There is evidence that subclinical hypothyroidism is associated with infertility, miscarriage and obstetric complications. However, there is controversy regarding the optimal TSH value in women seeking pregnancy.
Current guidelines recommend that hypothyroid women with levothyroxine replacement who are planning pregnancy should optimise the dose of levothyroxine to achieve thyrotrophin (TSH) levels <2.5 mU/l, since these requirements increase in pregnancy, thus reducing the risk of TSH elevation during the first trimester.
In women with infertility, who undergo highly complex treatments and have positive thyroid autoimmunity, values of TSH <2.5 mU/l prior to fertility treatment are suggested.
Although this is a different population, these «optimal» TSH levels were also extended to euthyroid women without evidence of infertility, who are seeking pregnancy.
ObjectivesDetermine whether preconception TSH levels between 2.5 and 4.64 mIU/l are associated with adverse obstetric outcomes in euthyroid women.
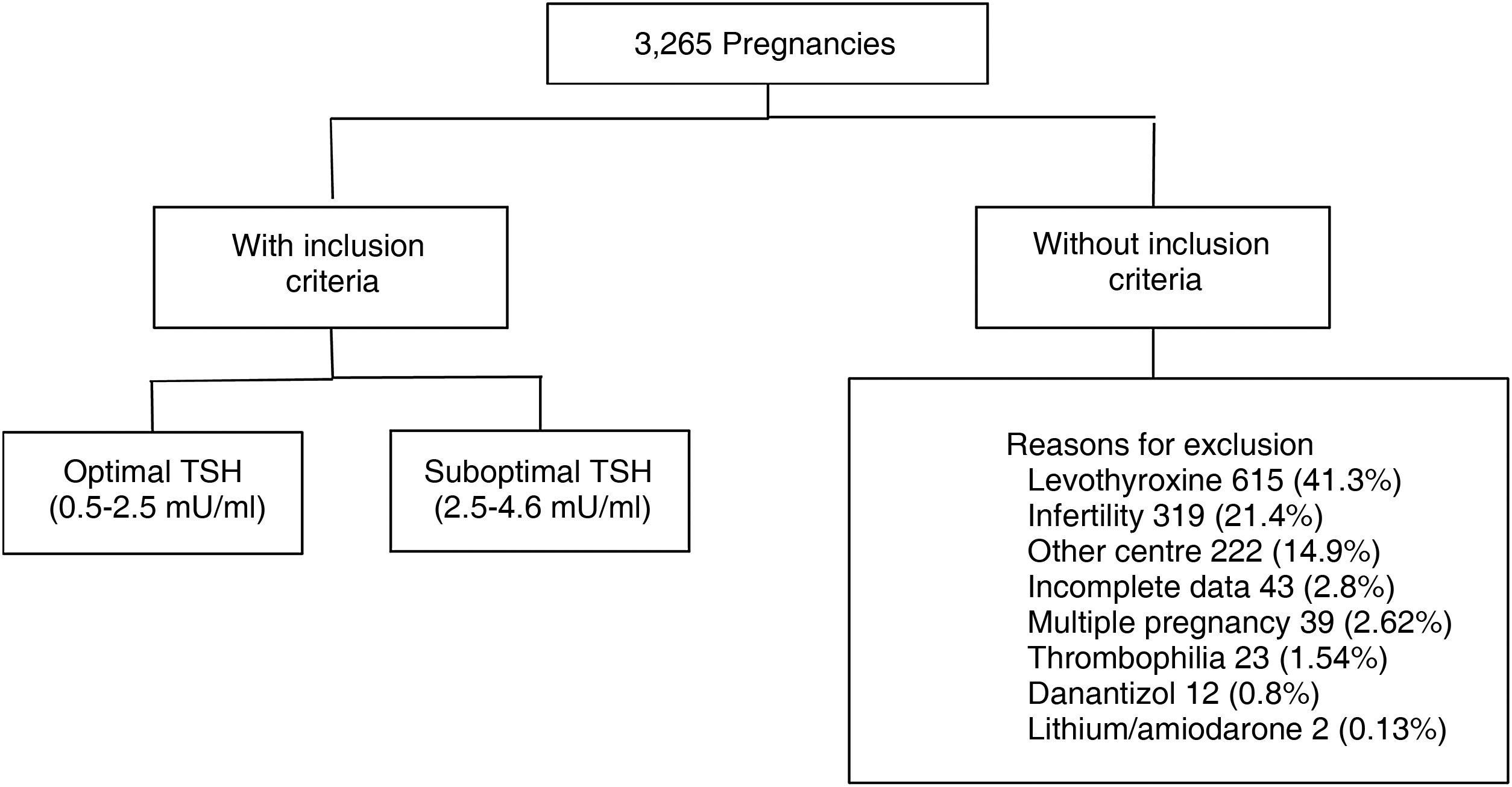
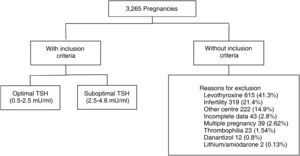
Materials and methodsRetrospective cohort study. We evaluated 3265 medical records of pregnant women aged 18–40 years, euthyroid (TSH 0.5–4.64 mU/ml), with TSH measurement at least one year before gestation. 1779 met inclusion criteria. The population was divided according to categories: TSH 0.5–2.4 mU/l (optimal) and TSH 2.5–4.6 mU/l (suboptimal). Information on maternal and fetal obstetric outcomes was collected from each group.
ResultsWe found no statistical difference in the occurrence of adverse obstetric events between the two groups. There was also no difference when adjusting for thyroid autoimmunity, age, body mass index, previous diabetes and previous arterial hypertension.
ConclusionOur results suggest that the reference range of TSH used in the general population could be used in women seeking pregnancy, even in the presence of thyroid autoimmunity. Treatment with levothyroxine should be considered only in patients with special situations.
Existe evidencia de que el hipotiroidismo subclínico se asocia con infertilidad, aborto y complicaciones obstétricas. Sin embargo, hay controversias con respecto al valor óptimo de tirotropina (TSH) en mujeres que buscan embarazo.
Las guías actuales recomiendan en mujeres hipotiroideas en sustitución con levotiroxina que planifiquen embarazo, optimizar la dosis de levotiroxina para lograr niveles de TSH<2,5 mU/L ya que los requerimientos aumentan en el embarazo y, de esta manera, se busca reducir el riesgo de elevación de TSH durante el primer trimestre.
En mujeres con infertilidad que realicen tratamientos de alta complejidad y que tengan autoinmunidad tiroidea positiva, se sugieren valores de TSH<2,5 mU/L previos al tratamiento de fertilidad.
Aunque se trate de una población diferente, estos niveles «óptimos» de TSH se extendieron también a mujeres eutiroideas, sin evidencias de infertilidad, que buscan embarazo.
ObjetivosDeterminar si los niveles preconcepcionales de TSH entre 2,5 y 4,64 mIU/L están asociados a resultados obstétricos adversos en mujeres eutiroideas.
Materiales y métodosEstudio de cohorte retrospectiva. Se evaluaron 3.265 historias clínicas de mujeres embarazadas de 18 a 40 años, eutiroideas (TSH 0,5–4,64 mU/mL), con medición de TSH al menos un año antes de la gestación. De estas pacientes, 1.779 cumplían criterios de inclusión. Se dividió a la población según categorías: TSH 0,5–2,4 mU/L (óptima) y TSH 2,5–4,6 mU/L (subóptima). De cada grupo se recabó información sobre resultados obstétricos maternos y fetales.
ResultadosNo encontramos diferencia estadística en la aparición de eventos obstétricos adversos entre ambos grupos. Tampoco se observó diferencia al ajustar por autoinmunidad tiroidea, edad, índice de masa corporal (IMC), diabetes (DBT) previa e hipertensión arterial (HTA) previa.
ConclusiónNuestros resultados sugieren que se podría utilizar el rango de referencia de TSH utilizado en población general en mujeres que buscan el embarazo, incluso en presencia de autoinmunidad tiroidea. El tratamiento con levotiroxina deberá ser considerado solo en pacientes con situaciones especiales.
There is evidence that clinical and subclinical hypothyroidism are associated with infertility, miscarriage and obstetric complications.1–4
During pregnancy, many adaptive changes take place in the thyroid axis, one of the most important being the decrease in levels of thyrotropin (thyroid-stimulating hormone [TSH]). This change is due to the action of chorionic gonadotropin (hCG) on the TSH receptor. TSH and hCG share structural homology in the B subunit, and when hCG concentrations increase (mainly in the first trimester of pregnancy), it acts at the TSH receptor level and decreases TSH synthesis due to a negative feedback mechanism. There is also an increase in thyroxine-binding globulin (TBG), with an associated increase in total T4 (TT4) values of around 50% with respect to pre-pregnancy levels from week 16. These changes are evident from week seven, with a peak at week 16, and are maintained until the end of pregnancy.1
At present, the 2017 Guidelines of the American Thyroid Association (ATA) for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum suggest having specific TSH reference values for each population. If not available, adjustments to the reference values used in the general population are recommended, reducing the lower limit by 0.4 mIU/l and the upper limit by 0.5 mIU/l; on our equipment this would equate to an upper limit of TSH of around 4 mU/l in the first trimester. Similarly, in the absence of specific reference values for TT4 during pregnancy, they also suggest a 5% increase in the upper reference limit per week from week seven and a 50% increase from week 16.1
There is debate about how representative free T4 levels are when measured during pregnancy by indirect immunoassay. This method may lose accuracy in the context of a pregnancy-related increase in TBG and decrease in albumin concentration, so specific reference values should be applied for each trimester of pregnancy.1
Thyroid autoimmunity occurs in 2%–17% of pregnant women. Current guidelines recommend that pregnant women who are positive for thyroid peroxidase or thyroglobulin antibodies should have measurement of TSH performed at the time of pregnancy confirmation and every four weeks until mid-pregnancy to rule out hypothyroidism.1
There is a large number of studies in the literature on TSH values and thyroid autoimmunity during pregnancy and their relationship with obstetric outcomes.5–7
Although they show an association between thyroid antibodies and spontaneous abortion, causality has not been demonstrated and the underlying mechanisms for such an association remain unclear.8–10
Results from studies on autoimmunity and pre-term birth have been inconsistent.8–10
The Thangaratinam et al. meta-analysis analysed five studies with 12,566 euthyroid women and evaluated the association of positive thyroid autoimmunity with pre-term delivery, plus 31 studies with 12,126 euthyroid women, evaluating its association with miscarriage. They found that positive thyroid autoimmunity tripled the risk of miscarriage in cohort studies (odds ratio [OR] 3.90, 95% CI 2.48–6.12; p < 0.001) and in case-control studies the OR was 1.80, CI 1.25–2.60; p = 0.002. When they evaluated pre-term delivery, the risk was doubled in the presence of thyroid autoimmunity (2.07, CI 1.17–3.68, p = 0.01).8
In contrast, Plowden et al. did not find a higher rate of pregnancy loss in their cohort of antibody-positive patients.11
The concept has emerged in recent years of the importance of preconception TSH levels associated with fertility treatments. So much so that the guidelines of different scientific societies (American Society of Reproductive Medicine [ASRM], published in 2015,12 the 2016 guidelines of the Federación Argentina de Sociedades de Endocrinología [FASEN] [Argentine Federation of Endocrinology Societies]13 and the ATA 2017 Guidelines)1 suggest treatment with levothyroxine in euthyroid patients undergoing highly complex fertility therapy and with pre-conception TSH levels above 2.5 mIU/ml associated with positive thyroid autoimmunity. Although with a low level of evidence, these guidelines suggest not treating women seeking spontaneous pregnancy with levothyroxine.
With new evidence available, in 2021 the European Thyroid Association published guidelines on thyroid disorders before and during pregnancy. Taking into account a recent meta-analysis by Wang et al.,14 which did not demonstrate improvement in obstetric outcomes in euthyroid subfertile women with positive autoimmunity treated with levothyroxine, they propose that all women with subfertility should have TSH and thyroid antibody tests prior to a highly complex procedure. If their TSH is >4 mIU/l they should be treated regardless of whether or not they have thyroid autoimmunity. If TSH is >2.5 mIU/l and they are positive for thyroid antibodies, treatment should be considered on a case-by-case basis, taking into account the causes of infertility and previous highly complex treatments.15
The guidelines do not, however, make recommendations on what is the “optimal” pre-pregnancy TSH value in euthyroid fertile women.
As levothyroxine requirements increase during pregnancy, the ATA 2011 guidelines recommend that women with hypothyroidism on hormone replacement who are planning pregnancy do so with optimal TSH values <2.5 mIU/l, as this reduces the risk of elevated TSH during the first trimester.16
These proposed TSH values in women with hypothyroidism began to be used in euthyroid women planning pregnancy, despite the test not having been endorsed by any of the clinical practice guidelines.
Studies analysing obstetric outcomes in relation to pre-conception TSH levels reported conflicting results.11,17
In the light of this debate, we aimed to find out how appropriate the indication is for pre-pregnancy levothyroxine in fertile women with “suboptimal” TSH, while determining whether TSH levels are associated with adverse obstetric outcomes.
Primary endpointTo determine whether or not pre-conception TSH levels from 2.5 to 4.64 mU/l are associated with adverse obstetric outcomes (miscarriage or pre-term delivery).
Secondary endpoints- 1.
To determine whether or not pre-conception TSH levels from 2.5 to 4.64 mU/l are associated with other maternal and fetal obstetric risks such as gestational diabetes (DBT), fetal macrosomia, low birth weight and gestational hypertension (HT)/pre-eclampsia.
- 2.
To establish whether this risk is dependent or independent of being positive for peroxidase antibodies.
Design: retrospective cohort study of pregnant women aged 18–40 assessed at the Hospital Italiano de Buenos Aires from 1 January 2010 to 31 December 2015, who were euthyroid (TSH 0.5–4.64 mU/ml), with TSH measurement result at least one year before pregnancy from the hospital’s Endocrinology laboratory. The population was divided according to TSH category: TSH 0.5–2.4 mU/l (optimal) and TSH 2.5–4.6 mU/l (suboptimal). Information was collected from each group on maternal and fetal obstetric outcomes.
Sphere: Hospital Italiano de Buenos Aires is a private high-complexity university hospital which cares for a middle social class population, with healthcare coverage from work-related or private medical insurance in the Autonomous City of Buenos Aires and the Greater Buenos Aires area, in the province of Buenos Aires, Argentina.
We included women with positive beta subunit values according to the data obtained from the record in the Hospital Information System or from the medical consultation for their pregnancy at the Obstetrics Department.
The patients had all their pregnancy monitoring, and gave birth or had the baby delivered by Caesarean section, at Hospital Italiano de Buenos Aires.
The following patients were excluded: those who were taking levothyroxine, lithium or amiodarone at the time of the analyses or had done so in the previous six months; those who had been indicated levothyroxine while trying to become pregnant or during their pregnancy; patients with pituitary disease which prevented the determination of TSH as a marker of hypothyroidism; patients who had other causes of obstetric complications such as thrombophilia; or those who had consulted for infertility and whose pregnancy was in the context of fertility treatment of low or high complexity or egg donation.
All the information to determine inclusion and exclusion criteria, as well as the outcome variables, was collected by manual review of the electronic medical records, the only repository of information on all healthcare interventions at our institution.
Outcome variables:
Miscarriage: pregnancy loss before week 20.
Pre-term birth: end of pregnancy before week 37.
Low birth weight: weight <2500 g at birth.
Fetal macrosomia: weight >4000 g at birth.
Gestational DBT: blood glucose abnormality diagnosed during pregnancy by two fasting blood glucose readings >100 or oral glucose tolerance test (OGTT) with blood glucose >140 at 120 min.
Gestational hypertension: blood pressure (BP) >130/80 mmHg with onset during pregnancy.
Pre-eclampsia: proteinuria associated with hypertension during pregnancy.
Exposure variablesTSH: the TSH value was collected as a continuous quantitative variable which was categorised as TSH 0.5–2.4 mU/l (optimal) and TSH 2.5–4.6 mU/l (suboptimal). We took the TSH test result closest to, in the year prior to, the pregnancy episode.
Thyroid antibodies: we categorised the determination of thyroid antibodies as requested or not requested. In those who had been tested for thyroid antibodies, we recorded whether they were positive or negative for autoimmunity.
Other variablesGeneral data were recorded: age, weight and height at the time of pregnancy; and body mass index (BMI). We also recorded maternal history: HT prior to pregnancy (BP >140/90 mmHg prior to pregnancy with or without medication); pre-pregnancy DBT; number of pregnancies; and previous spontaneous abortions.
Fetal/neonatal outcomes were collected: live newborn; gestational age; newborn weight; need for admission to neonatal unit if the newborn was not admitted to the ward with the mother.
Statistical analysisThe association between TSH values and obstetric complications was evaluated by multiple logistic regression adjusting for age, BMI, previous history of miscarriage, HT or DBT, and we evaluated the interaction between TSH values and each of the risk factors. We also analysed the association between the TSH level and adverse obstetric outcomes (miscarriage and pre-term delivery), and tested the interaction with TSH values using multiple logistic regression.
Coefficients and adjusted OR are shown with their 95% confidence interval (95% CI). When the interaction was significant, the OR were calculated by strata with their 95% CI using the Mantel-Haenszel method. Statistical significance was considered with a p-value less than 0.05.
Quantitative variables are expressed as mean and standard deviation (SD) and median and interquartile range (IQR) 25%–75% according to the normality of the data, which was tested graphically and with the Shapiro–Wilk test. Categorical variables are expressed as absolute and relative frequency.
For comparisons between optimal and suboptimal TSH groups, we used Student’s t test or the Mann–Whitney U test for the quantitative variables according to their distribution and X2 or Fisher’s exact test for the qualitative variables according to assumptions.
The analysis was performed with the software RStudio Version 1.1.383 – © 2009–2017 RStudio, Inc.
Ethical considerationsThe study was approved by the Hospital Italiano de Buenos Aires ethics committee. It was carried out fully in accordance with current national and international regulations: World Medical Association Declaration of Helsinki and ICH E6 Good Clinical Practice.
ResultsWe analysed the medical records of 3265 pregnant women with TSH measurement within the year prior to becoming pregnant. A total of 1779 met the inclusion criteria (Fig. 1).
Of these patients, 80.5% had a TSH value from 0.5 to 2.4 mIU/l (optimal) and 19.5% from 2.60 to 4.5 mIU/l (suboptimal). The median pre-conception TSH was 1.82 mIU/ml. We found a miscarriage rate of 13.5%, pre-term delivery rate of 7.7% and 3.4% had gestational DBT. Of the total, 513 patients (28.8%) were tested for thyroid antibodies (of whom 13.2% were positive).
We compared the two groups in terms of thyroid autoimmunity. Among the women who had suboptimal TSH, a higher percentage were positive for thyroid antibodies. Analysing other previous medical history in these groups, no difference was found between the patients with suboptimal TSH and those with optimal TSH in terms of age, BMI, previous pregnancy loss, HT or DBT (Table 1).
General characteristics of all the patients and by group according to TSH value.
| Variable general characteristics | Total (n = 1779) | Optimal TSH (n = 1432) | Suboptimal TSH (n = 347) | p Value |
|---|---|---|---|---|
| Agea | 32.6 (4.6) | 32.7 (4.63) | 32.5 (4.7) | 0.490 |
| BMIa | 24.2 (4.5) | 24.2 (4.5) | 24.1(4.3) | 0.866 |
| History of spontaneous abortionb | 290 (16.3) | 228 (15.9) | 62 (17.8) | 0.379 |
| HT pre-pregnancyb | 14 (0.8) | 12 (0.8) | 2 (0.6) | 0.637 |
| DBT pre-pregnancyb | 15 (0.8) | 10 (0.7) | 5 (1.4) | 0.189 |
| Multiparityb | 757 (42.5) | 602 (42) | 155 (44.6) | 0.374 |
| Positive thyroid autoimmunityb | 68 (13.2) | 36 (8.7) | 32 (30.7) | <0.001 |
DBT: diabetes; HT: hypertension; BMI: body mass index; TSH: thyroid stimulating hormone.
In our population, we did not find a statistically significant difference in adverse obstetric events (miscarriage, pre-term delivery, low birth weight, gestational DBT, gestational hypertension, fetal macrosomia or cholestasis) in women with pre-conception TSH of 2.5–4.64 mU/l, compared to those with TSH in the range 0.5–2.4 mU/l (Table 2). Nor did we find an increase in adverse obstetric outcomes when adjusting for thyroid autoimmunity, age, BMI, previous DBT and previous HT (Tables 3 and 4).
Obstetrics results: total population and by group according to TSH value.
| Variable | Total (n = 1779) | Optimal TSH (n = 1432) | Suboptimal TSH (n = 347) | p Value |
|---|---|---|---|---|
| Results | ||||
| Spontaneous abortiona | 240 (13.5) | 189 (13.2) | 51 (14.7) | 0.440 |
| Pre-term birtha | 117 (7.6) | 94 (7.6) | 23 (7.8) | 0.881 |
| Gestational HTa | 55 (3.1) | 45 (3.2) | 10 (2.9) | 0.836 |
| Pre-eclampsiaa | 26 (1.5) | 22 (1.2) | 4 (1.5) | 0.614 |
| Gestational DBTa | 57 (3.3) | 41 (3) | 16 (4.9) | 0.080 |
| Cholestasisa | 27 (1.7) | 19 (1.5) | 8 (2.7) | 0.159 |
| Fetal macrosomia | 113 (7.4) | 89 (7.2) | 24 (8.2) | 0.541 |
| Low birth weighta | 90 (5.9) | 72 (5.2) | 18 (6.2) | 0.860 |
DBT: diabetes; HT: hypertension; BMI: body mass index; TSH: thyroid stimulating hormone.
Miscarriage adjusted for TSH level, positive autoimmunity, age, BMI, DBT pre-pregnancy and HT pre-pregnancy (multivariate analysis).
| Miscarriage | OR | 95% CI | p Value |
|---|---|---|---|
| Suboptimal TSH | 1.25 | 0.66−2.36 | 0.478 |
| Positive for thyroid antibodies | 0.70 | 0.31−1.59 | 0.4 |
| Age | 1.08 | 1.01−1.15 | 0.12 |
| BMI | 1.01 | 0.92−1.04 | 0.595 |
| DBT pre-pregnancy | 1.44 | 0.30−6.91 | 0.644 |
| HT pre-pregnancy | 1.61 | 0.16−15.44 | 0.679 |
DBT: diabetes; HT: hypertension; BMI: body mass index; OR: odds ratio; TSH: thyrotropin.
Pre-term birth adjusted for TSH level, positive autoimmunity, age, BMI, DBT pre-pregnancy, and HT pre-pregnancy (multivariate analysis).
| Pre-term birth | OR | 95% CI | p Value |
|---|---|---|---|
| Suboptimal TSH | 0.57 | 0.21–1.52 | 0.265 |
| Positive for thyroid antibodies | 2.11 | 0.859–5.19 | 0.103 |
| Age | 1.05 | 0.97–1.14 | 0.204 |
| BMI | 1.02 | 0.94–1.1 | 0.55 |
| DBT pre-pregnancy | 3.18 | 0.628–16.16 | 0.162 |
| HT pre-pregnancy | 4 | 0.374–43.47 | 0.250 |
DBT: diabetes; HT: hypertension; BMI: body mass index; OR: odds ratio; TSH: thyrotropin.
Analysis of the subgroup of patients who had been tested for thyroid antibodies showed no increase in the rate of adverse obstetric events in relation to being positive for autoimmunity, even adjusting for age, BMI, prior DBT and prior hypertension.
DiscussionIn our study we found no difference between patients with optimal TSH and those with suboptimal TSH in the number of adverse obstetric events. Given the retrospective design of the study, we were unable to follow up TSH levels during pregnancy to establish how thyroid function evolved and determine the impact of those values on obstetric outcomes, although we did only include women who did not take levothyroxine during their pregnancy.
The negative impact of clinical hypothyroidism on conception and pregnancy (infertility, increased risk of miscarriage, premature birth, low IQ) is well known. However, the impact of subclinical hypothyroidism is less clear.
Some authors propose lowering the cut-off value of TSH in women planning pregnancy to <2.5 mU/l, primarily for infertile women undergoing fertility treatment or with a history of recurrent miscarriages. However, in the general population of fertile women, there is insufficient evidence of TSH values >2.5 mU/l having any implications for obstetric outcomes.
Findings on the impact of pre-conception TSH values are mixed. Some authors report no significant differences when comparing euthyroid women with values above or below 2.5 mU/ml.18–21
Seungdamrong et al. analysed 1468 euthyroid infertile patients, who were divided into two groups, according to TSH <2.5 mU/l or >2.5 mU/l. They also assessed the presence of antibodies. They found that pre-conception TSH values >2.5 mU/l were not a risk factor for adverse obstetric outcomes, unlike being positive for autoimmunity, which was associated with an increased miscarriage rate and a lower live birth rate.22
Plowden et al. found no association between the pregnancy, miscarriage and live birth rates in healthy women and TSH >2.5 mU/l or being positive for thyroid autoimmunity.11
In contrast, the Chen et al. cohort study evaluating 62,715 healthy euthyroid women from 30 provinces in China reported that the highest pre-conception TSH values, even when normal for non-pregnant women, are associated with a greater risk of miscarriage (OR 1.10; 95% CI, 1.03–1.18) and pre-term delivery (OR, 1.09; 95% CI, 1.04–1.15).10
In recent years, several population studies evaluating pre-conception TSH values have been published, with large numbers of patients. Most of these studies were carried out in China, where thyroid antibodies are not measured in screening, and did not therefore analyse the impact of thyroid autoimmunity on obstetric outcomes.
Yang et al. analysed 5,840,894 women during the Chinese National Free Pre-Pregnancy Checkups Project. They assessed pre-conception TSH values and related them to obstetric outcomes. The groups with low values (<0.10 and 0.10−0.37 mIU/l) and high values (4.88–9.99 mIU/l and >10 mIU/l) had a higher risk of pre-term delivery, low weight for gestational age and perinatal death; no differences were found in the groups in the ranges 0.37–2.49 mIU/l and 2.5–4.87 mIU/l.23
They also assessed the risk of miscarriage, including 4,678,679 pregnancies in the analysis. The groups with pre-conception TSH >2.5 mIU/l showed a higher risk compared to the group with levels at 0.37–2.49 mIU/l (TSH 4.88–9.99 mIU/l: OR, 1.33; 95% CI, 1.28–1.38; TSH 10.00 mIU/l: OR, 1.5; 95% CI, 1.14–1.36).24
The relevance of these studies lies in the evaluation of pre-pregnancy TSH values in a large number of women from the general population. However, they have several limitations, which are raised by the authors themselves in the discussion sections. One such limitation is that they do not have thyroid antibody results (the authors state that thyroid antibodies are not measured in routine pre-pregnancy tests in China); they only had their TSH level tested, not excluding patients who were on levothyroxine treatment, before or during pregnancy.
The above results differ from ours with respect to the miscarriage risk, but we have to take into account that the population belongs to a different ethnic group, and so the TSH reference values may not be comparable with our population.
We believe it is important that, although we did not have thyroid antibody results for all the patients in our study, in the group of patients in whom thyroid antibodies had been measured, we found no increase in the rate of adverse obstetric events.
Consulting the data on adverse obstetric events in Argentina (according to the National Ministry of Health), the prevalence of pre-term birth (<37 weeks) is 8%, similar to the figures for both groups in our population: optimal TSH (7.6%); and suboptimal TSH (7.8%).
Although our study is retrospective, we analysed a significant number of women with TSH in the normal pre-pregnancy range who were not on hormone replacement with levothyroxine either before or during pregnancy. The electronic medical records were reviewed manually to ensure the accuracy of these data.
When reviewing the medical records, we ruled out confounding factors, such as the patient having thrombophilia or taking medication, such as levothyroxine, methimazole, amiodarone or other drugs which could affect thyroid function.
Unlike the population studies, in which the number of women is very large and has a strong impact on the results, the lack of information on factors such as antibody levels, the taking of thyroid function-modifying medication, and others which increase the risk of miscarriage or pre-term delivery means that these studies have a lot of bias.
The value of our work lies in the good selection of patients included, as we removed confounding factors which could have caused selection or information bias.
ConclusionsIn our population of euthyroid pregnant women, we did not find a higher prevalence of miscarriage or adverse obstetric effects in patients with pre-conception TSH in the range 2.5–4.64 mU/l compared to patients with pre-conception TSH levels 0.5–2.49 mU/l, even when adjusting the data for age, BMI, DBT or prior HT. Nor did we find a higher prevalence of these events in patients positive for thyroid autoimmunity.
Based on the evidence published to date, and the results of our study, we believe that the same TSH reference range used for the general population should be considered in women planning a pregnancy, even if they have thyroid autoimmunity. As other results have reported conflicting results, the history of each patient should be taken into account and the decision made to treat with levothyroxine only in special situations, such as previous miscarriage or adverse obstetric outcomes in previous pregnancies.
Conflicts of interestThe authors declare that they have no conflicts of interest.