7±5.9 | 0.516 |
| Waist circumference (cm) | 108.3±15.4 | 105.6±12.6 | 0.257 |
| HbA1c (%) | 8.2±1.7 | 8.6±1.7 | 0.017 |
| Systolic blood pressure (mmHg) | 140.6±20.1 | 143.1±21 | 0.509 |
| Diastolic blood pressure (mmHg) | 80.7±11.8 | 82.2±12.5 | 0.147 |
| Total cholesterol (mg/dl) | 180.5±28.8 | 213.7±40.6 | <0.001 |
| LDL-cholesterol (mg/dl) | 103.3±21.5 | 134.8±35 | <0.001 |
| HDL-cholesterol (mg/dl) | 48.7±15.4 | 48.2±13.4 | 0.673 |
| Triglycerides (mg/dl) | 142.5±60.6 | 152.7±59.7 | 0.114 |
| Smoking (% smokers) | 23.5 | 21.7 | 0.823 |
| Diabetic retinopathy | 36.1 | 37.3 | 0.925 |
| Cataracts | 27.3 | 23.1 | 0.539 |
| Chronic kidney disease | 11.5a | 15.2a | 0.651 |
| 1.6b | 1.3b | ||
| Albuminuria (mg/g) | 70.7±249.4 | 94.2±355.2 | 0.480 |
| Diabetic polyneuropathy | 27.9 | 40.8 | 0.035 |
| Ischemic | 16.4 | 16.5 | 0.999 |
| Heart disease | |||
| Arrhythmias | 1.6c | 3.2c | 0.677 |
| 3.3d | 2.5d | ||
| Stroke | 9.8 | 5.7 | 0.282 |
| Peripheral artery disease | 12.3e | 19.6e | 0.255 |
| 2.5f | 1.9f | ||
| Use of anxiolytic agents | 6.6 | 13.3 | 0.102 |
| Use of antidepressants | 13.9 | 17.1 | 0.580 |
| Use of hypnotics | 7.4 | 7.6 | 0.999 |
| Hypothyroidism | 18.9 | 17.1 | 0.822 |
Continuous variables are expressed as the mean±standard deviation (SD), and categorical variables as a percentage.
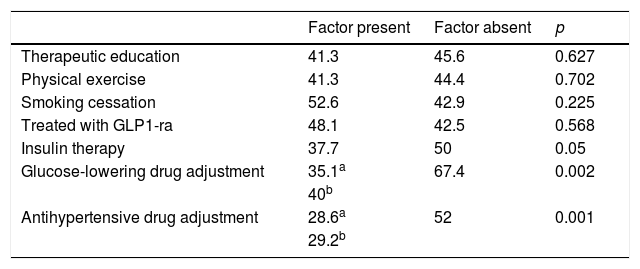
Table 5 reports therapeutic inertia with regard to lipid-lowering drugs according to the concomitant treatments received. Patients exposed to inertia received an average of 8.5±4.1 drugs a day versus 9.2±3.8 in the case of those who were not (p=0.186). The patients exposed to inertia required an average of 3.6±2.3 visits versus 4.4±3.1 in the case of those without inertia (p=0.029). The number of cases with therapeutic inertia and the percentage the number represented for each lipid-lowering drug were: atorvastatin 38 cases (29.2%), simvastatin 22 cases (41.5%), rosuvastatin 2 cases (7.1%), fluvastatin 2 cases (40%), pravastatin 1 case (25%), lovastatin 3 cases (100%), fenofibrate 3 cases (30%), gemfibrozil 3 cases (60%) and omega-3 fatty acids 2 cases (28.6%). Inadequate initial statin prescription resulted in a 2.6-fold increase in the probability of therapeutic inertia at successive visits (72.7% inertia in the case of inadequate statins versus 27.6% inertia in the case of adequate statin prescription; p<0.001). In turn, inertia proved 8 times less frequent among those treated with ezetimibe versus those that did not receive this associated lipid-lowering drug (6.4% versus 51.1%; p<0.001). A lack of adherence to lipid-lowering therapy was associated with lesser therapeutic inertia (46.7% among adherent patients versus 4.2% in non-adherent patients; p<0.001).
Percentage of patients with lipid-lowering therapeutic inertia according to concomitant treatments.
| Factor present | Factor absent | p | |
|---|---|---|---|
| Therapeutic education | 41.3 | 45.6 | 0.627 |
| Physical exercise | 41.3 | 44.4 | 0.702 |
| Smoking cessation | 52.6 | 42.9 | 0.225 |
| Treated with GLP1-ra | 48.1 | 42.5 | 0.568 |
| Insulin therapy | 37.7 | 50 | 0.05 |
| Glucose-lowering drug adjustment | 35.1a | 67.4 | 0.002 |
| 40b | |||
| Antihypertensive drug adjustment | 28.6a | 52 | 0.001 |
| 29.2b |
GLP1-ra: GLP-1 receptor agonists.
On examining the determinants of the lack of therapeutic inertia regarding lipid-lowering drugs based on binary logistic regression analysis, we found the independent predictors to be initial LDL-cholesterol (odds ratio [OR]=1.053; p<0.001), polyneuropathy (OR=2.976; p=0.014), antihypertensive drug adjustment (OR=4.132; p=0.002), lipid-lowering drug adequacy (OR=8.771; p<0.001), lack of adherence to lipid-lowering therapy (OR=18.942; p=0.015) and the use of ezetimibe (OR=35.714; p=0.001).
DiscussionIn the present study, 27.5% of the diabetic patients treated at an Endocrinology clinic achieved LDL-cholesterol concentrations on-target, and the improvement in LDL-cholesterol over follow-up (-19.5±38.7mg/dl on average) was primarily conditioned by the initial LDL-cholesterol level, the prescribed statin intensity, patient adherence to lipid-lowering therapy, and therapeutic inertia.
A limitation of this study was the relatively short patient follow-up involved (11.1±11.2 months). A contribution of the study was the identification of those factors with the strongest influence upon therapeutic inertia, i.e., initial LDL-cholesterol concentration, diabetic polyneuropathy, antihypertensive treatment adjustment, lipid-lowering drug suitability, the use of ezetimibe, and therapeutic adherence.
The CARDS study randomised 1428 diabetic patients to atorvastatin (10mg) and another 1410 patients to placebo, with the observation of a 38% decrease in cardiovascular events among the patients with initial LDL-cholesterol≥119.8mg/dl versus 37% among those with initial LDL-cholesterol<119.8mg/dl (p=0.96).15 Similarly, the Heart Protection Study involving simvastatin (40mg) documented a 27% decrease in major vascular events among diabetic patients with initial LDL-cholesterol<116mg/dl, this improvement being comparable to that seen in patients with LDL-cholesterol>116mg/dl (p=0.5).16 The persistence of the cardiovascular benefit of statins independently of the initial LDL-cholesterol concentration adds interest to our observation that those patients with therapeutic inertia with regard to lipid-lowering drugs presented initial LDL-cholesterol 103.3±21.5mg/dl, while those without such inertia presented initial LDL-cholesterol 134.8±35mg/dl (p<0.001). This finding indicates that in real-life clinical practice a significant proportion of patients may not be benefiting from the cardiovascular advantages shown by statins in clinical trials. It points to a need for more in-depth analysis of the predictors of therapeutic inertia, with a view to adopting measures designed to reduce its incidence.
One of these predictors could be the presence of diabetic polyneuropathy. Lipotoxicity has been implicated as a major factor in the etiopathogenesis of polyneuropathy in patients with DM2.17 In our study, the absence of therapeutic inertia regarding lipid-lowering drugs was found to be almost three times more likely among patients with diabetic polyneuropathy. This is important in the light of the findings of the SEARCH study, which reported a 22% prevalence of polyneuropathy in young individuals with DM2, and proposed the treatment of dyslipidemia as a priority for preventing or delaying the consequences of polyneuropathy in these patients.18 Exploring the causes that have contributed to lessened therapeutic inertia with regard to lipid-lowering medication among patients with polyneuropathy represents an interesting field for future prospective studies.
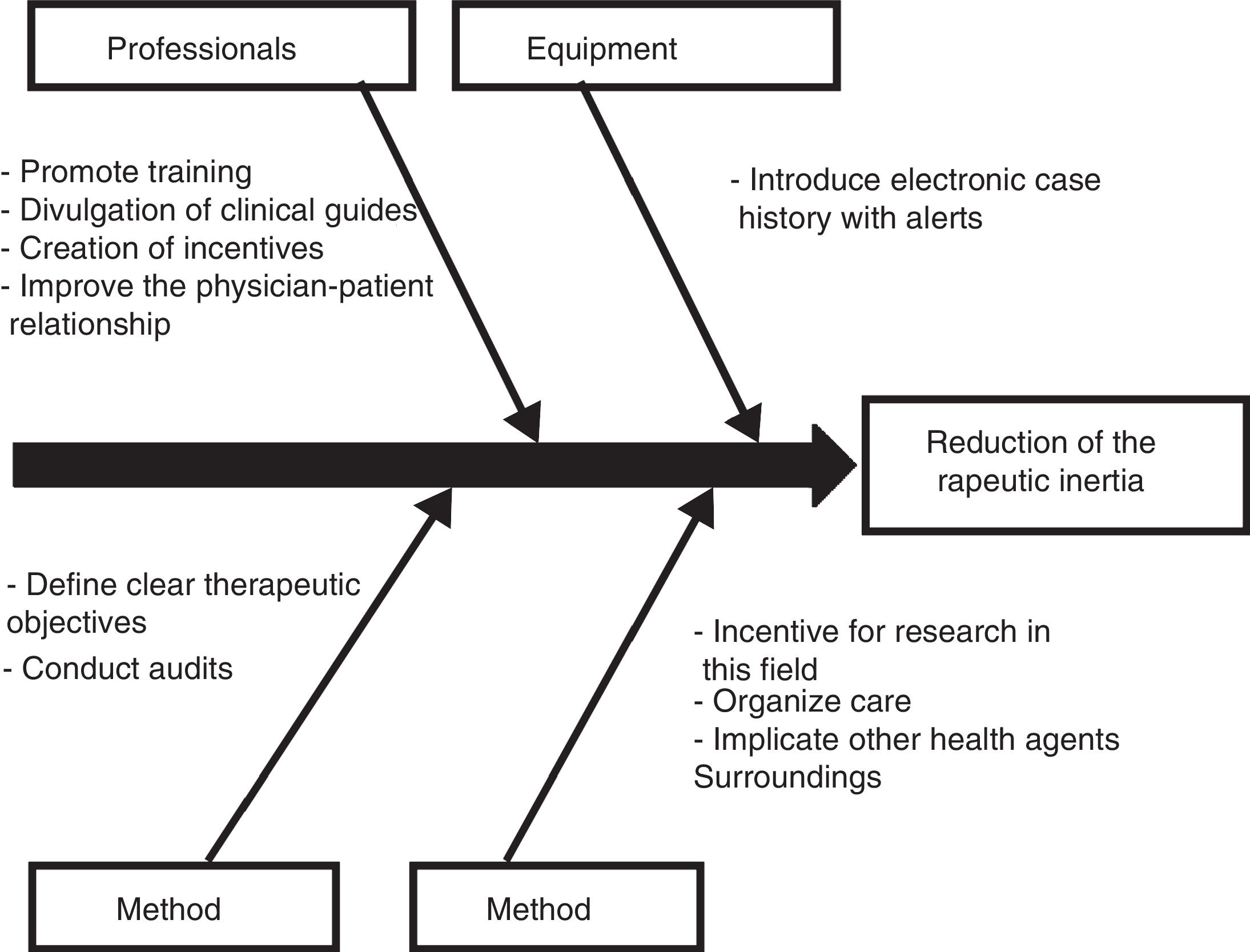
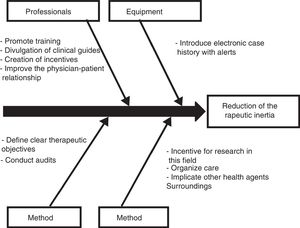
Those diabetic patients subjected to antihypertensive drug adjustment were characterized by a 4.1-fold greater likelihood of not being exposed to inertia with their lipid-lowering treatments. This link between lesser therapeutic inertia with antihypertensive drugs and lesser inertia with lipid-lowering drugs is consistent with the care strategy used in the STENO-2 study. This trial involved an intensified intervention targeted to multiple cardiovascular risk factors in patients with DM2 and 7.8 years of oligoalbuminuria, and who were followed-up on for 21.2 years. The patients subjected to intensified therapy gained an average of 7.9 life years compared to the patients receiving conventional treatment.19 In the same way that a joint approach to the different cardiovascular risk factors is recommended in diabetic patients, the measures designed to control therapeutic inertia could also be multifactorial and integrate actions intended to secure parallel reductions in inertia regarding lipid-lowering, antihypertensive and glucose-lowering drugs. On this subject, the Spanish Society of Arteriosclerosis has proposed 10 measures for reducing therapeutic inertia,20 represented in Fig. 1 as a cause–effect diagram.
The organization of care plays a key role in securing therapeutic objectives, as demonstrated by the observation that the likelihood of not becoming exposed to therapeutic inertia is 8.7 times greater in the case of adequate satin prescription and 35.7 times greater among those patients treated with ezetimibe. Masana et al. proposed cholesterol-lowering treatment planning based on a table to determine the statin or combination therapy (statin plus ezetimibe) best suited to each patient, with the published potency of the different lipid-lowering drugs, the starting LDL-cholesterol level of the patient, and the established therapeutic objective or target being taken into consideration.21 The SHARP study reported a 22% decrease in major atherosclerotic events in patients with diabetes and chronic kidney disease who received simvastatin (20mg)/ezetimibe (10mg) instead of placebo.22 Another stimulus for combining lipid-lowering drugs is found in the results of the IMPROVE-IT trial, which reported a 14.4% decrease in its composite primary endpoint (cardiovascular mortality, nonfatal myocardial infarction, unstable angina with reentry, coronary revascularization or nonfatal stroke) in diabetic patients hospitalized due to coronary ischemic syndrome and treated with simvastatin (40mg)/ezetimibe (10mg) instead of simvastatin alone (40mg).23 The cardiovascular benefit of this combination in the aforementioned study was significantly greater in the patients with diabetes than in the other participants (p=0.023).
Further studies are needed to delimit therapeutic inertia-generating mechanisms in the management of dyslipidemia in patients with DM2, and to understand their relationship with other complex phenomena such as therapeutic adherence. Grant et al. conducted a study in 2065 diabetic patients followed-up on between 1992 and 2001 and found the patient quartile with the greatest adherence to the prescribed glucose-lowering drugs to be 53% more likely to receive treatment intensification in the event of high HbA1c than the patient quartile with the lowest adherence (p=0.01).24 In our study, involving a cohort of 639 diabetic patients followed-up on between 2011 and 2016, a lack of adherence to lipid-lowering medication was found to be associated with lesser therapeutic inertia. These differences, which could be explained by the different methods used for assessing adherence (dispensed and prescribed medication records in the study of Grant et al. versus the use of the Haynes–Sackett question in our study) and by the different drugs analyzed (glucose-lowering agents in one case, lipid-lowering drugs in another), invite us to reflect upon the physician response when detecting a lack of patient adherence to therapy.
ConclusionInertia in the lipid-lowering treatment of a diabetic patient is less common in the presence of higher initial LDL-cholesterol values, in cases complicated by diabetic polyneuropathy, when antihypertensive agents are adjusted or replaced, and when a lack of adherence to therapy is detected. The adequate initial prescription of statins and their combination with ezetimibe reduce the likelihood of therapeutic inertia.
Authorship/collaborationsEGD contributed to the study design, followed-up on patients, analyzed the data, and drafted the manuscript. DRM, OMMP and JLCM followed-up on patients and reviewed the manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest in relation to this study.
Please cite this article as: García Díaz E, Ramírez Medina D, Morera Porras ÓM, Cabrera Mateos JL. Determinantes de la inercia en el tratamiento hipolipidemiante de pacientes con diabetes mellitus tipo 2. Endocrinol Diabetes Nutr. 2019;66:223–231.