A 45-year-old woman with grade IV obesity was subjected in 2010 to Roux-en-Y gastric bypass surgery with a common loop of 270cm. After surgery, she presented deficient levels of iron, vitamin B12, vitamins D and A, zinc and calcium, which required oral supplementation. She achieved a weight loss of 40kg (previous weight: 130kg, current weight: 90kg), with a current body mass index (BMI) of 34kg/m2.
Referral to Haematology was required to start intravenous iron supplementation in October 2016, despite oral supplementation at maximum doses. Iron saccharose was initially prescribed (20mg/ml monthly), but in view of the scant improvement in the iron profile, treatment with iron carboxymaltose 50mg/ml monthly was introduced starting in August 2017. After 5 doses, she presented severe hypophosphatemia with a phosphorus concentration of 0.9mg/dl (reference range 2.3–4.7mg/dl), which was incidentally detected in the laboratory tests. The other results showed vitamin D: 56ng/ml (30–100ng/ml); PTH: 179pg/ml (11.10–79.50pg/ml) similar to previous tests; plasma calcium corrected for albumin: 8.7mg/dl (8.4–10.2mg/dl); magnesium: 2mg/dl (1.7–2.8mg/dl); plasma creatinine: 0.5mg/dl (0.5–1.1mg/dl); and 24-h urine phosphorus: 1500mg/24h. She presented an adequate nutritional status with plasma proteins: 6.6g/dl (6.4–8.3g/dl); and albumin: 3.9g/dl (3.5–5.0g/dl). The patient was receiving no drug treatment capable of accounting for the condition, such as diuretics, and experienced no vomiting or diarrhoea.
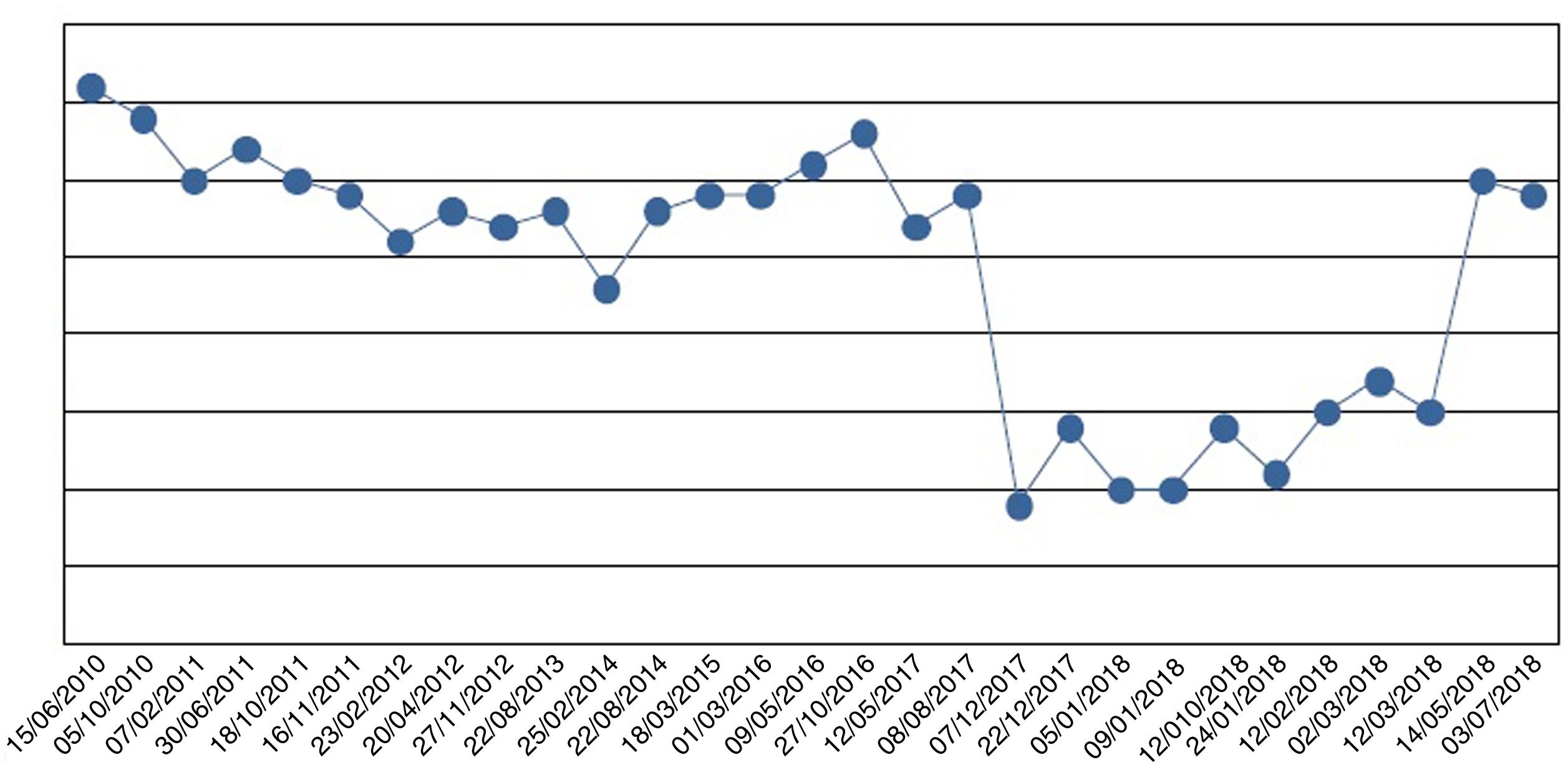
We started oral phosphate supplementation in the form of sodium phosphate up to a dose of 4000mg of phosphorus ion/day (5sachets/day). Because of her intestinal malabsorption secondary to bariatric surgery, the improvement in plasma phosphorus levels was very slow (Fig. 1). After four weeks of severe hypophosphatemia, the patient developed symptoms of asthenia and weakness, with generalized muscle pain that gradually improved as the phosphorus levels normalized.
Oral phosphate supplementation was required for 8 months at decreasing doses until final discontinuation. Subsequently, the patient did not require reintroduction of the drug, and her plasma phosphorus levels remain within normal ranges.
Her iron profile improved considerably after the administration of iron carboxymaltose. After 10 months, she maintains adequate levels without oral or intravenous supplementation.
The incidence of iron deficiency in patients subjected to bariatric surgery ranges from 30% to 50%, and is most common in women of childbearing age, in patients with iron deficiency before surgery, and in procedures involving a malabsorptive component.1
Hypophosphatemia is an adverse effect which should be taken into account after the administration of different forms of intravenous iron. It has been reported after the administration of iron saccharose and carboxymaltose, but not after iron dextran.2 The incidence of hypophosphatemia in different studies has been estimated at 41–70%.3 In patients with inflammatory bowel disease it is the most common complication.4 Specifically, the Summary of Product Characteristics of iron carboxymaltose describes this adverse effect in up to 27% of all cases.
The etiopathogenesis appears to be a decrease in phosphate reabsorption and in 1-α hydroxylation of vitamin D mediated by an increase in fibroblast growth factor-23 (FGF-23) that reaches a maximum in the first week after administration, and can last for up to 5 weeks. The mechanism underlying the increase in FGF-23 is not fully understood. It has been postulated that intravenous iron may produce a nephrotoxic effect upon renal cells or a specific proximal tubule effect in relation to phosphate homeostasis.3,5 It is also believed that rapid phosphate ion influx to the cell, stimulated by an increase in erythropoiesis after iron therapy, may by a leading causal factor.6,7
The symptoms are nonspecific, comprising muscle pain and weakness, bone pain and confusion. Our patient developed muscle weakness after four weeks of hypophosphatemia, but the condition gradually improved with treatment.
Within the differential diagnosis of hypophosphatemia with increased phosphate excretion, as in our case, consideration is required of hyperparathyroidism, oncogenic osteomalacia, Fanconi syndrome, and drugs such as acetazolamide or tenofovir. Our patient presented hyperparathyroidism secondary to vitamin D deficiency (corrected at the time of diagnosis) and intestinal malabsorption characteristic of gastric bypass surgery. However, she had not experienced a worsening coinciding with the appearance of hypophosphatemia; the condition therefore did not appear to be justified. All other possible causes were ruled out, based on the clinical history.
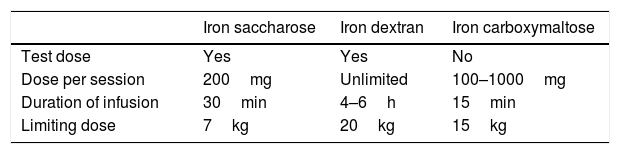
Efficacy, but also the adverse effects and costs should be taken into account when choosing the intravenous iron formulation. Iron saccharose offers adequate clinical tolerance, but requires several infusions not exceeding 200mg (with a maximum of 600mg/week) administered over 30min, in order to avoid toxicity. Other molecules such as low molecular weight iron dextran, iron carboxymaltose, or iron isomaltoside allow for the administration of higher intravenous iron doses, thereby fully resolving the iron deficiency with a single infusion1,8,9 (Table 1). Iron carboxymaltose is one of the newest formulations. Malone et al. reported superior efficacy with this preparation in 123 bariatric surgery patients. Similar findings have also been found in other diseases characterized by iron deficiency. The possibility of fully resolving iron deficiency with a single injection, together with its efficacy, has confirmed iron carboxymaltose as a cost-effective management option.1,10,11
In our patient, the administration of iron carboxymaltose was decided upon because of the initial lack of efficacy of oral iron and subsequently of iron saccharose via the intravenous route. Both were well tolerated, but severe hypophosphatemia only occurred with iron carboxymaltose.
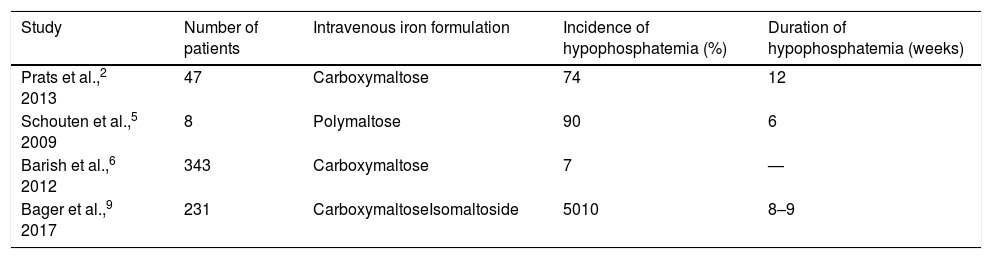
As regards the incidence of hypophosphatemia, very different values are found in the literature (Table 2).
Comparison of the incidence of hypophosphatemia after the administration of different intravenous iron formulations.
| Study | Number of patients | Intravenous iron formulation | Incidence of hypophosphatemia (%) | Duration of hypophosphatemia (weeks) |
|---|---|---|---|---|
| Prats et al.,2 2013 | 47 | Carboxymaltose | 74 | 12 |
| Schouten et al.,5 2009 | 8 | Polymaltose | 90 | 6 |
| Barish et al.,6 2012 | 343 | Carboxymaltose | 7 | — |
| Bager et al.,9 2017 | 231 | CarboxymaltoseIsomaltoside | 5010 | 8–9 |
In conclusion, although the incidence of hypophosphatemia after intravenous iron administration has not been fully established, it is not negligible, and we therefore recommend phosphorus measurement in patients receiving intravenous iron, particularly in those who develop muscle weakness and pain.
Please cite this article as: Gómez Rodríguez S, Castro Ramos JC, Abreu Padín C, Gómez Peralta F. Hipofosfatemia severa por hierro intravenoso en paciente intervenida de bypass gástrico. Endocrinol Diabetes Nutr. 2019;66:340–342.