Vitamin D deficiency is a serious public health problem worldwide that affects not only skeletal health, but also a wide range of acute and chronic diseases. However, there is still skepticism because of the lack of randomized, controlled trials to support association studies on the benefits of vitamin D for non-skeletal health. This review was based on articles published during the 1980–2015 obtained from the Cochrane Central Register of controlled trials, MEDLINE and PubMed, and focuses on recent challenges with regard to the definition of vitamin D deficiency and how to achieve optimal serum 25-hydroxyvitamin D levels from dietary sources, supplements, and sun exposure. The effect of vitamin D on epigenetic fetal programming and regulation of genes that may potentially explain why vitamin D could have such lifelong comprehensive health benefits is reviewed. Optimization of vitamin D levels in children and adults around the world has potential benefits to improve skeletal health and to reduce the risk of chronic diseases, including some types of cancer, autoimmune diseases, infectious diseases, type 2 diabetes mellitus, and severe cardiovascular disorders such as atherothrombosis, neurocognitive disorders, and mortality.
La deficiencia de vitamina D es un problema grave de salud pública en todo el mundo y que afecta no solo la salud músculo-esquelética sino también una amplia gama de enfermedades agudas y crónicas, incluyendo las no trasmisibles de riesgo cardiovascular, algunas enfermedades autoinmunes, metabólicas y mecanismos fisiopatológicos en obesidad. Sin embargo, subsiste el escepticismo de la falta de ensayos controlados aleatorizados para apoyar los estudios de asociación sobre los beneficios de salud no esqueléticos de vitamina D. Esta revisión fue obtenida de las bases de datos de MEDLINE, PubMed de 1980–2015 con respecto a la definición de deficiencia de vitamina D y su participación en trastornos proinflamatorios, inmunometabólicos y factores de riesgo cardiovascular. Se revisan las acciones de la vitamina D sobre la programación epigenética fetal y regulación de los genes que potencialmente podrían explicar por qué la vitamina D podría tener tales beneficios para la salud a lo largo de la vida en el humano. Hay potencialmente una ventaja en optimizar los niveles de la vitamina D de niños y adultos mayores en todo el mundo para mejorar no solo la salud músculo-esquelética, sino también para reducir el riesgo de enfermedades crónicas, incluyendo algunos factores de riesgo cardiovascular, así como ciertos tipos de cáncer, enfermedades autoinmunes, enfermedades infecciosas, diabetes mellitus tipo 2, trastornos cardiovasculares, incluyendo aterotrombosis, alteraciones neurocognitivas y, como consecuencia sobre la morbimortalidad global.
After having a significant impact on rickets followed by a period of silence, vitamin D deficiency in humans is again being paid increasing attention and is currently considered a worldwide pandemic.1–3 According to a recent report from the National Center for Health Statistics of the United States covering the 2001–2006 period, from 32% to 46% of the US population had serum 25 (OH) D3 levels less than 20ng/mL, and 8% values lower than 12ng/mL.4
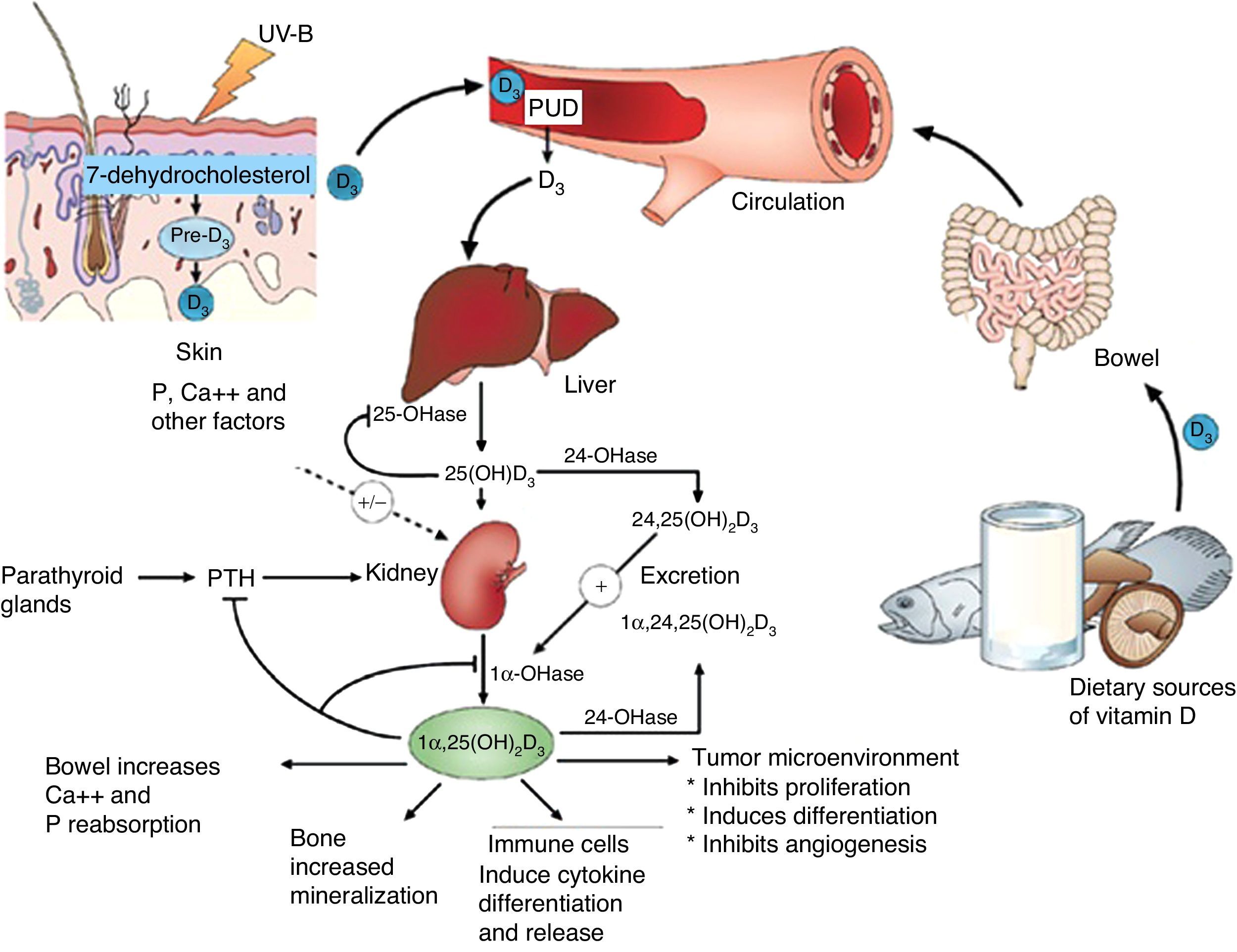
Vitamin D is known to play a role in bone metabolism, being important for maintaining calcium homeostasis, ensuring its physiological absorption from the bowel.1 The discovery of the vitamin D nuclear receptor (VDR), ubiquitously expressed in almost all body cells, including immune, vascular and myocardial cells, suggested an implication of effects mediated by vitamin D in several systems other than musculoskeletal tissues2 (Fig. 1).
Action sites of vitamin D. Note extraosseous effects. ©Modified from Hossein-Nezhad and Holick.99
This led to comprehensive research into vitamin D as a factor with an influence on the pathogenesis of various non-skeletal acute and chronic diseases such as infection, cancer, psychiatric disorders, and autoimmune diseases, as well as cardiovascular risk factors such as high blood pressure, dyslipidemia, diabetes mellitus, obesity, and atherosclerosis.4–6
Cardiovascular (CV) risk factors, and cardiovascular diseases, including myocardial infarction, coronary artery disease and stroke, are recognized as the most common diseases and represent the leading causes of death worldwide, particularly in Western countries.7 In Mexico, there has been a clear increase in cardiovascular risk factors in the past two decades, and the same pandemic has occurred elsewhere in Latin America. This emphasizes the importance of clarifying the role of vitamin D in the context of cardiovascular diseases and their most common risk factors.
It has recently been reported that coronary artery disease, especially myocardial infarction and heart failure, affects 7.9 and 5.7 million adults in the United States respectively.8,9 Left ventricular abnormalities related to remodeling (progressive dilation of the predominant chamber, fibrosis, and systolic/diastolic dysfunction) and heart failure often complicate an acute myocardial infarction.10,11 Recent data suggest that the prevalence of ventricular dysfunction related to myocardial infarction and heart failure is associated with decreased vitamin D levels.11 Thus, increasing attention is being paid to the role of vitamin D deficiency as a risk marker in the development of heart failure.
As early as in 1981, a study had reported seasonal variations in CV mortality and the potential positive effects of protection from UVB (ultraviolet B) radiation on CV risk.8 The association of vitamin D with CV risk factors and their associated diseases has been widely investigated in recent years. Multiple observational studies, prospective meta-analyses, and some interventional studies have addressed the potential link with vitamin D deficiency.9–12
Patients with advanced heart failure are known to have significantly lower serum 25 (OH) D levels, an independent risk factor associated with poor clinical outcomes and prolonged decompensation of heart failure.7,13–15 VDR agonists such as calcitriol and its analogs, including doxercalciferol, have been developed to treat secondary hyperparathyroidism in chronic kidney disease,8 osteoporosis,11 and psoriasis.13
Researchers are currently developing another candidate as a selective and effective VDR agonist for treating vitamin D deficiency in heart failure and the phenotypes associated with dysfunction in the renin–angiotensin–aldosterone system (RAAS).15
One of our central objectives is to lend scientific support to the urgent need to understand the prevalence of vitamin D deficiency in countries such as Mexico and elsewhere in Latin America, where the pandemic of non-communicable chronic diseases is the main factor responsible for overall morbidity and mortality.16
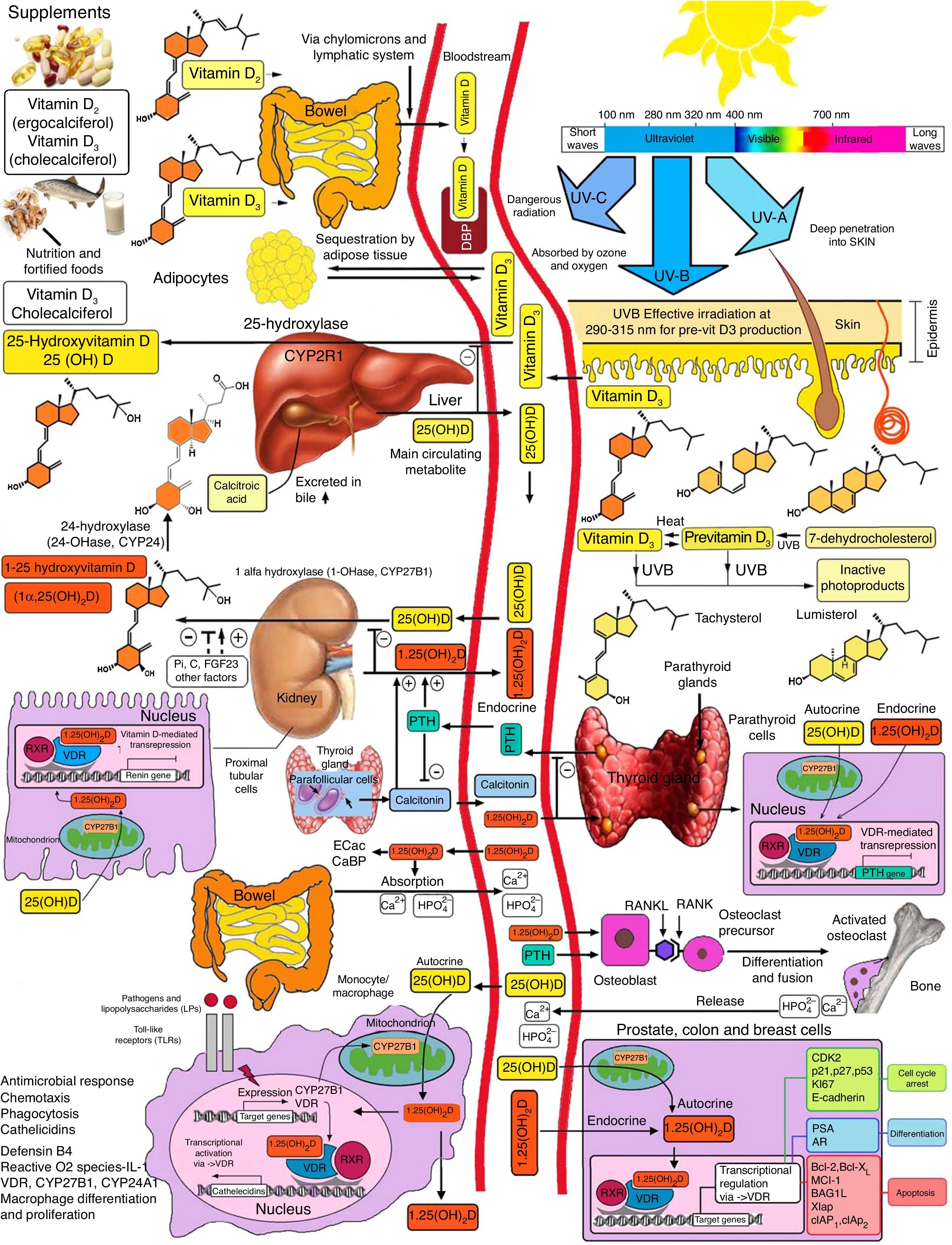
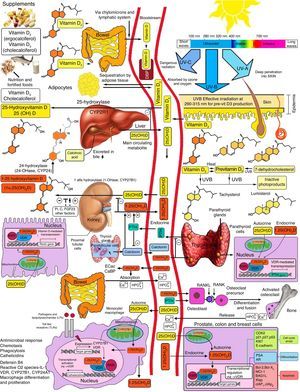
Basic vitamin D metabolismVitamin D3 is a steroid prohormone mainly derived from the synthesis induced by UVB radiation on dehydrocholesterol in the skin (Fig. 2).
Schematic representation of vitamin D synthesis and metabolism for skeletal and non-skeletal functions. 1-OHase: 25 (OH) D 1α-hydroxylase; 24-OHasa: 24 hydroxylase; 25(OH)D: 25-hydroxyvitamin D; 1,25(OH)2D: 1,25-dihydroxyvitamin D; CaBP: calcium binding protein; DBP: vitamin D binding protein; ECaC: epithelial calcium channel; FGF-23: fibroblast growth factor-23; PTH: parathyroid hormone; RANK: receptor activator of nuclear factor kB (NFkB); RANKL: receptor activator of NFkB ligand; RXR: retinoic acid receptor; TLR2/1: toll-like receptor 2/1; VDR: vitamin D receptor; vitamin D: vitamin D2 or D3. ©Copyright Holick MF, 2013, coauthor of this study.99
Endogenous synthesis is the main source of vitamin D in the body and accounts for approximately 80% of the vitamin. After synthesis in the skin or absorption from diet, vitamin D is transported by a specific binding protein to the liver, where it is hydroxylated to 25-hydroxyvitamin D. This inactive form is the main metabolite circulating in blood, and is also used to determine vitamin D status.1–3
25-Hydroxyvitamin D is hydroxylated by the enzyme 1α-hydroxylase (1α-OHase) to its most active metabolite, 1α-25 (OH)2 D3, mainly in the kidneys. From this 1α-hydroxylation it is known that it can also be activated by extrarenal 1α-hydroxylation throughout the body13,14 (Fig. 2). This has led to the assumption that vitamin D plays a wider role in overall health, including tissues beyond the musculoskeletal system such as the heart and blood vessels.15–17
Classification of vitamin D deficiencyVitamin D status is categorized based on serum 25 (OH) D levels, and the half-life of the vitamin is approximately 2–4 weeks. However, this half-life may be decreased even to less than a week in the event of hemodilution, infection, or other catabolic diseases, or after the administration of corticosteroids.
A report by the United States Institute of Medicine (IOM) defines vitamin D deficiency as 25 (OH) D levels less then 12ng/mL (multiply by 2496 to convert ng/mL to nmol/L), while values of 20ng/mL or higher are considered adequate. On the other hand, the guidelines of the Endocrine Society of America14 suggest that 25 (OH) D levels ≤20ng/mL are deficient and that values ≥30ng/mL are sufficient.14–16
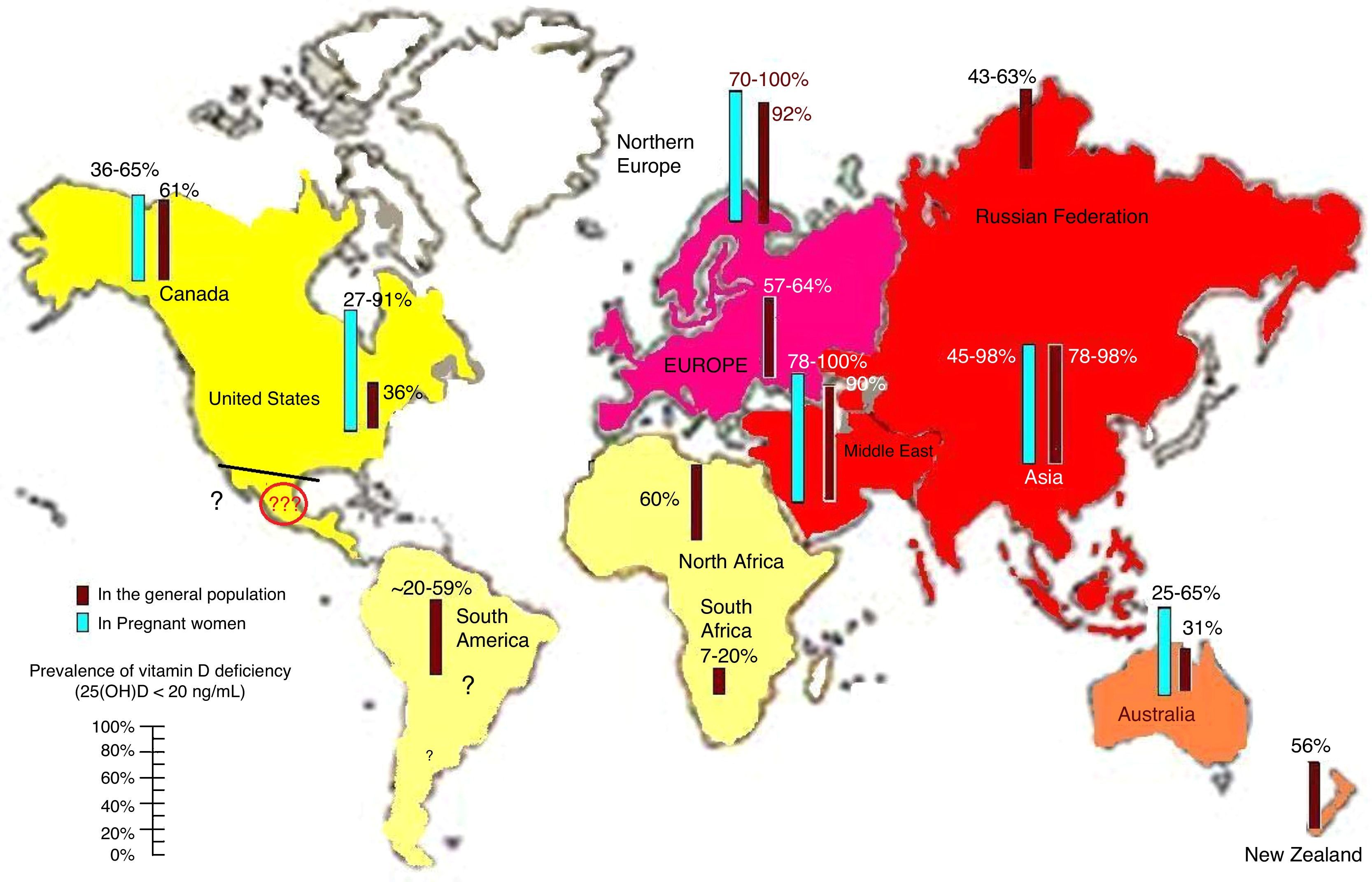
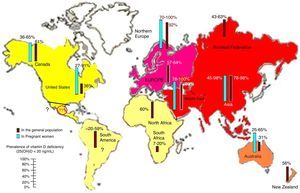
Prevalence of vitamin D deficiencyVitamin D deficiency and insufficiency are highly prevalent, as is shown by the fact that more than half of the population worldwide has levels lower than 30ng/mL13,16 (Fig. 3). However, the prevalence of the deficiency in countries such as Mexico is not known, which should alert the medical community. There are various risk factors for vitamin D deficiency that should always be taken into account, including age, female sex, pigmentation in darker skin, decreased sun exposure, seasonal variation and distance from the Equator. Special mention should be made of air pollution.17,18 Studies on the impact of pollution on vitamin D should be conducted in cities with high pollution levels because of their unique population and geodemographic conditions.11,19–21
Reported prevalence of vitamin D deficiency, defined as 25-hydroxyvitamin D levels less than 20ng/mL in the worldwide population of pregnant women and the general population. Note the lack of information for Latin America. To convert 25 (OH) D levels to nmol/L, multiply by 2496. ©Copyright Holick 2013, reproduced with permission.99
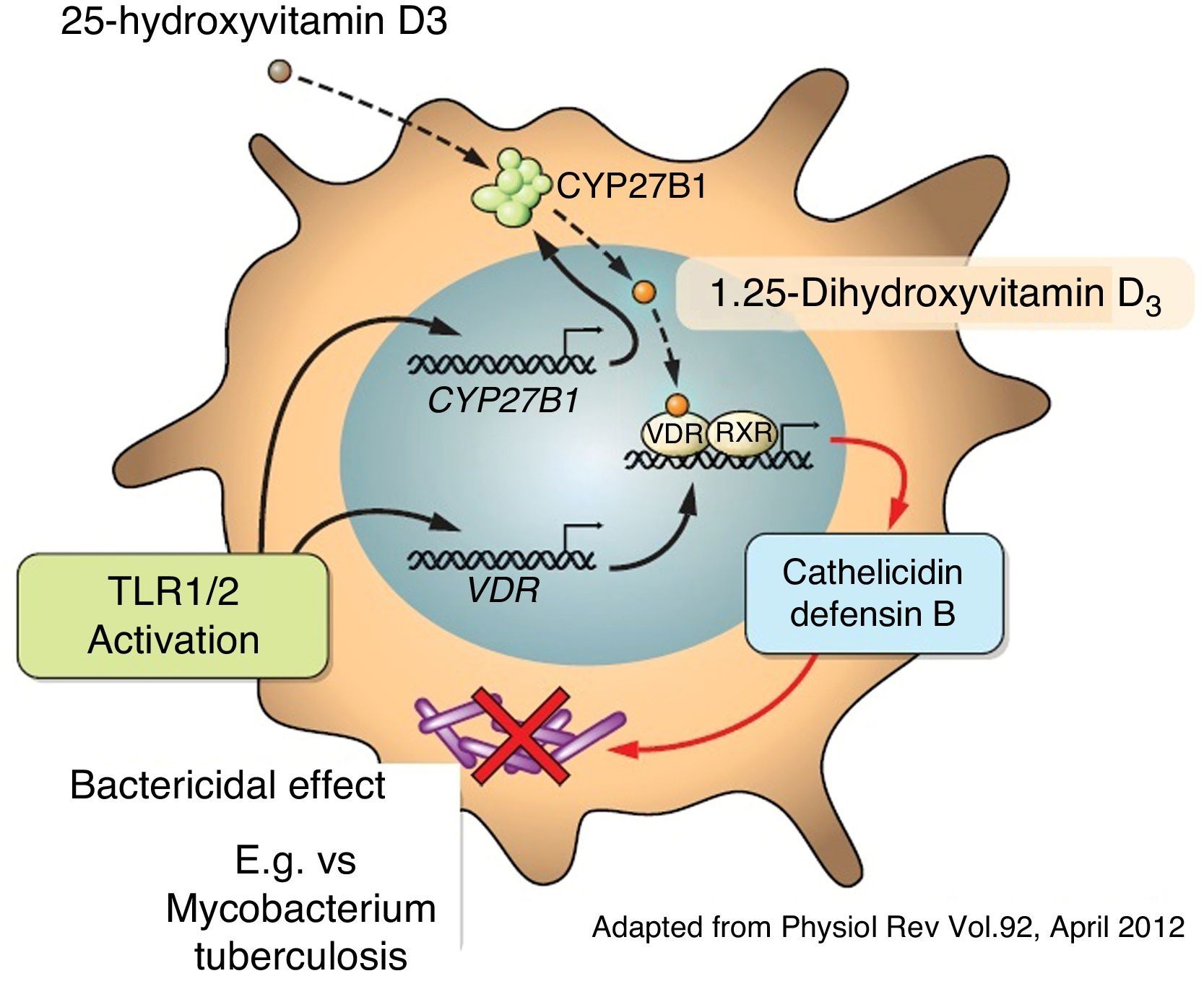
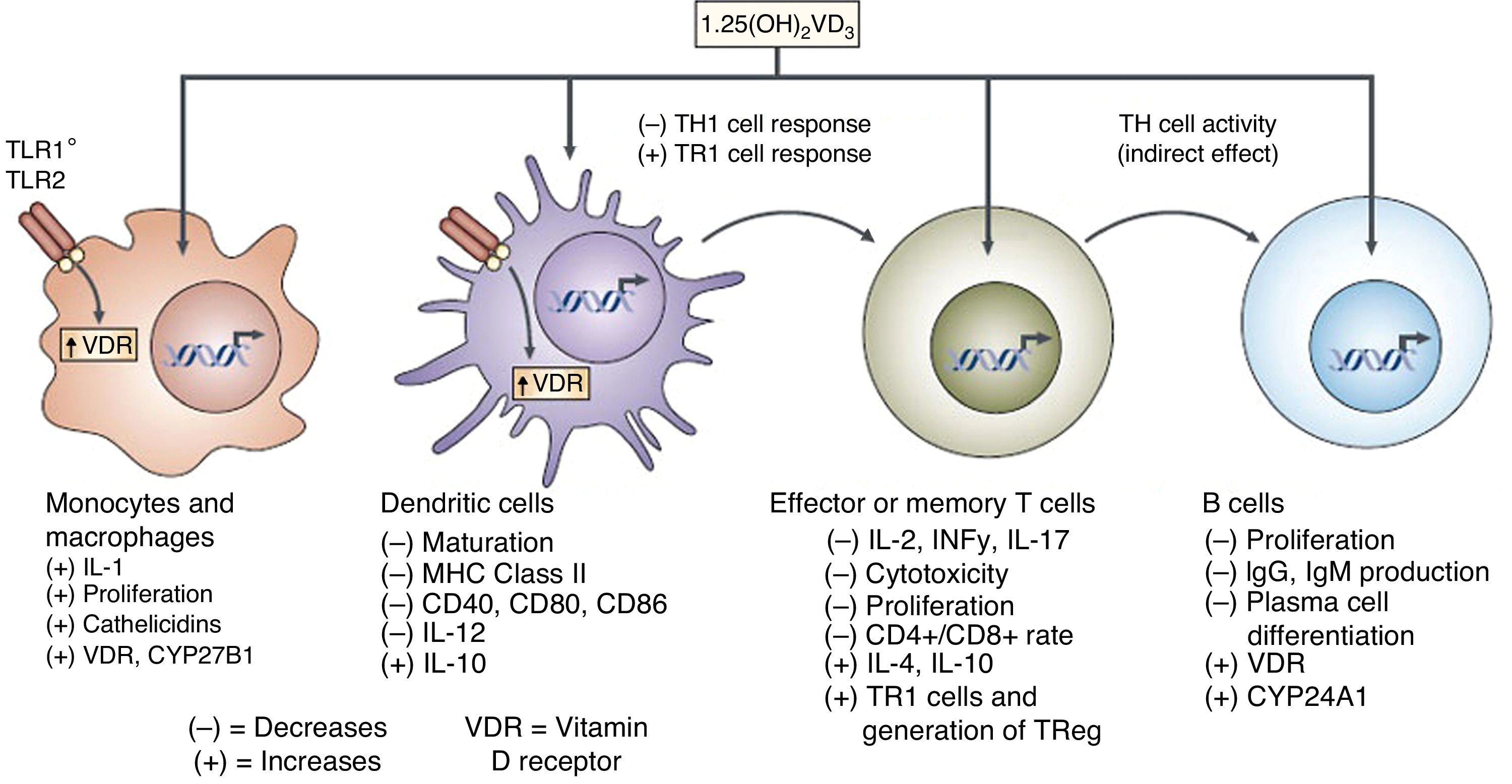
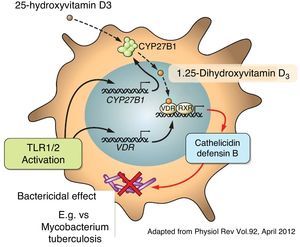
Apart from its traditional role as a modulator of calcium metabolism and bone homeostasis, vitamin D has been shown to have potent anti-inflammatory effects. Because of this, it has been considered as adjuvant therapy for many chronic diseases such as asthma, arthritis, prostate cancer and psoriasis, amongst others.4,22,23 A variety of both pro-inflammatory and anti-inflammatory effects of vitamin D had previously been reported.24,25 It has also been shown that vitamin D may directly induce the production of important antimicrobial peptides such as cathelicidins and defensin β4 in monocytes/macrophages and in human epithelial cells13,26 (Fig. 4).
1,25-(OH)2 D3 has been shown to have antiproliferative and proapoptotic activity in tumor cells due to the induction of the cyclin-dependent kinase inhibitors p27Kip1 and p21Waf/Cip1 and the inhibition of c-Myc and antiapoptotics such as Bcl-2.23 Moreover, vitamin D was shown to suppress the action pathways of prostaglandins in tumor cell lines through the inhibition of cyclooxygenase-2 production and the stimulation of 15-hydroxyprostaglandin production by the cells.23
Vitamin D has also been shown to interfere with the activation and signaling of nuclear factor (NF) kB by increasing the expression of IκBα in activated cells, and to interfere with the nuclear translocation of subunits of NF-κB.4
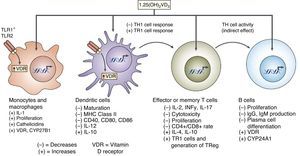
It has also been reported that vitamin D may influence dendritic cell maturation and function.27 Several studies have shown the ability of vitamin D to modulate the population and role of FOXP3-positive regulatory T cells and the production of IL-1027 (Fig. 5).
The interaction of peroxisome proliferator-activated receptors (PPARs) and the VDR has been widely studied, because they both form heterodimers with LXR. The potential modulation of PPARy activity has been implicated in many metabolic processes, especially in lipids and in mechanisms for insulin release from β cells in the islets of Langerhans. Additional results of this transcriptome have shown that vitamin D regulates proadipogenic PPARy in activated macrophages. PPARy agonists reverse the antiadipogenic and antimicrobial effects attributed to the vitamin D receptor, which suggests a link between the VDR and signaling in the regulation of processes of two-directional functions between vitamin D and PPARy.28
Vitamin D and cardiovascular risk factorsHigh blood pressure and vitamin D deficiencyVitamin D deficiency has been associated with higher blood pressure levels, as is shown in most prospective studies and in meta-analyses of observational studies.9,29,30 Reported blood pressure variations may be explained by differences in vitamin D levels between the studies, which have often been relatively small. However, the additive role in antihypertensive drug treatment has clinical relevance.
The potential mechanisms for this association of vitamin D and blood pressure include an inverse association of vitamin D with RAAS activity, the effect of improved endothelial function, and the prevention of secondary hyperparathyroidism, all of them mechanisms of interest.24–27
In this regard, it should be noted that high parathyroid hormone levels are a distinctive feature of vitamin D deficiency, and are known to be associated with myocardial infarction and higher blood pressure levels.28 In addition, there is an increasing evidence to suggest that mutual interactions between PTH, vitamin D, and aldosterone are involved in damage to the cardiovascular system independently of the RAAS.31,32
A significant inverse association has been reported between circulating serum vitamin D levels and the incident risk of hypertension. The combined relative risk (RR) was 0.70; the confidence interval (95% confidence interval: 0.57–0.86) after comparing the highest value to the lowest tertile of the baseline 25 (OH) D levels, with no evidence of heterogeneity between the results. Therefore, when dosage response in the five studies that reported RRs for exposure to vitamin D was assessed, the authors found that the risk of high blood pressure decreased 12% with each 10ng/mL increase in 25 (OH) D levels.29 Several randomized clinical trials assessing the effect of vitamin D on blood pressure levels published in 2012 reported different results.23–25 A controlled clinical study was conducted in Denmark on 130 hypertensive patients who during the winter months received supplements of 3000IU of vitamin D versus placebo.23 Ambulatory blood pressure monitoring (ABPM) for 24h showed a non-significant decrease in blood pressure (−3mmHg, p=0.26–1mmHg, p=0.18). Interestingly, when only patients with vitamin D deficiency (25 [OH] D less than 32ng/mL) were analyzed, systolic and diastolic BP values significantly decreased in 24-h ABPM (−4mmHg, p=0.05–3mmHg, p=0.01) in the treatment group as compared to the placebo group.23
In an unselected Black population, vitamin D supplementation decreased systolic BP by 0.2mmHg per each 1ng/mL increase in vitamin D during the 3 months of treatment (p=0.02).24
Obesity and vitamin D deficiencyObesity is closely related to vitamin D deficiency.25 It has been suggested that this may be due to vitamin D sequestration in adipose tissue, which results in lower circulating 25 (OH) D levels.31 Other authors have hypothesized a causal relationship with vitamin D deficiency that may lead to the promotion of obesity.27
A bidirectional Mendelian randomization study was conducted to address this question. The study showed a unidirectional causal relationship, which suggests that obesity leads to a reduction of vitamin D levels, rather than the opposite.28 That research, including 21 cohort studies with a total sample of 42,024 patients, found 12 established single nucleotide polymorphisms (SNP) related to the BMI and four typical SNPs associated with vitamin D status. It cannot be ignored that there has been a great increase in the prevalence of overweight and obesity in countries such as Mexico and in Latin America in general. On the other hand, genetically determined 25 (OH) D levels are not significantly related to the body mass index. Thus, obesity is known to be a pro-inflammatory state that has been related to abnormally high C-reactive levels, with a significant role being played by pro-inflammatory mediation in vitamin D deficiency.31
Glucose metabolism, vitamin D, and type 2 diabetes mellitusIn observational and prospective studies, low vitamin D levels have been related to changes in glucose metabolism and to a greater risk of the development of diabetes in the future.5,30–33 It should also be noted that vitamin D deficiency in diabetic patients may be partly due to a reduction of physical activity and to obesity, as well as to limited sun exposure. Thus, residual confusion in observational studies due to the close relationship of obesity with glucose intolerance and vitamin D deficiency cannot be ruled out with certainty.26,34 On the other hand, inverse causality may exist, as there are data suggesting that an inflammatory state may be decreased by 25 (OH) D.33,35–37
There are however several potential mechanisms that could explain the association of vitamin D deficiency with changes in glucose homeostasis and diabetes mellitus. Both the VDR and 1-α-hydroxylase are expressed in pancreatic beta cells, which suggests a potential role of vitamin D in beta cell function. In addition, the interaction of VDR and PPARy has been suggested as a mechanism related to adequate insulin release.2,38
However, randomized trials have not shown that vitamin D supplements clearly improve blood glucose or insulin resistance.37,38 It remains to be clarified whether drug treatment for diabetes may help optimize metabolic control. To address this issue, a randomized clinical study was conducted in subjects with prediabetes and hypovitaminosis D.39 The study participants were randomized to treatment with high vitamin D doses compared to placebo (mean weekly dose, 88,865IU). After the administration of vitamin D for 1 year, no differences were seen in plasma glucose, insulin secretion and sensitivity, or in the development of diabetes between the treatment and placebo groups.39
Vitamin D and serum lipidsSome observational studies suggest an association of vitamin D deficiency with low high density lipoprotein (HDL) levels, high triglyceride levels, and higher apolipoprotein E levels.40,41 A prospective evaluation of vitamin D and lipid levels showed a significant association between low 25 [OH] D levels and hypercholesterolemia.42 It should be noted, however, that blood lipid and vitamin D levels may be confounded by the above mentioned relationship between vitamin D and obesity.28 Recent clinical studies evaluating the effect of vitamin D supplementation on blood lipids provided no conclusive evidence.24,43–45
Chronic kidney disease and vitamin D deficiencyPatients with chronic kidney disease have significantly lower vitamin D levels as compared to the overall population. Thus, a high prevalence of vitamin D deficiency, with levels less than 20ng/mL, was seen in more than 70% of patients on dialysis.46
In addition, several epidemiological studies showed that low 25 (OH) D levels are associated with albuminuria or progression to renal failure. Vitamin D deficiency has also been identified in patients with chronic kidney disease as an independent risk factor for increased mortality, which may mainly be attributed to cardiovascular death.47–51
In addition to low 25 (OH) D levels, 1,25 (OH)2 D has been associated with higher mortality rates in most observational studies in patients with chronic kidney disease.51,52 Thus, special attention has been paid to vitamin D in the field of nephrology, given the classical known effect of vitamin D supplementation on the reduction of parathyroid hormone (PTH) levels. This is of great clinical significance, because PTH itself is an independent cardiovascular risk factor,53 and secondary hyperparathyroidism is very common in patients with chronic kidney failure. It should be noted that this therapeutic effect of PTH decrease is achieved with both the active form (1,25 [OH]2 D) and with natural supplementation with vitamin D, although greater effects are seen with 1,25 [OH]2 D.54
To ascertain the role of active vitamin D in chronic kidney failure, a systematic review was conducted of seven studies with meta-analysis and seven retrospective observational studies in patients with chronic kidney failure supplemented with 1,25 (OH)2 D or with the different analogs of active vitamin D. The authors found a significant reduction in all-cause mortality (RR 0.73, 95% CI: 0.65–0.82), and a 37% decrease in cardiovascular mortality (RR 0.63; 95% CI 0.44–0.92) in patients treated with active vitamin D.55
While these data suggest the beneficial effects of treatment with vitamin D in patients with chronic kidney failure, the greatest impact was seen in the cardiovascular area. By contrast, a meta-analysis of randomized trials in the overall study populations, a great part of whom had kidney failure, also showed decreases in fractures and all-cause mortality with vitamin D supplementation.56,57
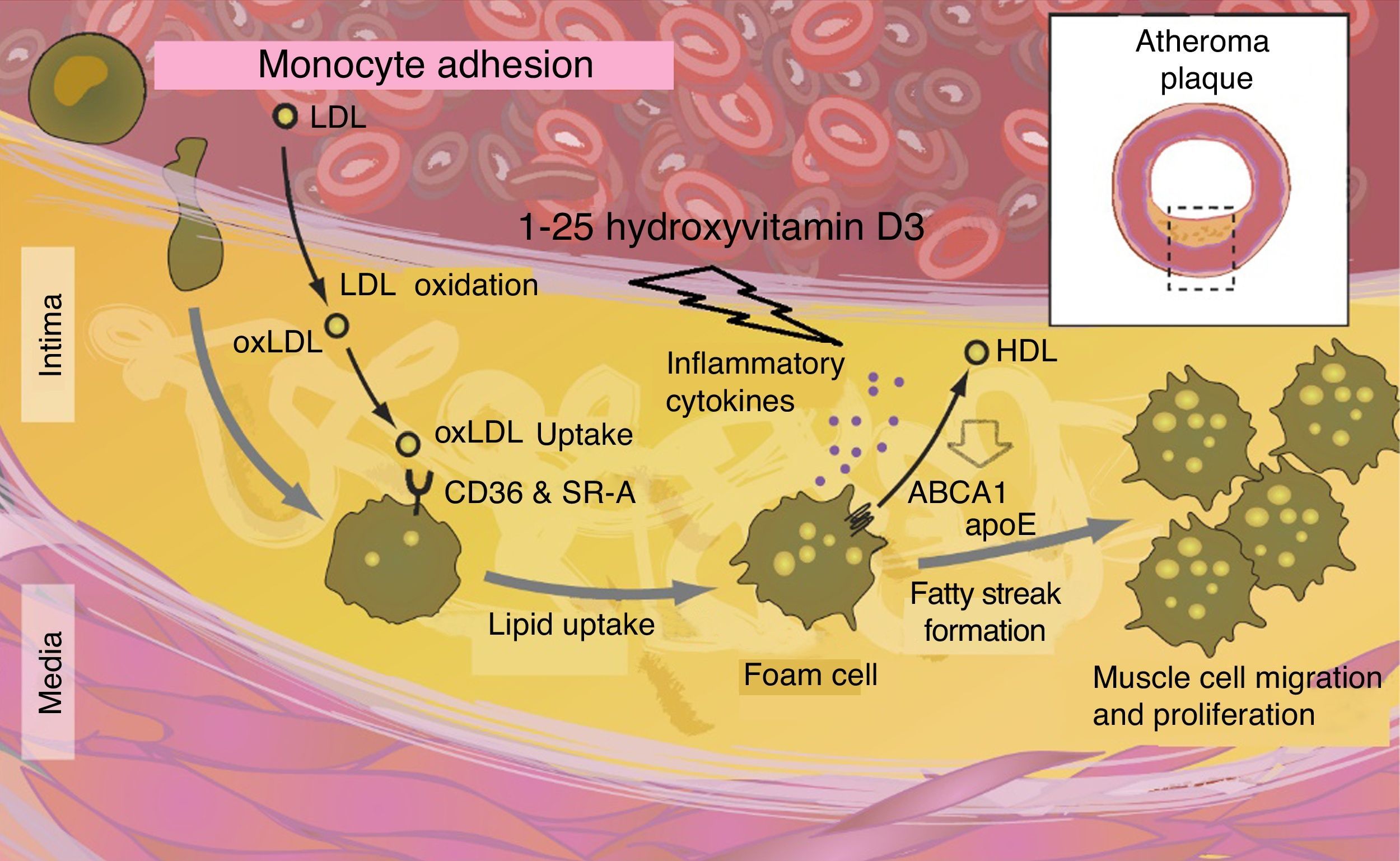
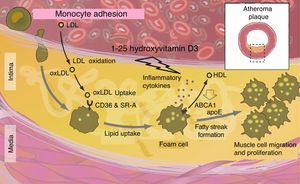
Atherosclerosis and endothelial dysfunctionSince the VDR is also expressed in the vasculature, the hypothesis that vitamin D may also protect against vascular diseases, including atherosclerosis and endothelial dysfunction, is tempting.31 According to experimental studies, some vasculoprotective actions attributable to vitamin D may be mediated by increased nitric oxide production, the inhibition of macrophages in foam cell formation, or the decreased expression of adhesion molecules in endothelial cells.58–71 This is in agreement with cross-sectional observational studies showing that low vitamin D levels are associated with endothelial dysfunction and with increased arterial stiffness.6,27
Vitamin D deficiency has been shown to have a harmful effect on the macrophage ABCA1 system, altering its function and preventing cholesterol efflux promoting foam cell formation. Cytokines also speed up cholesterol synthesis within macrophages (Fig. 6).
The data from controlled clinical trials addressing the effects of vitamin D on vascular diseases are limited and show inconsistent results. However, encouraging effects in endothelial function have been reported with vitamin D in randomized clinical trials, although not all of them showed that vitamin D improves endothelial function.72–74
Vitamin D and cardiovascular eventsAs previously noted, decreased vitamin D levels have been associated with a greater incidence of cardiovascular events.10,55,75–77 Even asymptomatic coronary artery disease is associated with lower vitamin D levels in high-risk patients such as patients with T2DM (adjusted OR 2.9 [95% CI 2.0–7.0]), as reported in a recent observational study.78 Thus, vitamin D deficiency has been associated with an increased risk of myocardial infarction and a significant inverse relationship between 25 (OH) D and matrix metalloproteinase-9, a marker of myocardial infarction after myocardial remodeling.79,80
Vitamin D levels also appear to predict the risk of adverse events after acute infarction and cardiac surgery, thus suggesting a greater risk for subjects with very low vitamin D levels, as reported in recent publications.81,82
Data from prospective observational studies suggest that low vitamin D levels are a risk factor for stroke83–85. In a meta-analysis of seven studies comprising 47,809 subjects and 926 strokes, the risk of cerebrovascular disease was significantly lower in subjects with higher 25 (OH) D levels as compared to those with inadequate vitamin D levels.84
Other authors investigated 19 studies comprising 65,994 patients and 6123 strokes and found that the lower the 25 (OH) D levels, the greater the risk (12). It should be noted, however, that not all individual studies showed a significant association between low 25 (OH) D levels and a greater risk of cardiovascular disease.86
When all the above-mentioned studies are reviewed, confounding factors such as decreased mobility in patients with chronic diseases should be carefully weighted. Other confounding factors such as age or obesity rate, as well as PTH, renin, calcium, and phosphorus, cannot be completely ruled out as long as they are included as potential confounding factors in most analyses.15,87
Vitamin D supplementation and cardiovascular diseaseSome small, controlled clinical trials reported mixed vitamin D results in cardiovascular events,34,88 while mention should be made of some meta-analyses of these controlled clinical trials that found no significant trends in CV event reduction in patients who received vitamin D supplements as compared to placebo.89,90
It should be noted that vitamin D supplementation was combined with calcium supplementation, and interpretation of the results of controlled clinical trials is therefore difficult, especially when we know that calcium intake may be associated with greater cardiovascular risk, as suggested by a previous meta-analysis.90–93
Discussion and early epidemiological alert on the effects of vitamin D deficiency and cardiovascular risk in AmericaBased on the evidence currently available in the literature, it may be concluded that vitamin D deficiency is an independent cardiovascular risk factor that is associated with a greater risk of cardiovascular events. It is not clear, however, if these associations are causal in nature. Vitamin D should not be used to treat chronic diseases. It may be of help, but that is all. However, it appears plausible to take vitamin D deficiency as a very relevant marker of poor health, especially in patients with chronic diseases, including cardiovascular diseases and subjects with greater cardiovascular risk, so that, in this way, vitamin D may have a direct impact on cardiovascular results.94
Despite these conflicting results, it is quite clear that vitamin D supplements have significant benefits in healthy populations, and also in patients with various comorbidities, particularly as regards prevention. It should be noted that most clinical studies have shown that the most beneficial effects of vitamin D supplementation occur in patients with very low 25 (OH) D levels. Such patients appear to have a greater risk of suffering cardiovascular diseases.11,12,22,23,62,95
Large scale studies are ongoing, including a study of 1000 patients with heart failure in Germany, another study assessing vitamin D in more than 5000 subjects in New Zealand, and a final study (the VITAL trial) using vitamin D and omega-3 to assess cardiovascular mortality and cancer in more than 20,000 US adults with no cancer or cardiovascular diseases at study start.96–98
ConclusionsVitamin D deficiency has multiple causes, and affects not only the inhabitants of countries at latitudes above 35°–40°, where there are no ultraviolet B rays for a good part of the year, but also the inhabitants of tropical countries, people with darker skin, obese subjects, and the elderly. Other causes include pollution in big cities, as in Mexico and elsewhere, which decreases ultraviolet radiation on the skin, and clothes and sun protectors, which reduce the capacity of the skin to synthesize vitamin D.
It may thus be concluded that vitamin D deficiency is a potential independent cardiovascular risk factor and is involved in immunomodulation events which are extremely significant for inflammation and in the processes that accelerate cell proliferation (cancer). It also participates actively in the stimulation of the release of substances with antibacterial properties, cathelicidins, and defensin B4.
Crossed communication between vitamin D receptors and other LXR heterodimers such as PPARy appears to determine significant functions in lipid metabolism and insulin release, and also an indirect protective effect of β cells of the islets of Langerhans. The cumulative evidence suggests a potential role of vitamin D in heart failure.
Mexico and other countries in Latin America urgently need adequate assessment of the prevalence of vitamin D deficiency and its association with the risk of chronic diseases and cardiovascular risk, but especially of the role of vitamin D in the prevention of such conditions. 25 (OH) D levels are already a potential risk marker of great value in cardiology, but specific randomized clinical trials, some of which are ongoing, are required to ascertain if vitamin D supplementation may significantly increase cardiovascular outcomes. However, vitamin D should not be considered as a mainstay treatment for chronic diseases.
Conflict of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Rosas-Peralta M, Holick MF, Borrayo-Sánchez G, Madrid-Miller A, Ramírez-Árias E, Arizmendi-Uribe E. Efectos inmunometabólicos disfuncionales de la deficiencia de vitamina D y aumento de riesgo cardiometabólico. ¿Potencial alerta epidemiológica en América? Endocrinol Diabetes Nutr. 2017;64:162–173.