Bullous pemphigoid is an acquired autoimmune disease characterized by the production of IgG antibodies targeted to hemidesmosome proteins of the epidermal basal membrane (antigens BP180 and BP230). It primarily affects the elderly, and clinically manifests as subepidermal blisters.1 While there is a long list of both physical agents and drugs related to the pathogenesis of bullous pemphigoid, in many cases the causal agent cannot be identified. However, the diagnosis should be suspected when bullous lesions appear in a patient who has recently started to receive a new drug.
In recent years, cases of bullous pemphigoid have been reported in association with the administration of DPP4 inhibitors in diabetic patients, mainly vildagliptin and sitagliptin,2–5 with the implication of linagliptin in exceptional cases.3–6 A recent article by Kridin and Bergman found treatment with vildagliptin to increase the risk of bullous pemphigoid (odds ratio [OR]: 9.28; 95% confidence interval [95%CI]: 4.54–18.99) versus linagliptin (OR: 6.61; 95%CI: 2.28–19.17).5
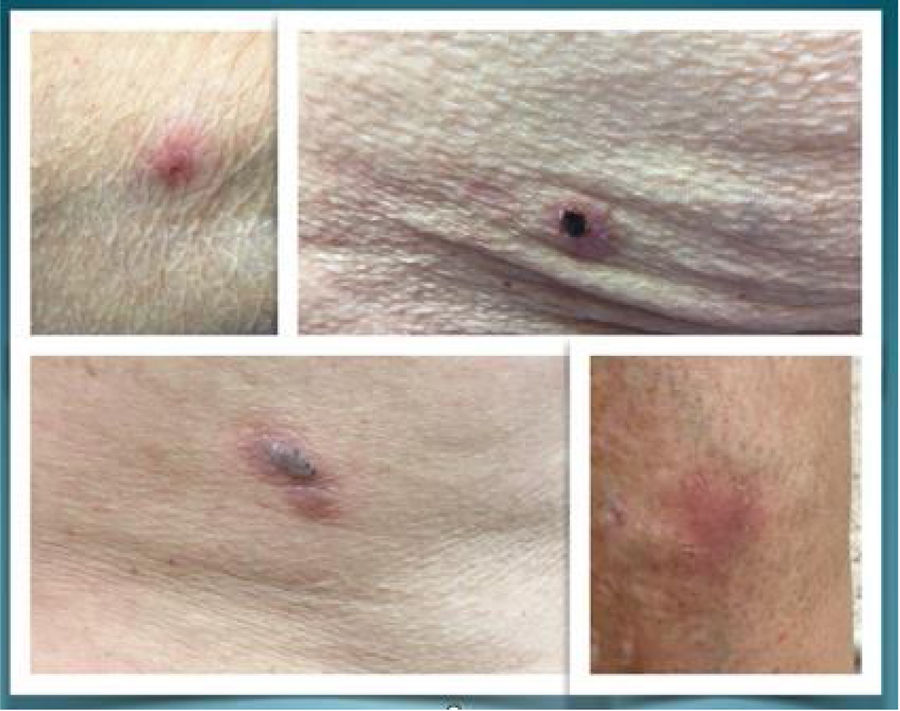
We present three cases of bullous pemphigoid associated with the use of linagliptin in patients with type 2 diabetes (DM2) and chronic kidney disease (CKD) secondary to diabetic nephropathy (expressed in glomerular filtration [G] and albuminuria stages [A]) (Fig. 1).
Case 1. An 84-year-old male presented with a history of arterial hypertension (AHT), DM2, mixed dyslipidemia, hyperuricemia with gout, and left nephrectomy secondary to renal carcinoma. There was no evidence of diabetic retinopathy or neuropathy, and no macrovascular complications. The latest recorded HbA1c value was 6.7%. The patient was receiving antidiabetic treatment with metformin 1000mg/24h and repaglinide 0.5mg/24h. After the diagnosis of chronic kidney disease (G4A3), metformin was discontinued and linagliptin was started at a dose of 5mg/day. Six months later, the patient developed bullous lesions on the upper extremities and trunk, and after evaluation two weeks later by Dermatology, a clinical diagnosis of bullous pemphigoid was established. Prednisone 30mg was administered and linagliptin was discontinued, with complete resolution after three weeks.
Case 2. An 83-year-old woman presented with a history of AHT, DM2, obesity, mixed dyslipidemia, hyperuricemia without gout, and no other known disease. There was no evidence of diabetic retinopathy or neuropathy, and no macrovascular complications; HbA1c 7.2%. The patient had been receiving antidiabetic treatment with metformin 850mg/12h and vildagliptin 50mg/12h over the previous two years. After establishing a diagnosis of chronic kidney disease (G4A2), metformin was discontinued and vildagliptin was replaced by linagliptin 5mg/24h. One month later she reported to the emergency room due to blister lesions on her limbs. With a clinical diagnosis of possible bullous pemphigoid, oral prednisone was started at a dose of 30mg/day. One month later she was evaluated by Dermatology, with confirmation of the diagnosis of bullous pemphigoid; treatment with prednisone was maintained at a dose of 15mg/day, with the addition of a topical corticosteroid and a cycle of oral doxycycline. At examination two months later, the pemphigoid outbreak was seen to persist, and linagliptin was consequently discontinued, with the introduction of repaglinide. One month later, following considerable clinical improvement, prednisone was discontinued. The clinical condition was seen to have resolved at evaluation two months later.
Case 3. A 78-year-old woman presented with a history of AHT, DM2, chronic kidney disease (G4A2) and non-obstructive hypertrophic cardiomyopathy. There was no evidence of diabetic retinopathy or neuropathy, and no macrovascular complications. The patient consulted due to poor metabolic control, with persistent HbA1c >8.5%. Linagliptin was therefore added to her antidiabetic therapy (insulin glargine 72IU/24h and repaglinide 1mg/8h). After four months she consulted her primary care physician due to the development of pruritic bullous lesions over an erythematous base distributed over her entire body surface, starting two weeks before. She was referred to Dermatology, with confirmation of the diagnosis of bullous pemphigoid probably triggered by linagliptin. No biopsy was obtained. Linagliptin was discontinued after one month and a short cycle of topical and systemic corticosteroids (prednisone 10mg via the oral route in a tapered dosing regimen) was added, followed by the disappearance of the lesions in one month.
In our three patients we observed a time relationship between linagliptin administration and the development of bullous pemphigoid, as well as the complete clearance of the lesions after drug discontinuation (one week to one month, i.e., similar to previous studies).7 In addition, in all the cases the absence of disease recurrence was found at re-evaluation months after treatment discontinuation.2
The physiopathological mechanism whereby patients treated with DPP4 inhibitors develop pemphigoid is unclear, though gliptins are known to have multiple biological actions. Many types of skin cells, including keratinocytes, express DPP4, which participates in cytokine production, tissue differentiation and collagen metabolism. In addition, DPP4 inhibitors could also increase dermal eosinophil recruitment mediated by the CCL11/eotaxin-2 chemokine.2 All these properties could favor the appearance of bullous pemphigoid in susceptible individuals, either by modifying the immune response and/or by altering the antigenic properties of the epidermal basal membrane.6
To date, there is no evidence that patients with DM2 and chronic kidney disease due to diabetic nephropathy are more susceptible to developing bullous pemphigoid or producing autoantibodies against BP180.8 It should be noted that in patient 2, who had been treated with vildagliptin for two years, renal functional impairment and a change in DPP4 inhibitor triggered the clinical condition, though it is not discarded that she may have been previously immunized after treatment with vildagliptin. Although we have found no justification in the reviewed literature, we postulate that the worsened renal function may have influenced the appearance of this clinical condition.
These three cases confirm that bullous pemphigoid induced by DPP4 inhibitors should be considered as a class adverse effect, though further studies are needed to establish an unequivocal causal relationship.7
AuthorshipBelén Sánchez López-Muelas: principal author. Salomé Muray-Cases, Fátima Illán-Gómez, Gloria García-Guzmán, María Elena Arjonilla-Sampedro: participation in study conception, data acquisition and review of the manuscript draft.
Please cite this article as: Sánchez López-Muelas B, Muray Cases S, Illán Gómez F, García Guzmán G, Arjonilla Sampedro ME. Penfigoide ampolloso asociado al tratamiento con linagliptina en pacientes con diabetes y enfermedad renal crónica. Endocrinol Diabetes Nutr. 2019;66:338–339.