Bullous pemphigoid (BP) is an acquired, autoimmune bullous and subepidermal disease characterized by a humoral and cellular response against hemidesmosomes.1 There are cases of drug-related BP, and in this regard dipeptidyl peptidase-4 inhibitors (DPP4i) have recently been reported as being a cause of the disease.2 We present a case of BP associated with vildagliptin.
A 91-year-old male with no known drug allergies presented with a history of Alzheimer's type dementia and type 2 diabetes mellitus (DM2) starting over 20 years ago, with no associated macro- or microangiopathic complications. Treatment was prescribed in the form of metformin 850mg/12h. Vildagliptin 50mg/12h was added to his antidiabetic therapy starting in June 2018. The patient was receiving no other type of drug treatment.
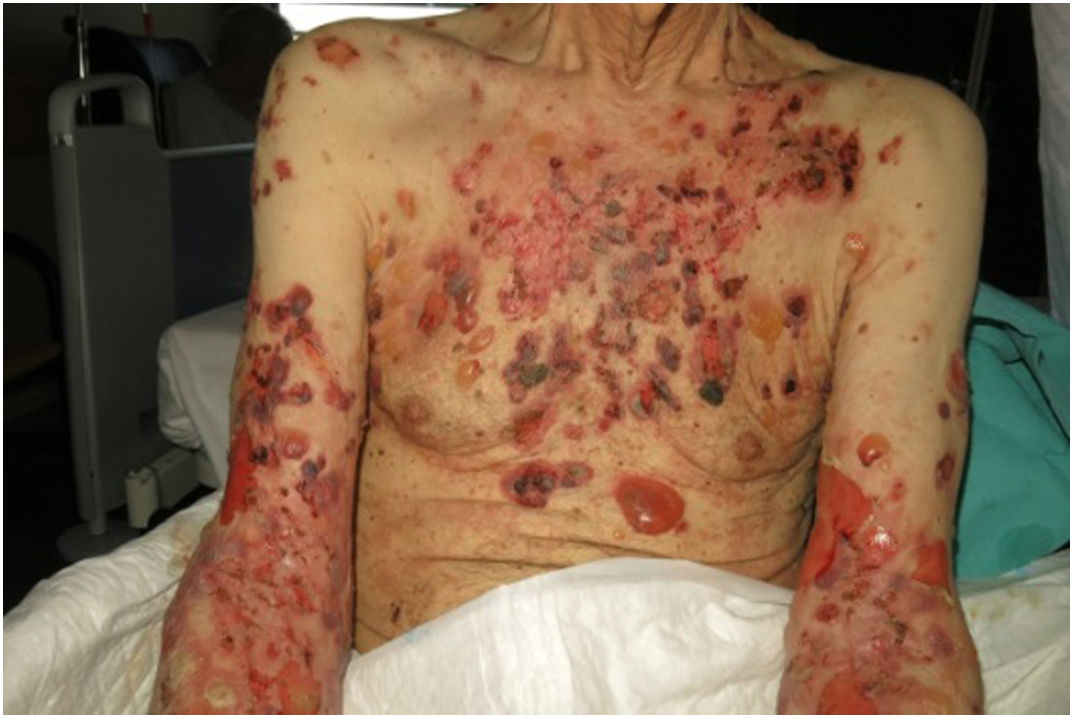
He was admitted to Dermatology in August 2018 due to tense, bullous pruriginous lesions with an erythematous base, located on the trunk and extremities (Fig. 1), with a marked skin fold involvement. There were no mucosal or systemic alterations. The patient proved negative for desmoglein and dermoepidermal-binding antibodies, with positive direct immunofluorescence for IgG, C3 and fibrinogen at basal membrane level.
Endocrinology was consulted regarding treatment adjustment. Due to suspected bullous pemphigoid secondary to vildagliptin, the latter drug was discontinued. During hospitalization, the patient presented an HbA1c concentration of 7.3%. The skin lesions gradually resolved after 6 days of Urbasón (methylprednisolone) 60mg/day via the oral route, and blood glucose was controlled with NPH insulin (10 units at lunchtime). At discharge, treatment was provided with metformin 850mg every 12h, insulin NPH (10 units at lunchtime), prednisone 50mg and Imurel (azathioprine) 50mg every 24h. Corticosteroid dosing was subsequently lowered over 5 weeks, with the suspension of Imurel; there was no relapse of the skin lesions.
Dipeptidyl peptidase-4 inhibitors (DPP4i) are a marketed drug class approved for the treatment of DM2, either alone or in combination (usually with metformin). These drugs increase endogenous GLP1 and postprandial GIP secretion, due to DPP4 inhibition, reducing glucagon secretion and increasing insulin secretion, resulting in lowered postprandial glycemia.2 Dipeptidyl peptidase-4 is a glycoprotein expressed on cell surfaces throughout the body, and its inhibition alters the antigenic properties of the epidermal basal membrane.3
The first marketed gliptin was sitagliptin (in 2006). The association between DPP4i and BP was not known at that time, though preclinical studies of vildagliptin described the appearance of necrotic lesions in monkeys.4
During the years following the marketing authorization of these drugs, case reports and pharmacovigilance analyses revealed an association between their use and the risk of BP.
In 2011, Pasmatzi et al. described two cases of BP in diabetic patients treated for two months with vildagliptin and metformin.5
In 2012, Skandalis et al. reported 5 cases of BP after 2–13 months of treatment with vildagliptin (4 cases) and sitagliptin (one case) in combination with metformin.6
Béné et al. in turn reported three cases of BP in patients using vildagliptin in different drug combinations (2 with metformin and another with sulfonylurea). All three patients showed BP regression following the withdrawal of DPP4i, despite the continuation of metformin. For this reason, and since metformin has been marketed for over 20 years with no reports of associated BP, a causal relationship between pemphigoid and metformin appears unlikely.7
In response to these publications and regarding the safety of DPP4i, the European Medicines Agency (EMA) introduced a BP alert in the summaries of the product characteristics of these drugs.8
In a cohort of 168,774 patients, Douros et al. found the use of DPP4i (mainly linagliptin and vildagliptin) to be associated with at least a two-fold rise in the risk of BP compared with the use of other second- or third-line drugs.9
Kridin et al. reported a greater association with BP in males and individuals under 70 years of age. These authors observed no increased risk with the administration of metformin.10
As of 6 July 2019, the EudraVigilance database documents 641 suspected cases of BP associated with the use of vildagliptin. Two hundred and seventy-seven cases are in the resolution period and 138 cases have resolved without sequelae.
The results of the literature review show that BP is an adverse effect probably shared by all types of DPP4i. The diagnosis of BP is established from both the clinical and the histopathological findings. In these patients, an early diagnosis and treatment discontinuation result in an improved prognosis and lower mortality.9
Lastly, it can be concluded that the aging of the population and the prescription of combination drug therapies result in the increasingly widespread use of DPP4i. These drugs modify the antigenic response of the epidermal basement membrane, favoring the appearance of BP.
Early diagnosis and prompt drug suspension are important, since they improve the prognosis.
Bullous pemphigoid is a type of adverse reaction with an unknown frequency in the summary of product characteristics. When suspected, reporting to the pharmacovigilance system is therefore advisable.
Please cite this article as: González Arnáiz E, Olmos Nieva C, Ariadel Cobo D, Alejo Ramos M, Ballesteros Pomar MD. Un caso clínico de penfigoide ampolloso inducido por vildagliptina. Endocrinol Diabetes Nutr. 2020;67:613–614.