To assess the degree of compliance with the European ESC/EAS 2016 and 2019 dyslipidaemia guidelines in patients with type 2 diabetes mellitus (T2DM).
MethodsMulticentre retrospective cross-sectional study, conducted in 380 adults with T2DM and dyslipidaemia in 7 Spanish health areas. Inclusion criteria: minimum follow-up of one year in Endocrinology Units, at least one visit in 2020 and a lipid profile measurement in the last 3 months. Exclusion criteria: familial hypercholesterolaemia, recent hospitalisation, active oncological pathology and dialysis.
ResultsAccording to the 2016 and 2019 guidelines the majority of patients were classified as being at very high cardiovascular risk (86.8% vs. 72.1%, respectively). LDL-c compliance was adequate in 62.1% of patients according to the 2016 guidelines and 39.7% according to the 2019 guidelines (p<0.001). Clinical conditions such as history of cardiovascular disease and therapy-related aspects (use of statins, especially high-potency statins, combination therapies and good adherence) were significantly associated with greater achievement of lipid targets.
ConclusionThere is a discrepancy between dyslipidaemia guideline recommendations and the reality of lipid control in patients with T2DM, despite most of these patients being at very high cardiovascular risk. Strategies to optimise lipid-lowering treatments need to be implemented.
El control lipídico es clave en el manejo del riesgo cardiovascular en personas con diabetes mellitus tipo 2 (DMT2). El objetivo de este trabajo es evaluar el grado de acuerdo con las Guías Europeas ESC/EAS de 2016 y 2019, en cuanto a objetivos lipídicos.
MétodosEstudio transversal multicéntrico retrospectivo. Criterios de inclusión: DMT2, con seguimiento en endocrinología de al menos un año, mínimo una visita en 2020 y una medición de perfil lipídico en los últimos 3 meses. Criterios de exclusión: hipercolesterolemia familiar, hospitalización reciente, enfermedad tumoral activa y diálisis.
ResultadosSe incluyeron 380 pacientes. De acuerdo con las guías ESC/EAS de 2016 y 2019 la mayoría de los pacientes fueron clasificados como de muy alto riesgo cardiovascular (86,8 y 72,1%, respectivamente). Cumplían el objetivo de LDL-c un 62,1% (2016) y un 39,7% (2019), p<0,001. Los valores de LDL-c fueron inferiores en pacientes con muy alto riesgo cardiovascular, aunque el porcentaje de pacientes en objetivos LDL-c, fue inferior que en otros grupos. Se relacionaron de forma significativa con consecución de los objetivos lipídicos la historia de enfermedad coronaria o insuficiencia cardíaca, uso de estatinas de alta potencia, terapia combinada y buena adherencia.
ConclusionesExiste una discrepancia entre las recomendaciones de las guías clínicas sobre el control lipídico en pacientes con DMT2 y la realidad del manejo de estos pacientes, a pesar de que la mayoría son de muy alto riesgo cardiovascular. Es necesario implementar estrategias para optimizar el tratamiento hipolipemiante.
Type 2 diabetes mellitus (T2DM) treatment must be approached comprehensively with the primary goal of reducing the morbidity and mortality associated with the disease. Atherosclerotic cardiovascular disease (ASCVD) is the main cause of morbidity and mortality in people with T2DM1 and optimal control of cardiovascular risk (CVR) factors is crucial in improving patients’ prognosis.
Regarding lipid control, there is strong, quality evidence to support the efficacy and safety of lipid lowering therapy (LLT) in people with T2DM for ASCVD outcomes, both in patients with and without coronary artery disease.2–4 This substantial evidence has prompted scientific societies to propose optimised LDL-c levels according to risk-based goals and to recommend statins as the first-line pharmacotherapy to lower LDL-c. In 2016, the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) jointly published their Guidelines for the management of dyslipidaemias.5 Subsequently, in 2019, they updated their recommendations to adapt them to the new available evidence,6 with risk-based targets for LDL-c that are more demanding and may be difficult to achieve in actual clinical practice.
Several studies have revealed a gap between the ESC/EAS recommendations and healthcare provider adherence to guidelines in people with T2DM. In some studies, the 2016 LDL-c goal attainment according to risk-based levels ranged between 36% and 69%, with the best results in secondary prevention.7,8 There are even studies where fewer than 20% achieved the LDL targets.9 The REPAR study in Spain, conducted in patients with stable coronary artery disease, reported a 43.2% prevalence of patients with T2DM and LDL-c levels below 70mg/dl.10
Based on the ESC/EAS 2019 recommendations, the researchers of the DA VINCI study11 provided contemporary data on LDL-c goal achievement across different risk levels, including 39% of T2DM patients. In the DA VINCI study, the degree of goal attainment was 33% for the overall population and 17% for very high-risk patients, with differences across LLTs in the subgroups.
Despite this evidence, very few real-world studies emphasise the existing gap between the recommendations contained in the guidelines and LDL-c achieved levels in patients with T2DM in real clinical practice. This study was designed to assess data regarding LDL-c goal achievement in T2DM patients attending Endocrinology Units in the Galician Healthcare System, Spain, based on 2016 and 2019 ESC/EAS endorsed recommendations.
Patients and methodsStudy designThis is a multicentre, cross-sectional retrospective study carried out in the Endocrinology Units of the seven healthcare areas of our region (Galicia, Spain), which cover a population of 2,700,629 inhabitants.
The sample size calculation was performed according to the results of the REPAR study.10 Establishing an expected proportion of patients with LDL-c on target of 43%, with a confidence interval of 95%, a precision of 5% and a power of 80%, a total of 377 patients was obtained.
The data from the medical records of patients with T2DM from the participating hospitals who were followed up in the Endocrinology clinic during 2020 were collected. The inclusion criteria were patients with T2DM diagnosed according to the usual criteria,1 over 18 years of age, with at least one visit in 2020, who had been followed up in Endocrinology in the previous year and had had a lipid profile in the last 3 months. We evaluated the cardiovascular risk category according to the 2016 and 2019 ESC/EAS guidelines5,15 and based on that we assessed LDL-c target achievement. Patients with familial hypercholesterolemia, those who had suffered an acute process with hospitalisation in the previous 3 months, patients with an active oncological pathology and patients undergoing dialysis treatment were excluded.
The Ethics and Clinical Research Committee of the University Hospital Complex (Santiago de Compostela, Galicia) approved the study protocol, and patient anonymity was preserved.
Aims and outcomesOur aim was to evaluate the degree of compliance with the LDL-c control recommendations according to the “Guide for the management of Dyslipidaemias of the European Society of Cardiology and the European Society of Atherosclerosis of 2016 and 2019” in a sample of patients with T2DM and dyslipidaemia from Galicia (Spain). The primary outcome was the proportion of patients achieving the LDL-c target recommended by the 2016 and 2019 ESC/EAS guidelines.
Individuals defined as primary prevention at LDL-c measurement were categorised according to the ESC/EAS risk categories (low, moderate, high, very high). All patients defined as secondary prevention were categorised as very high risk.
Secondary objectives included: categorising CVR, evaluating the mean values of each of the lipids and the percentage of patients who meet the goals of total cholesterol, non-HDL cholesterol, HDL cholesterol and triglycerides, as recommended in the 2016 and 2019 dyslipidaemia guidelines. The LLT used is described and the frequency of statin intolerance in our sample is analysed. Furthermore, the achievement of LDL-c objectives was studied in different subgroups of patients, depending on their different CVR factors and the type of LLT prescribed.
Data collectionStudy parameters included baseline demographic variables and DM characteristics (age, sex, comorbidities, type, and duration of DM). Data was collected on personal history of classical and non-classical macro- and microvascular complications including heart failure (HF) or non-alcoholic steatohepatitis (NASH) as well as the presence of other CVR factors (obesity, high blood pressure or smoking). Anthropometric measurements and analytical variables observed during the study visit were also collected: weight, height, body mass index (BMI), waist circumference, HbA1c, lipid profile (total cholesterol, LDL cholesterol, non-HDL cholesterol, HDL cholesterol and triglycerides), glomerular filtration rate, albuminuria. The background diabetes therapy and the use of lipid-lowering drugs (type, dosage, and combinations) were described. The presence of statin intolerance was assessed according to the definition of the National Lipid Association guidelines (inability to tolerate at least two statins, one at the lowest possible dose, due to the appearance of symptoms or laboratory abnormalities, which are temporarily related and reversible upon discontinuation of treatment).12 The term “partial intolerance” was included for those patients who did not tolerate statins with the necessary intensity according to their CVR category or with any condition that would contraindicate them (e.g., chronic kidney disease). The degree of adherence to LLT was measured as the rate of possession of medication in the last 12 months (%); this is the ratio between the number of total units dispensed and the number of total theoretical units prescribed in that period×100.
The CVR of patients was calculated, following the recommendations of the ESC/EAS guidelines of 2016 and 2019. In the 2019 Guide for the management of Dyslipidaemias of the ESC/EAS, patients with DM and three major risk factors are categorised as very high-risk patients. However, the guide does not specify what these factors are. Therefore, the major cardiovascular risk factors listed in the 2016 Guide for the management of Dyslipidaemias of the ESC/EAS were selected: smoking, obesity, and hypertension.
The 2016 guidelines categorised patients as very high-risk if they had DM and a major cardiovascular risk factor such as smoking, marked hypercholesterolaemia or marked hypertension. However, the 2016 guidelines do not specify these items. Thus, the standards of the Spanish Society of Atherosclerosis (SEA) were applied and marked hypercholesterolaemia was considered a total cholesterol ≥250mg/dl and marked HTN as grade 2 HTN or higher (≥160/100mmHg).13
Statistical analysisQuantitative variables are presented as mean and standard deviation (SD) and categorical variables are expressed as percentages. The following analyses were performed: the percentage of patients meeting the targets for total cholesterol, non-HDL-c, HDL-c and triglycerides, and the descriptive values for each lipid and for the characteristic of the patients (e.g., age) were calculated. The degree of compliance and the mean levels of the different lipids were compared according to different population characteristics with the Chi-square test, Student's t-test and ANOVA, respectively. The mean values of each lipid according to the CVR category were compared using ANOVA. The degree of compliance with the LDL-c targets in different subgroups of patients was analysed according to the CVR category or the lipid-lowering treatment, using the Chi-square test. The factors associated with lipid control were studied calculating the odds ratio (OR) with their 95% confidence interval (CI95%). A multivariate analysis was performed using logistic regression. A p-value of less than 0.05 was considered statistically significant. Statistical analysis was performed with SPSS 19.0 (SPSS Inc., Chicago, IL, USA).
ResultsGeneral patient characteristicsAccording to the sample calculation, 380 patients with type 2 diabetes and dyslipidaemia under follow-up by Endocrinology units were included. Their demographic characteristics, comorbidities and cardiovascular risk factors are described in Table 1.
Demographic and general characteristics of the patients.
| (n=380) | |
|---|---|
| Age (years) | 65.9 (10.0) |
| Sex (n, male) | 203 (53.4%) |
| Follow-up time in endocrinology (years) | 5.9 (3.9) |
| Weight (kg) | 86.6 (13.4) |
| Body mass index (kg/m2) | 30.6 (4.2) |
| Waist circumference (cm) | 110.4 (13.4) |
| Diabetes duration | |
| <5 years | 35 (9.2%) |
| 5–10 years | 82 (21.6%) |
| >10 years | 263 (69.2%) |
| Diabetes treatment | |
| Metformin | 309 (81.3%) |
| SGLT2i | 212 (55.8%) |
| GLP1-RA | 153 (40.3%) |
| DPP4i | 107 (28.2%) |
| Sulphonylureas | 28 (7.4%) |
| Repaglinide | 13 (3.4%) |
| Pioglitazone | 8 (2.1%) |
| Insulin | 237 (62.4%) |
| Cardiovascular risk factors | |
| Hypertension | 299 (78.7%) |
| Obesity | 239 (62.9%) |
| Grade 1 | 140 (36.8%) |
| Grade 2 | 58 (15.3%) |
| Grade 3 or superior | 40 (10.5%) |
| Smoking habit | |
| Active smoker | 59 (15.5%) |
| Former smoker | 98 (25.8%) |
| Macrovascular disease | |
| Ischaemic cardiopathy | 76 (20%) |
| Cerebrovascular disease | 26 (6.8%) |
| Peripheral arteriopathy | 34 (8.9%) |
| Microvascular disease | |
| Nephropathy | 113 (29.7%) |
| Retinopathy | 109 (28.7%) |
| Neuropathy | 46 (12.1%) |
| Other comorbidities | |
| Heart failure | 38 (10%) |
| NASH | 55 (14.5%) |
| COPD | 25 (6.6%) |
Data are expressed as n (%) values or mean (SD).
SGLT2i: sodium-glucose cotransporter-2 inhibitors; GLP1-RA: glucagon-like peptide 1 receptor agonists; DPP4i: dipeptidyl peptidase 4 inhibitors; NASH: non-alcoholic steatohepatitis; COPD: chronic obstructive pulmonary disease.
Most patients had long-standing diabetes with an average duration of 15.8 (9.5) years, with 69.2% presenting more than 10 years of evolution of the disease. Glycaemic control among the population, assessed as the mean HbA1c, was 7.1% (1.0%) (54.1mmol/mol). Table 1 shows the frequency of the different anti-diabetic treatments in our patients, highlighting the high rate of insulin use (in two thirds of the cases).
Nephropathy was the most frequent microvascular complication, with 69 patients (18.2%) presenting chronic kidney disease and 44 (11.0%) albuminuria without glomerular filtration rate impairment. The mean glomerular filtration rate in the sample was 78.7 (29.0) ml/min and the albumin-to-creatinine ratio was 105.9 (26.0)mg/g. Retinopathy affected 109 patients (28.7%) (20.3% non-proliferative and 8.4% proliferative), whereas the diagnosis of neuropathy was only established in 46 cases (12.1%).
Lipid profile and treatment of dyslipidaemiaThe status of dyslipidaemia at the time of the study and the treatments used for its management are listed in Table 2.
Status of dyslipidaemia and treatment.
| (n=380) | |
|---|---|
| Lipid profile | |
| Total cholesterol (mg/dl) | 144.1 (32.1) |
| HDL cholesterol (mg/dl) | 44.5 (12.7) |
| Non-HDL cholesterol (mg/dl) | 98.0 (28.8) |
| LDL cholesterol (mg/dl) | 68.7 (26.0) |
| Triglycerides (mg/dl) | 162.0 (112.2) |
| Statin therapy | |
| Use of statins | 355 (93.4%) |
| Subtype of statin | |
| Atorvastatin | 204 (53.7%) |
| Rosuvastatin | 84 (22.1%) |
| Simvastatin | 45 (11.8%) |
| Pravastatin | 16 (4.2%) |
| Pitavastatin | 5 (1.3%) |
| Fluvastatin | 2 (0.5%) |
| Statin potency | |
| Low | 30 (7.9%) |
| Medium | 179 (47.1%) |
| High | 146 (38.4%) |
| Statin dose (mg/day) | |
| Atorvastatin | 33.5 (20.5) |
| Rosuvastatin | 17.3 (7.5) |
| Simvastatin | 24.4 (11.1) |
| Pravastatin | 32.5 (10) |
| Pitavastatin | 2.6 (1.3) |
| Fluvastatin | 80 (0) |
| Use of other lipid-lowering treatments | |
| Ezetimibe 10mg | 115 (30.3%) |
| Fenofibrate | 55 (14.5%) |
| Fenofibrate dose (mg/day) | 162.9 (28.4) |
| Omega-3 | 8 (2.1%) |
| Omega-3 dose (mg/day) | 2714.2 (1253.5) |
| PCSK9 inhibitors (only alirocumab) | 2 (0.5%) |
| Alirocumab dose (mg/14 days) | 112.5 (53.0) |
| Monotherapy (statins) | 215 (56.6%) |
| Combination treatments | |
| Statin plus ezetimibe | 94 (24.7%) |
| Statin plus fenofibrate | 27 (7.1%) |
| Statin plus ezetimibe plus fenofibrate | 13 (3.4%) |
| Other combinations | 18 (4.8%) |
Data are expressed as n (%) values or mean (SD).
Aspects of adherence to statin treatment and the frequency of intolerance to statins were also collected. Adherence to statins, measured as the percentage of prescriptions collected in the last 12 months was 94.4% (13.4%). Only 27 patients (7.1%) had poor adherence to treatment, defined as less than 80% prescription collection. Fourteen patients (3.6%) met the criteria for statin intolerance in their clinical history, with total intolerance in 9 cases (2.4%) and partial intolerance in the remaining 5 (1.3%).
Attainment of recommended LDL cholesterol goals according to the 2016 European Society of Cardiology/European Atherosclerosis Society GuidelinesAccording to the 2016 guidelines, most patients were classified as being at very high cardiovascular risk (330 patients, 86.8%), while only 45 patients (11.8%) and 5 patients (1.3%) were considered to be at high or moderate risk, respectively. The levels of the different lipid parameters as a function of the patient's cardiovascular risk are described in Table 3.
Comparison of lipid parameters in patients at very high, high and moderate cardiovascular risk according to the 2016 and 2019 guidelines.
| Lipid parameters | 2016 ESC/EAS guidelines | 2019 ESC/EAS guidelines | ||
|---|---|---|---|---|
| Total cholesterol (mg/dl) | VHR: 142.0 (32.3) | VHR: 139.2 (31.5) | ||
| HR: 156.6 (28.4) | p=0.005 | HR: 156.9 (30.9) | p<0.001 | |
| MR: 165.6 (13.5) | MR: 152.2 (21.8) | |||
| Non-HDL cholesterol (mg/dl) | VHR: 96.5 (28.2) | VHR: 95.0 (29.0) | ||
| HR: 109.2 (31.9) | p=0.019 | HR: 106.2 (27.3) | p=0.004 | |
| MR: 113.0 (12.2) | MR: 108.5 (14.9) | |||
| LDL cholesterol (mg/dl) | VHR: 67.3 (36.7) | VHR: 64.4 (25.2) | ||
| HR: 76.9 (17.8) | p=0.019 | HR: 79.2 (25.6) | p<0.001 | |
| MR: 88.0 (14.6) | MR: 81.7 (17.2) | |||
| HDL cholesterol (mg/dl) | VHR: 43.8 (12.7) | VHR: 42.8 (12.4) | ||
| HR: 48.6 (11.3) | p=0.020 | HR: 48.8 (12.6) | p<0.001 | |
| MR: 52.6 (11.2) | MR: 47.1 (10.4) | |||
| Triglycerides (mg/dl) | VHR: 161.6 (99.8) | VHR: 168.2 (121.8) | ||
| HR: 169.5 (183.1) | p=0.696 | HR: 148.1 (82.9) | p=0.182 | |
| MR: 125.8 (43.0) | MR: 118.5 (32.8) | |||
Data are expressed as mean (SD).
VHR: very high risk; HR: high risk; MR: moderate risk.
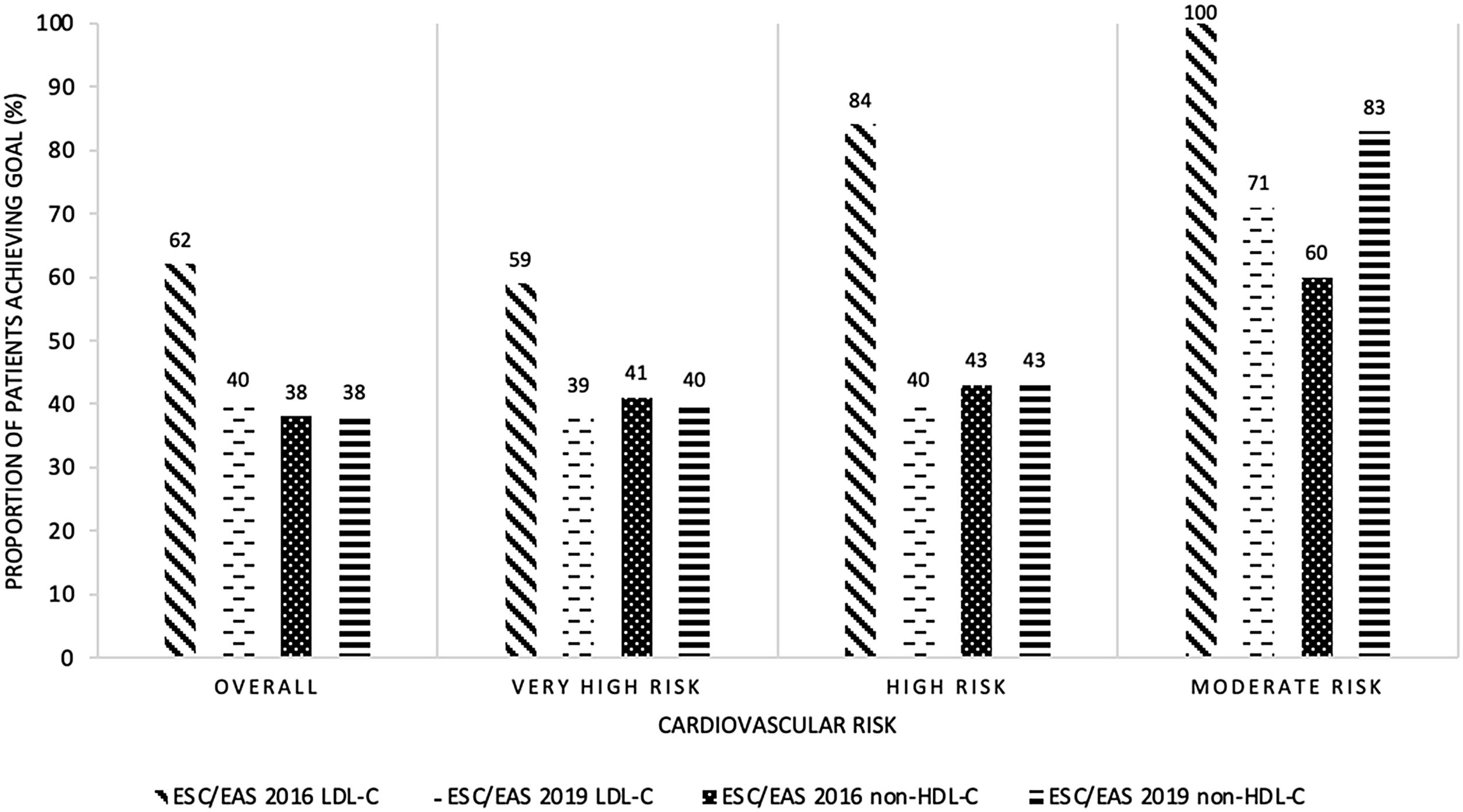
In 236 patients, the LDL control target set by the 2016 guidelines was considered to be adequately met (62.1%), with 40% attaining the non-HDL cholesterol goal. Fig. 1 represents the degree of adherence to the guidelines according to the patients’ cardiovascular risk classification, showing significant differences in LDL control between groups (p=0.001), but no differences in non-HDL cholesterol control.
Proportion of patients achieving the LDL-C goal or the non-HDL-C goal according to ESC/EAS 2016 and 2019 guidelines and cardiovascular risk. LDL-C: low-density lipoprotein cholesterol; non-HDL-C: high-density lipoprotein cholesterol; ESC: European Society of Cardiology; EAS: European Atherosclerosis Society.
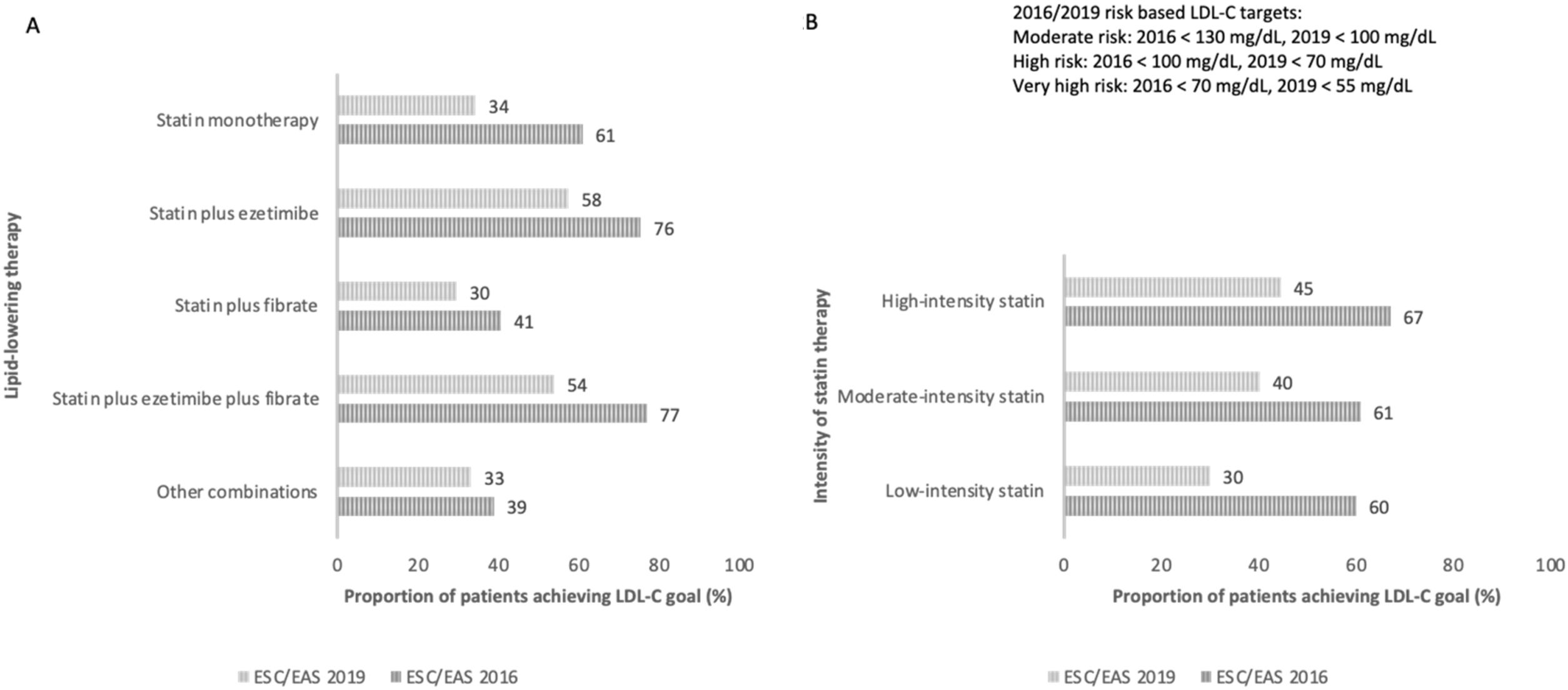
Fig. 2 shows the proportion of patients who achieved LDL control targets according to the type of lipid-lowering treatment (monotherapy versus combination therapies or statin potency used).
Proportion of patients achieving LDL-C goal according to ESC/EAS 2016 and 2019 guidelines and type of lipid-lowering therapy. (A) Proportion of patients achieving the LDL-C goal according to ESC/EAS 2016 and 2019 guidelines and lipid-lowering therapy. (B) Proportion of patients achieving the LDL-C goal according to ESC/EAS 2016 and 2019 guidelines and intensity of the statin therapy. LDL-C: low-density lipoprotein cholesterol; ESC: European Society of Cardiology; EAS: European Atherosclerosis Society.
Of the 144 patients who did not meet control targets, lipid lowering medication was modified in 45.5% of the cases.
Attainment of recommended LDL cholesterol goals according to the 2019 European Society of Cardiology/European Atherosclerosis Society GuidelinesBased on the 2019 guidelines, 274 patients were classified as being at very high cardiovascular risk (72.1%), 99 at high risk (26.1%) and 7 at moderate risk (1.8%). The levels of the different lipid parameters as a function of the patients’ cardiovascular risk are described in Table 3.
Compliance with the LDL target proposed by these recommendations was considered adequate in 151 cases (39.7%), a significantly lower percentage than the 62.1% obtained in the 2016 guidelines (p<0.001). According to both guidelines only 147 patients (38.6%) achieved the LDL target, while 89 patients (23.4%) met the 2016 but not the 2019 control criteria and 4 patients (1.0%) met the 2019 but not the 2016 targets. In addition, 145 patients (38.2%) met the non-HDL cholesterol goals set out in the 2019 guidelines.
Achievement of the LDL and non-HDL cholesterol targets according to the patients’ cardiovascular risk classification is shown in Fig. 1, and achievement of LDL targets according to the lipid-lowering-therapy used is represented in Fig. 2. No significant differences in LDL or non-HDL cholesterol control were found between the different cardiovascular risk groups. Although in the moderate risk category there appears to be a higher percentage of patients with good non-HDL cholesterol control using the 2019 guidelines compared to the 2016 guidelines, this difference is not significant. The low number of patients in this risk category makes the difference in percentage terms larger, but in absolute terms it is minimal (three patients with good non-HDL cholesterol control according to the 2016 guidelines and five patients according to the 2019 guidelines). Therefore, this finding does not appear to be clinically relevant.
In the univariate analysis, the factors associated with attaining the LDL and non-HDL cholesterol goals recommended by the 2019 guidelines were mostly related to the lipid-lowering therapy. The use of statins (especially high-potency statins), combination therapies and good adherence were significantly associated with greater achievement of lipid targets (Table 4). Other factors that were also associated with good LDL control were a history of ischaemic heart disease and heart failure (p=0.009 and p=0.039, respectively).
Main factors associated with attainment of the goals for LDL and non-HDL cholesterol according to ESC/EAS 2019 guidelines.
| LDL cholesterol | Non-HDL cholesterol | |||||||
|---|---|---|---|---|---|---|---|---|
| Patients on target (n=151) | Patients off-target (n=229) | OR (95% CI) | p | Patients on target (n=145) | Patients off-target (n=206) | OR (95% CI) | p | |
| Statin therapy | 146 (96.7) | 209 (91.3) | 2.7 (1.0–7.6) | 0.037* | 142 (97.9) | 186 (89.9) | 5.3 (1.15–18.2) | 0.003* |
| Statin type | ||||||||
| Atorvastatin | 88 (60.3) | 116 (55.2) | 1.52 (0.77–2.99) | 0.049* | 92 (64.8) | 101 (54.0) | 1.68 (0.80–3.50) | 0.035* |
| Rosuvastatin | 40 (27.4) | 44 (21.0) | 1.82 (0.86–3.86) | 35 (24.6) | 44 (23.5) | 1.47 (0.65–3.29) | ||
| Simvastatin | 15 (10.3) | 30 (14.3) | 1.00 | 13 (9.2) | 24 (12.8) | 1.00 | ||
| Pravastatin | 3 (2.1) | 13 (6.2) | – | 2 (1.4) | 11 (5.9) | – | ||
| Pitavastatin | 0 (0.0) | 5 (2.4) | – | 0 (0.0) | 5 (2.7) | – | ||
| Fluvastatin | 0 (0.0) | 2 (1.0) | – | 0 (0.0) | 2 (1.1) | – | ||
| Statin potency | ||||||||
| High | 65 (44.5) | 81 (38.8) | 1.87 (0.80–4.36) | 0.152 | 68 (47.9) | 73 (39.2) | 2.52 (1.00–6.39) | 0.045* |
| Moderate | 72 (49.3) | 107 (51.2) | 1.57 (0.68–3.62) | 67 (47.2) | 94 (50.5) | 1.93 (0.77–4.86) | ||
| Low | 9 (6.2) | 21 (10) | 1.00 | 7 (4.9) | 19 (10.2) | 1.00 | ||
| Ezetimibe therapy | 63 (41.7) | 52 (22.7) | 2.4 (1.5–3.8) | <0.0001* | 60 (41.4) | 49 (23.7) | 2.2 (1.4–3.6) | <0.0001* |
| Fenofibrate therapy | 16 (10.6) | 39 (17.0) | 0.5 (0.3–1.0) | 0.081 | 10 (6.9) | 38 (18.4) | 0.3 (0.1–0.6) | 0.002* |
| Combination therapy | 75 (50.3) | 77 (35.5) | 1.86 (1.21–2.84) | 0.004* | 65 (45.5) | 82 (41.8) | 1.16 (0.75–1.79) | 0.002* |
| High adherence to treatment (>80%) | 144 (97.3) | 194 (89.4) | 4.2 (1.4–2.6) | 0.005* | 134 (94.4) | 179 (91.8) | 1.4 (0.6–3.6) | 0.365 |
| Adherence rate (mean, SD)a | 97.3 (6.4) | 92.5 (16.2) | – | 0.001* | 96.6 (8.2) | 92.7 (16.2) | – | 0.009* |
| Treatment changes | 1 (0.7) | 82 (36.1) | 0.12 (0.02–0.09) | <0.0001* | 7 (4.9) | 68 (33.0) | 0.1 (0.04–0.6) | <0.0001* |
| Ischaemic heart disease | 40 (52.6) | 36 (47.4) | 1.95 (1.17–3.33) | 0.009* | 35 (24.1) | 36 (17.5) | 1.50 (0.89–2.54) | 0.126 |
| Heart failure | 21 (13.9) | 17 (7.4) | 2.01 (1.03–3.96) | 0.039* | 15 (10.3) | 22 (10.6) | 0.97 (0.49–1.94) | 0.932 |
Data are expressed as n (%) values or mean (SD).
Multivariate analysis showed that the determinants of achieving the 2019 LDL-c targets were adherence to statin therapy (OR 4.67, 95% CI: 1.55–14.10, p=0.006) and use of ezetimibe (OR 2.45, 95% CI: 1.54–3.91, p<0.001).
In the 229 patients who did not meet the LDL targets according to the 2019 guidelines, the adjustment of lipid-lowering treatment was assessed, finding that only 36.1% had had their treatment modified.
Attainment of other lipid goalsThe general goal of total cholesterol <200mg/dl was achieved in almost all of the patients (366, 96.3%). However, the rate of HDL cholesterol or triglyceride control was lower; 161 patients (42.4%) presented levels of HDL cholesterol on target (>40mg/dl for men and >50mg/dl for women) and 219 (57.6%) achieved the goal of triglycerides <150mg/dl.
DiscussionThe GALIPDIA study is a multicentre, cross-sectional study to analyse the degree of lipid control in a population of patients with long-standing T2DM. In this large sample evaluated, there is a predominance of patients at very high CVR according to the criteria of the two guidelines (ESC/EAS 2016 and 2019). The results show good glycaemic control and frequent use of anti-diabetic drugs with proven cardiovascular benefit. Lipid control was adequate in two thirds of the patients according to the 2016 guideline recommendations but was reduced to close to 40% when applying the 2019 guideline recommendations. The key determinants of the degree of lipid control were the type of LLT used and the degree of patient adherence.
To our knowledge, there are few studies that have analysed the degree of LDL-c cholesterol control according to cardiovascular risk (moderate, high or very high) and specifically the LDL-c target (<100mg/dl, <70mg/dl and <55mg/dl, respectively) in a population of T2DM, as has been analysed in our study. In the recently published high-impact DA VINCI study, only 54% and 33% of the subjects achieved their risk-based 2016 or 2019 LDL-c goal, respectively.11 However, our population is not comparable with that of the DA VINCI study as the percentage of patients with T2DM in that study comprised only 39% of the sample. A sub-analysis of EUROSPIRE V showed that 37% of the 2452 patients with T2DM and established coronary artery disease (very high CVR) had an LDL-c <70mg/dl (versus 59% in our study). However, the percentage of patients with LDL-c<55mg/dl, which is the target according to ESC/EAS 2019 guidelines, was not assessed.14,15
Two cross-sectional studies with a similar design to ours and comparable in patient characteristics have recently been published. One was carried out in Endocrinology clinics in Italy16 and the other in Cardiology centres in Buenos Aires (Argentina).17 They included patients comparable to our study in terms of age, percentage of males, time of T2DM evolution and mean HbA1c. Compared to the Argentinian study, our study shows a higher percentage of patients classified as being at very high CVR (72.1% vs. 54.2%), a lower proportion of patients categorised at high CVR (26% vs. 43.4%), and a similar number of patients considered to be at moderate CVR (1.8% vs. 2.4%).17 The percentage of patients who achieved the LDL-c control target was higher in our study (for all of the risk categories) than that observed in the other two studies: very high CVR 39% vs. 11.6% and 16.4% in the Italian and Argentinian studies, respectively; high CVR 40% vs. 20.4% and 18.8%. The higher percentage of patients achieving their LDL-c goal could be due to differences in the LLT received. Our study had a higher percentage of patients treated with statins (93.4% vs. 61% and 62.5% in the Italian and Argentinian studies, respectively), a higher proportion of patients with high-intensity statins (38.4% vs. 36% and 15.6%) and more patients treated with statins in combination with ezetimibe (24.7% vs. 5.6% in the Italian study).
Several factors have been associated in the literature with compliance to the guidelines. Our study has shown that the type of LLT used clearly determines the patients’ degree of control (use of statins, their type and potency, combination with other drugs such as ezetimibe, etc.). However, other factors are related to difficulties in achieving objectives. Therapeutic inertia, which is the lack of initiation or intensification of the lipid-lowering agents by the physician when indicated due to LDL-c being outside the target, is certainly a key factor that may determine the achievement of recommended lipid targets. In this study, of the patients who did not meet LDL targets according to the 2019 guidelines, only 36.1% had their treatment modified. Data from the literature is even higher. A Spanish study performed on 639 patients with diabetes showed that medical inertia in terms of lipid control occurred in 43.6% of the population.18 A study based on the Health Improvement Network (THIN) database, which provides longitudinal patient-level primary care data in the UK, showed a prevalence of dyslipidaemia of 66% at diabetes diagnosis. Among those with high risk of ASCVD, the median time to initiation of therapy was 20.4, 10.9 and 9.5 months for the 18–39, 40–49, and 50–59 age groups, respectively, demonstrating a significant delay in the start of LLT. Similar data were reported in the primary prevention setting across all T2DM diagnosis age groups, irrespective of ASCVD risk status, resulting in poor lipid control.19 Despite the fact that inertia may have multifactorial causes, one of the most important reasons might be the underestimation of CVR.20 In our study, a history of ischaemic heart disease was associated with better lipid control, probably because the diagnosis of very high CVR is more evident in this subgroup of patients. However, the confluence of T2DM and target organ damage or multiple major risk factors, which also imply very high CVR according to the 2019 guidelines, can sometimes be misclassified as a lower degree of risk. This underestimation of patient risk would explain why the mean dose of atorvastatin (the most commonly used statin in our sample) was relatively low (33.5mg), while the mean LDL-c was 68.7mg/dl (thus off-target for most patients, who should have been categorised as very high risk, with a target LDL-c of less than 55mg/dl). Thus, clinicians should be more attentive to such patients. Moreover, there is some imprecision in certain aspects of the guidelines that may contribute to an underestimation of risk. As explained in our methods section, one of the difficulties encountered when classifying CVR was the lack of specificity in the 2019 guidelines in the definition of what are considered to be major CVR factors, making it necessary to assume those defined in the previous 2016 guidelines. There are also other factors, such as central obesity, which do not appear but should be taken into account. Likewise, the 2016 guidelines do not specify the definitions of smoking, hypertension and marked hypercholesterolaemia, which may be criteria for very high CVR when associated with diabetes. In the absence of these definitions, the researchers used the criteria of the Spanish Society of Arteriosclerosis to resolve uncertainties in CVR assessment.13
Another factor associated with poor compliance of lipid objectives is the lack of adherence to the LLT. A large analysis of a secondary prevention database showed that in the population with diabetes, adherence only reached 55%, and the group of high-risk patients had the lowest levels of adherence.21 In our study, however, adherence to treatment measured as the percentage of prescriptions collected in the last 12 months was high (94.4%). Only 14% had poor adherence, defined as less than 80% prescription collection. Nevertheless, correct medication intake in all patients cannot be ensured. Intolerance to statins, like muscle-related symptoms, are considered one of the main reasons for poor adherence.22 However, this reason seems to be less important in our subjects as only 2.4% had total intolerance and 1.3% partial intolerance.
The difference between the treatment suggested by the guidelines and eligibility for reimbursement by national health systems or insurance16,23 should also be taken into account. Among the lipid-lowering drugs used, a very low use of proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) (0.5%) was detected, probably related to the strict funding criteria in Spain's national health system (population with established cardiovascular disease or familial hypercholesterolaemia, who, with statin treatment that they can tolerate, maintain LDL levels above 100mg/dl). These criteria are far from the strict targets proposed by the guidelines. Thus, treatments may not be sufficiently optimised.
Finally, some studies have linked diabetes to a lower response to statin treatment, probably related to an increase in intestinal cholesterol absorption observed in patients with T2DM (hypo-responsive subjects), although this factor seems less relevant.17
One of the strengths of this study is its multicentre nature, with a population including patients from all the health areas of our Autonomous Community. In addition, a large sample of patients was recruited with sufficient power to achieve the objectives set.
This study is a good opportunity to obtain real-life results of clinical practice in general endocrinology consultations, where patients with T2DM and at very-high CVR are concentrated. The results show good adherence to current guideline recommendations regarding the choice of antidiabetic drugs in this population, with a high use of SGLT2i and GLP1-RA.
On the other hand, there are certain limitations to this study. One is its retrospective nature, which limits certain aspects, such as the assessment of non-classical complications or comorbidities of the disease due to the lack of data in the patients’ medical history. This may lead to the underestimation of some of these conditions (e.g., heart failure or NASH).
Another limitation when assessing the degree of adherence to the guidelines is that baseline LDL-c levels were not available for all patients to calculate the LDL-c reduction with treatment, which is one of the criteria assessed by both dyslipidaemia guidelines, beyond the final LDL-c result achieved. This may have had a negative influence on the observed LDL-c target compliance rate.
Finally, the study was designed and conducted just one year after the publication of the 2019 guidelines and two years after the publication of the ESC Guidelines on cardiovascular disease prevention.24 As a result, this could justify the lower compliance with respect to the previous guidelines from 2016, since it is reasonable to assume that the implementation of new criteria requires time in daily clinical practice.
In conclusion, our study demonstrated a still insufficient compliance with LDL-c goals in patients with T2DM according to the main guidelines for lipid management, despite the fact that most of these patients are at very high cardiovascular risk. Previous ischaemic cardiac disease, treatment with high potency statins and combination lipid therapy were associated with a higher frequency in achieving objectives. Strategies to avoid the underestimation of CVR in daily clinical practice and to optimise lipid-lowering treatment should be implemented.
FundingThis research has not received any public or private financial support.
Conflict of interestThe authors declare no conflict of interest.
Thanks to Pilar Guijarro for her support and trust.