Iodine is an essential component of thyroid hormones, and iodine deficit is the leading cause of preventable mental retardation worldwide. Spain was considered iodine-deficient until 2003. Although iodine urinary levels have been in the optimal range in Spain since 2004, the WHO recognizes that our country does not meet the necessary requirements to ensure that the whole population is not at risk of an iodine deficiency disorder. The aim of this article is to review the current iodine status in Spain. Data from several studies emphasize the low consumption of iodized salt at home. Despite the progress made in recent decades, Spanish children are not exempt from suffering an iodine deficiency disorder. Policies that allow for controlling iodine nutrition and promote universal consumption of iodized salt should therefore be implemented.
El yodo es un componente esencial de las hormonas tiroideas y su déficit es la causa principal de retraso mental prevenible en el mundo. España ha sido considera yododeficiente hasta 2003. A pesar de que desde 2004, la yoduria está en rango óptimo, la OMS reconoce que no se cumplen los requisitos necesarios para garantizar que la población no pueda sufrir un trastorno por déficit de yodo. El objetivo de este artículo es realizar una revisión de la situación nacional de este micronutriente. Los datos obtenidos en diversos estudios destacan el bajo consumo domiciliario de sal yodada. A pesar de los avances conseguidos en las últimas décadas, los niños españoles no están exentos de sufrir un trastorno por déficit de yodo. Es necesario, por tanto, implementar políticas que permitan controlar la nutrición yódica así como impulsar el consumo de sal yodada de manera universal.
Iodine is the essential component of thyroid hormones, which are needed for life in all mammals. According to the World Health Organization (WHO), iodine deficiency is currently the leading cause of preventable mental retardation in the world. Different animal models have demonstrated histological changes in the cerebral cortexes of mammals with severe iodine deprivation: small and compact neuronal bodies, reduced dendritic prolongations, myelinization defects and delayed cell proliferation and migration.1 When iodine levels are insufficient, a number of functional and developmental changes known as iodine deficiency disorders (IDDs) occur (Table 1).2 The severity of such disorders depends on the period of life at which they occur: the younger the age, the more serious the disorder. The greatest threat is irreversible brain damage, which can occur in utero if maternal iodine intake proves insufficient. These disorders cover a broad clinical spectrum from cretinism, with severe mental retardation, to mild cognitive dysfunction, hearing loss, or somatic or pubertal delay. The latter alterations are much more prevalent worldwide.
Iodine deficiency disorders according to the period of life.
| Period | Consequence |
|---|---|
| Fetus | |
| Increased number of miscarriages | |
| Stillbirths | |
| Congenital anomalies | |
| Increased perinatal mortality | |
| Neurological cretinism: - Mental impairment - Deafness-muteness - Diplegia, spastic tetraplegia - Strabismus | |
| Myxedematous cretinism: - Dwarfism - Mental impairment | |
| Mental retardation of apparently normal inhabitants | |
| Increased vulnerability in the event of nuclear accidentsa | |
| Newborn infants | |
| Psychomotor defects | |
| Neonatal goiter | |
| Neonatal hypothyroidism | |
| Delayed body development | |
| Increased vulnerability in the event of nuclear accidentsa | |
| Children and adolescents | |
| Goiter | |
| Juvenile hypothyroidism | |
| Impaired mental capacity | |
| Delayed body development | |
| Increased vulnerability in the event of nuclear accidentsa | |
| Adults | |
| Goiter and its complications | |
| Hypothyroidism | |
| Impaired mental capacity | |
| Tendency toward hyperthyroidism when prophylactic measures are introduced | |
| Increased vulnerability in the event of nuclear accidentsa | |
Iodine (in the form of iodide) is widely distributed over the planet, but in many regions and due to glaciations, flooding and erosions, the element has been removed from the terrain and is now mostly found in seas and oceans. All foods of sea origin (algae, fish and seafood) naturally contain a large amount of iodine, with the exception of sea salt. Sea salt or table salt is derived from seawater and is harvested from salt flats; however, although such salt is naturally iodine-containing, the amount supplied is insufficient, unless artificially fortified. The use of iodine to fortify feed for cows and laying hens, or as an antiseptic for udders and containers explains why in many countries animal products such as milk and dairy products or eggs can contain a large amount of iodine.3,4 Other non-dietary sources of iodine include providence–iodine, amiodarone, some antitussive products containing potassium iodide as a mucolytic agent, some vitamin supplements, and radiographic contrast media.5
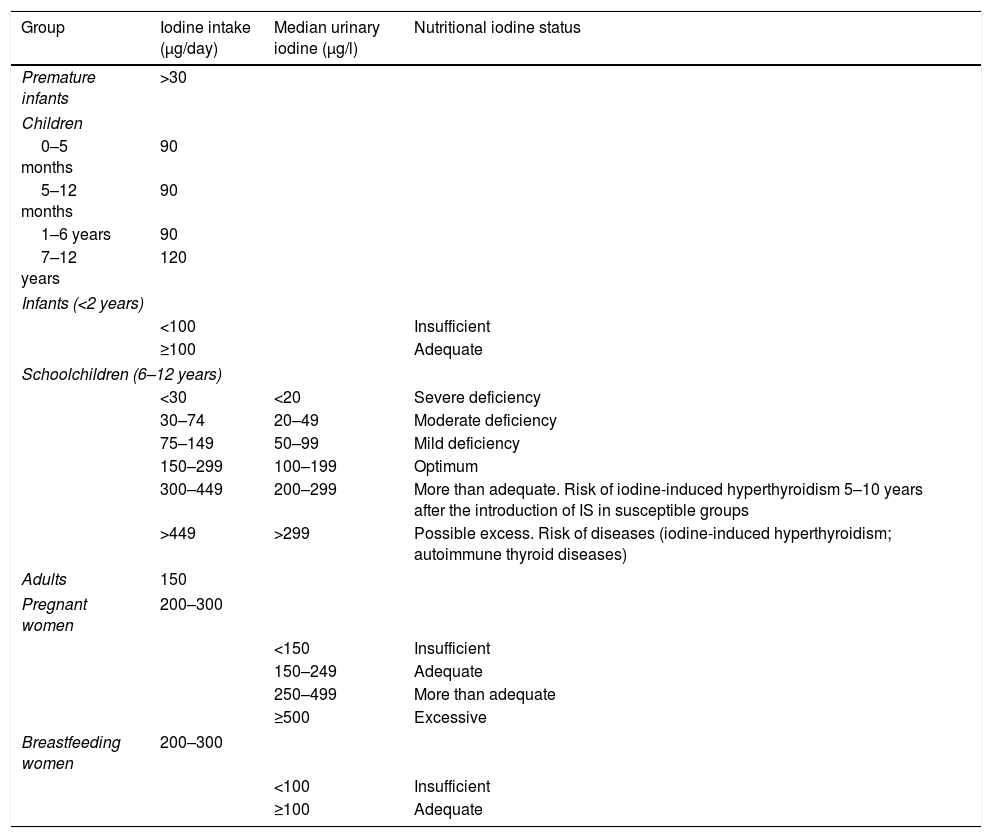
Daily iodine requirements according to ageBased on the WHO recommendations, the required daily iodine dose varies according to the different stages of life (Table 2).6–8 It is important to note that the needs of preterm infants, neonates and young children are greater in relation to their body weights. The amount of iodine needed for an entire lifetime is only 4g, but the element must be ingested on a daily basis, because it cannot be stored in the body.
Recommended minimum iodine intake, median urinary iodine levels, and clinical relevance according to the type of population.
| Group | Iodine intake (μg/day) | Median urinary iodine (μg/l) | Nutritional iodine status |
|---|---|---|---|
| Premature infants | >30 | ||
| Children | |||
| 0–5 months | 90 | ||
| 5–12 months | 90 | ||
| 1–6 years | 90 | ||
| 7–12 years | 120 | ||
| Infants (<2 years) | |||
| <100 | Insufficient | ||
| ≥100 | Adequate | ||
| Schoolchildren (6–12 years) | |||
| <30 | <20 | Severe deficiency | |
| 30–74 | 20–49 | Moderate deficiency | |
| 75–149 | 50–99 | Mild deficiency | |
| 150–299 | 100–199 | Optimum | |
| 300–449 | 200–299 | More than adequate. Risk of iodine-induced hyperthyroidism 5–10 years after the introduction of IS in susceptible groups | |
| >449 | >299 | Possible excess. Risk of diseases (iodine-induced hyperthyroidism; autoimmune thyroid diseases) | |
| Adults | 150 | ||
| Pregnant women | 200–300 | ||
| <150 | Insufficient | ||
| 150–249 | Adequate | ||
| 250–499 | More than adequate | ||
| ≥500 | Excessive | ||
| Breastfeeding women | 200–300 | ||
| <100 | Insufficient | ||
| ≥100 | Adequate | ||
Three criteria are used to determine the iodine nutritional status in a region or in a given population group. The direct method is to assess iodine concentration in urine, while the indirect methods involve the determination of the percentage of schoolchildren with goiter and the prevalence of TSH>5mU/l detected in metabolic tests performed in newborn infants. Since more than 90% of ingested iodine is excreted in urine, urinary iodine concentration (UIC) or ioduria is a good biomarker of recent iodine intake and the best indicator for detecting IDD. The measurement of UIC in an isolated micturition in each individual in the sample and its median is the parameter recommended by both the WHO and the International Council for Control of Iodine Deficiency Disorders (ICCIDD) in order to ascertain iodine nutritional status in a population group. A population median of <100μg/l suggests a high risk of developing different thyroid disorders in that population.9,10 The analysis of UIC can be made using different systems: (1) the colorimetric study of chloric acid according to the technique of Zak and modified by Benotti; (2) the Dunn colorimetric technique; (3) the semiquantitative method described by Gnat; (4) the Sandell–Kolthoff method; and (5) high performance liquid chromatography (HPLC).11–13
The best indicators that a population is iodine deficient are a median UIC in the school population of <100μg/l; a school goiter rate of >5%; and a prevalence of TSH >5mU/l in whole blood in neonates of >3%. It should be noted that isolated micturition iodine determination serves as an indicator of population iodine status, but is not useful as an individual marker. The measurement of UIC in 24-h urine is the best indicator at the individual level, but is less recommended for population studies because of its high cost.10 Some authors propose adjusting ioduria according to the ioduria/creatinine ratio, which is not required for population studies,14,15 though a recent article has shown that the degree of hydration can affect UIC.16
On a universal basis, the WHO proposes studying the urinary iodine levels of schoolchildren (6–12 years of age) in order to ascertain the nutritional status of a given community. Table 2 also shows the ioduria values in relation to iodine intake in schoolchildren. The values considered optimal in this population range from 100 to 199μg/l, corresponding to a daily iodine intake of 150–299μg. This same table expresses urinary iodine values for all age ranges, not only for schoolchildren.
Goiter occurs when the population ioduria is <100μg/l and, when detected at school age, such levels constitute an indicator of chronic iodine deficiency in that population.17 Thyroid volume may be assessed in two ways: through direct neck palpation (the classical procedure) or by neck ultrasound exploration performed by an expert radiologist. According to the latest international recommendations, ultrasound is more precise, particularly in individuals with a poor visible/palpable goiter ratio.17–19
In endemic areas, elevated blood TSH in newborn infants is an effective indicator of nutritional iodine status in the population. Neonatal TSH elevation is the single indicator that best predicts brain damage and the impairment of intellectual development over the long term. According to the WHO, in order for a given population to be considered iodine sufficient, it must meet the following criteria: median urinary iodine in the school population ≥100μg/l, a goiter prevalence in schoolchildren of <5%, and a prevalence of TSH >5mU/l in whole blood in neonates of <3%.20
Epidemiology of nutritional iodine status in the worldIodine deficiency and its associated disorders were identified 150 years ago, but it did not become a priority concern until well into the twentieth century. In the 1960s, the WHO described the European situation regarding cretinism, but it was not until 1980 when the European Thyroid Association re-evaluated the nutritional status of iodine.21 Estimates of iodine deficiency at the end of the twentieth century were so high that in 1986 the WHO, on the occasion of the 43rd World Assembly, indicated that “worldwide and after extreme starvation, iodine deficiency is the most common nutritional cause of preventable mental retardation”. It was suggested that the conception of goiter should be left to one side, and that the relationship requiring consideration was that between iodine and the brain. Since then, several meetings have been held worldwide addressing this subject, and Spain has participated in all of them.22
Goiter prevention through salt iodization began in Switzerland and in the United States in the 1920s. In 1990 only Switzerland, some Scandinavian countries, the United States, Canada and Australia were iodine-sufficient. It is estimated that 2000 million people still have UIC <100μg/l, of which 246 million are children, and 78 million live in Southeast Asia and 58 million in Africa. Twelve percent of the world population has goiter; 26 million people have some degree of neurological impairment secondary to iodine deficiency; and about 6 million suffer cretinism with severe mental retardation.23
In 2012, a total of 111 countries had normal urinary iodine levels, including Spain; 30 presented iodine deficiency (21 mild, 9 moderate and none severe); and 10 countries had excessive iodine ranges.23 It is important to note that even in countries currently considered to be iodine-sufficient, certain population groups such as vegetarians or vegans, infants under two years of age who are not breastfed and do not receive iodine-supplemented infant formulas, as well as certain regions with low iodized salt (IS) coverage, may still be iodine deficient.23
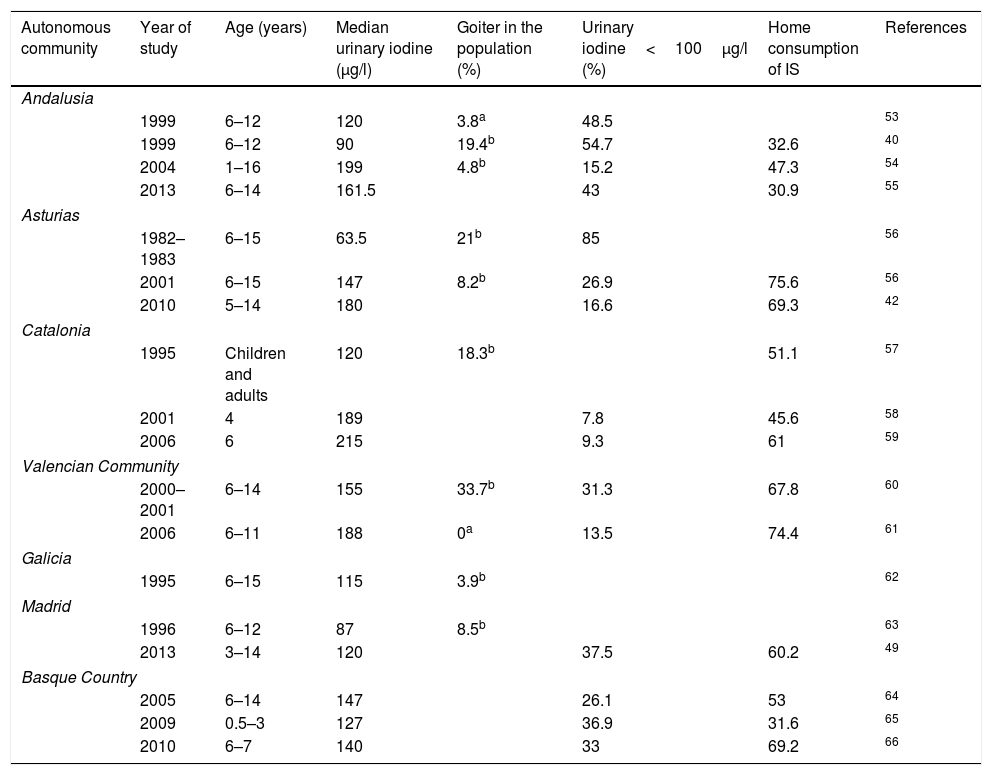
Epidemiology of nutritional iodine status in SpainThe first written report on the problem posed by goiter in Spain dates back to 1833, but it was in 1922, thanks to Dr. Marañón and his trip to Las Hurdes, when institutional awareness of the problem developed. Until the publication of the Tirokid study in 2016,24 the most recent data referring to the pediatric population in the entire country were published in 1993, when the investigators Morreale and Escobar collated all the information obtained locally since 1981 and found Spain to have endemic grade I–II goiter.25 Since then, a series of studies have been carried out in different Spanish regions, as shown in Table 3. It should be noted that only studies providing median ioduria data and not the arithmetic mean have been included, since UIC does not exhibit a normal distribution.
Iodine levels in children in different Spanish regions.
| Autonomous community | Year of study | Age (years) | Median urinary iodine (μg/l) | Goiter in the population (%) | Urinary iodine<100μg/l (%) | Home consumption of IS | References |
|---|---|---|---|---|---|---|---|
| Andalusia | |||||||
| 1999 | 6–12 | 120 | 3.8a | 48.5 | 53 | ||
| 1999 | 6–12 | 90 | 19.4b | 54.7 | 32.6 | 40 | |
| 2004 | 1–16 | 199 | 4.8b | 15.2 | 47.3 | 54 | |
| 2013 | 6–14 | 161.5 | 43 | 30.9 | 55 | ||
| Asturias | |||||||
| 1982–1983 | 6–15 | 63.5 | 21b | 85 | 56 | ||
| 2001 | 6–15 | 147 | 8.2b | 26.9 | 75.6 | 56 | |
| 2010 | 5–14 | 180 | 16.6 | 69.3 | 42 | ||
| Catalonia | |||||||
| 1995 | Children and adults | 120 | 18.3b | 51.1 | 57 | ||
| 2001 | 4 | 189 | 7.8 | 45.6 | 58 | ||
| 2006 | 6 | 215 | 9.3 | 61 | 59 | ||
| Valencian Community | |||||||
| 2000–2001 | 6–14 | 155 | 33.7b | 31.3 | 67.8 | 60 | |
| 2006 | 6–11 | 188 | 0a | 13.5 | 74.4 | 61 | |
| Galicia | |||||||
| 1995 | 6–15 | 115 | 3.9b | 62 | |||
| Madrid | |||||||
| 1996 | 6–12 | 87 | 8.5b | 63 | |||
| 2013 | 3–14 | 120 | 37.5 | 60.2 | 49 | ||
| Basque Country | |||||||
| 2005 | 6–14 | 147 | 26.1 | 53 | 64 | ||
| 2009 | 0.5–3 | 127 | 36.9 | 31.6 | 65 | ||
| 2010 | 6–7 | 140 | 33 | 69.2 | 66 | ||
Several studies based on nutritional surveys show iodine intake to be inadequate. In 2010, IS consumption in Valladolid was found to be lower than recommended.26 Data from the Valencian Community collected in schoolchildren and published in 2015 (the ANIVA study) found food questionnaires to reveal deficient iodine consumption.27 Finally, the National Food Survey in Children and Adolescents (Encuesta Nacional de Alimentación en la población Infantil y Adolescente, ENALIA), published in 2017, showed the intake of iodine-rich food to be clearly inadequate from 14 years of age onwards.28
It is important to note that two Spanish autonomous communities have been the pioneers in detecting the importance of IDDs and in implementing programs to combat them: one was Catalonia, which in 1986 launched a public health program informing about the benefits of IS and incentivizing salting and salt vending companies; and the other was Asturias, which in 1992 made the use of IS compulsory in all school lunchrooms.
The most current Spanish national data have been compiled in the following studies: (1) the Tirobus project (2010), targeted to people of all ages, which showed the median UIC in Spain to be 143.2μg/l29; (2) the Di@betes study (2012), conducted only in adults, which showed a median UIC of 117.2μg/l, with higher values among individuals who routinely consumed IS and milk30; and (3) the Tirokid Project, published in 2016, which represents the only national pediatric study conducted since 1993. Nearly 2000 children between 6 and 7 years of age were evaluated in the 17 autonomous communities in the country. The median UIC was 173μg/l, and thus in the optimum range, being higher in boys than in girls, and in those who regularly consumed IS and consumed at least two glasses of milk a day.24
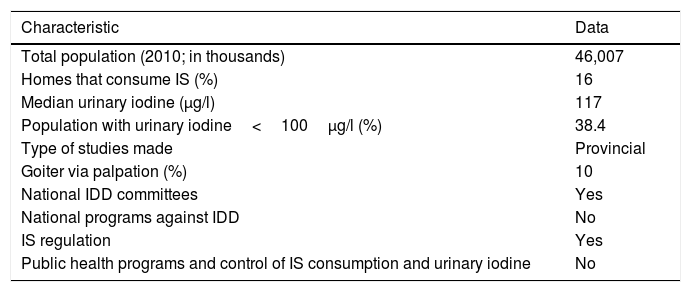
Until 2003, Spain was regarded as an iodine-deficient country.31 However, since 2004 the WHO has included it among the group of countries with optimum iodine levels, according to the median UIC. However, as will be seen below, this unfortunately does not mean that the entire population is protected from the risk of suffering IDD.2Table 4 shows the most recent Spanish data published by the WHO, highlighting the very low penetrance of IS in homes and underlining the fact that almost 40% of the population presents inadequate urinary iodine levels.23,32 It is important at this point to emphasize that UIC shows great variability throughout the course of the day and may be influenced by the degree of hydration. Consequently, an isolated UIC reading of <100μg/l in an individual does not mean that he or she is truly iodine deficient, since repeating the measurement at another time of day or on the following day may yield a different value.33 Therefore, such isolated UIC readings are only used as a global sample value, employing the population median, which in the case of Spain is normal. However, the number of samples below 100μg/l reflects a potential risk for both the global population and for specific population groups (pregnant women and nursing infants). The data published by the WHO in 2012 were improved upon in the Tirokid study published in 2016, which showed the national prevalence of IS consumption to be 69%. In turn, 17% of the children presented UIC<100μg/l.24 Having explained the above, it is important to mention that there are other criteria for ensuring that there is no long-term risk of suffering IDD (Table 5), and that our country does not fully meet these criteria.
Nutritional iodine status in Spain according to the data published by the WHO (2007 and 2012).
| Characteristic | Data |
|---|---|
| Total population (2010; in thousands) | 46,007 |
| Homes that consume IS (%) | 16 |
| Median urinary iodine (μg/l) | 117 |
| Population with urinary iodine<100μg/l (%) | 38.4 |
| Type of studies made | Provincial |
| Goiter via palpation (%) | 10 |
| National IDD committees | Yes |
| National programs against IDD | No |
| IS regulation | Yes |
| Public health programs and control of IS consumption and urinary iodine | No |
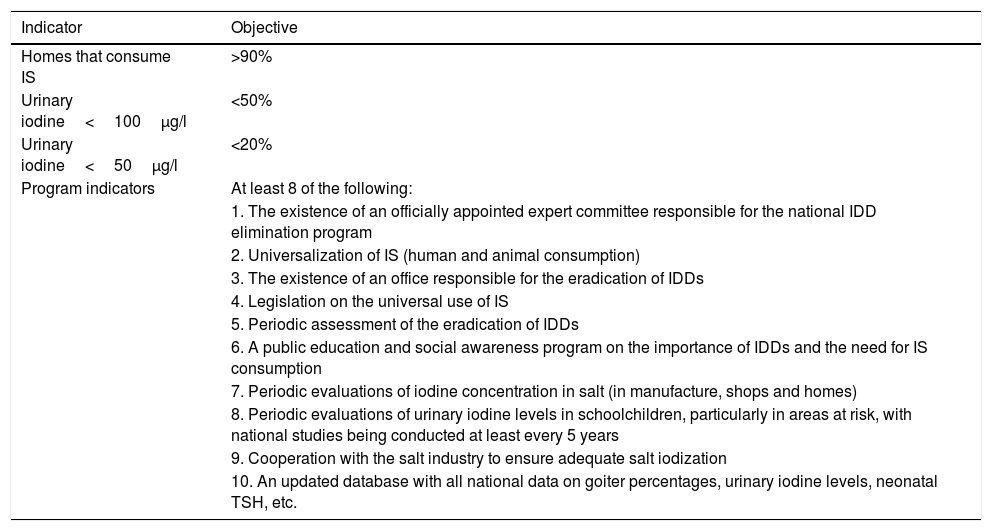
Objectives for the virtual eradication of iodine deficiency disorders.
| Indicator | Objective |
|---|---|
| Homes that consume IS | >90% |
| Urinary iodine<100μg/l | <50% |
| Urinary iodine<50μg/l | <20% |
| Program indicators | At least 8 of the following: |
| 1. The existence of an officially appointed expert committee responsible for the national IDD elimination program | |
| 2. Universalization of IS (human and animal consumption) | |
| 3. The existence of an office responsible for the eradication of IDDs | |
| 4. Legislation on the universal use of IS | |
| 5. Periodic assessment of the eradication of IDDs | |
| 6. A public education and social awareness program on the importance of IDDs and the need for IS consumption | |
| 7. Periodic evaluations of iodine concentration in salt (in manufacture, shops and homes) | |
| 8. Periodic evaluations of urinary iodine levels in schoolchildren, particularly in areas at risk, with national studies being conducted at least every 5 years | |
| 9. Cooperation with the salt industry to ensure adequate salt iodization | |
| 10. An updated database with all national data on goiter percentages, urinary iodine levels, neonatal TSH, etc. |
Iodine is found in relatively low amounts in everyday foods. The population therefore needs an additional source. The method recommended by the WHO for eliminating iodine deficiency and preventing IDD is based on the universal use of IS (iodine enrichment of all salt used for human consumption, both table salt and salt used in food manufacture, and of animal feed). Salt for human consumption should contain at least 15ppm of iodine and should be used in at least 90% of all households.34 Although in Spain the content is 60ppm (one of the highest levels in the world), several studies have shown that the actual iodine concentration is often lower than that reported on the packaging, or that the purported “iodized salt” contains less than 15ppm of iodine when analyzed.35 The causes of this may be inadequate industrial iodization processes, deficient containers and packaging, or environmental storage conditions that favor iodine degradation.
Worldwide IS coverage in homes varies, though according to WHO sources it has improved from less than 20–70% in two decades. In some countries the use of IS is legally mandatory. Examples are Denmark and China, where all salt sold in supermarkets and used in food manufacturing processes must be iodized. In Switzerland, Denmark, Germany and the Netherlands, the use of IS is mandatory in prepared food processing.36 In Spain, table IS has been available since 1983, but the fortification of salt and other foods is not regulated. It is therefore not mandatory to specify the type of salt used on manufactured food labeling.37 Although the data provided by the WHO in 2007 suggested IS penetration in Spanish homes to be 16%,51 the latest available data reflect a higher IS intake of 43.9% in adults30 and 68.9% in children.24
According to the Spanish Society of Endocrinology and Nutrition, the only contraindications to the use of IS are a full body scan with 131I; the administration of diagnostic or therapeutic doses of radioactive iodine; or patients with active Graves-Basedow disease or toxic multinodular goiter. Children with hypothyroidism treated with thyroxine should continue to maintain adequate IS intake to cover the recommended total daily intake. In children that have undergone thyroidectomy, with the administration of replacement therapy in the form of levothyroxine, even if iodine is not required, the use of IS is not harmful.38
Many studies reflect the economic benefits of iodization programs. The estimated cost of salt iodization programs worldwide ranges from 0.0025 to 0.10 USD per person per year, including not only the price of potassium iodide or iodate but also ad hoc building construction, equipment, workers, maintenance, administration and electricity. This represents one-third of the cost of long-term treatment and care for patients with IDD.23,39 The frequent intake of high-iodine foods such as fish and shellfish is also recommended. Iodine is routinely used to fortify feed for laying hens and cows. It is also used as an antiseptic for livestock or containers; as a result, animal products such as milk and dairy products or eggs can contain a large amount of iodine.40 This issue still lacks regulation, however. A study published in 2011 found great variability in iodine amounts even within each milk brand (each brand being supplied from different farms with different iodine usage policies). The mean iodine concentration in different milk batches was 259±58μg/l (minimum 79μg/l and maximum 409μg/l). It should be noted that the labeling information of the different brands did not specify the iodine contents or the processes involved.41
A recent study has shown milk consumption in children from Asturias to be significantly related to ioduria. The latter increased proportionally to the daily amount of glasses of milk, and for each glass of milk consumed daily, the mean urinary iodine levels were seen to increase by 24g/l.42
The median iodine content found in Basque brands of pasteurized cow's milk was 190 (159–235) μg/l, with no differences among full, semi-skimmed and skimmed milk.43 However, the median in organic UHT milk was 55 (50.5–61.5) μg/l, due to the different processing involved.41,43 Meat, cereals, vegetables, fruits and oil naturally or intrinsically contain little iodine (2–3μg iodine/100g of food).
Iodine deficiency disorder eradication criteriaStating that a country has an optimal nutritional iodine status (median population UIC≥100μg/l) is not the same as affirming that this same population is free from the risk of developing IDD. A population is considered to have a quantitatively sufficient iodine status if it meets the following criteria: median ioduria in the school population≥100μg/l; a goiter rate in schoolchildren of <5%; and a prevalence of TSH>5mU/l in whole blood in neonates of <3%, though this does not mean that iodine deficiency (and thus the risk of IDD) has been definitively eradicated.2 Consequently, the WHO considers that a number of objectives need to be met (see Table 5).44
Final considerationsAccording to Santiago et al., the current problem in the world is no longer cretinism, but mild alterations in intellectual development, which imply poorer school performance, with a consequent lesser social and economical development of the populations.40,45 It is very important to be aware that although the degree of iodine deficiency may be mild, this does not mean that the consequences are mild in terms of intellectual or hearing impairment. Although Spain is considered to have had adequate nutritional iodine levels since 2004, it cannot be concluded that our population is free from the risk of IDD. If we consider the criteria for eradicating IDD established by the WHO (Table 5), then Spain does not currently meet them. In this country, IS coverage is less than 90%, and only three of the 10 program indicators are met, namely the existence of an officially appointed committee of experts on the subject; regulation regarding the use of IS; and regular cooperation with the salt industry.44 However, there is no national public health program for this purpose; there are no public education programs; the amount of iodine in salt or ioduria in the population is not regularly monitored; and no sentinel studies have been performed.32,46,47 According to data from the Spanish Society of Endocrinology and Nutrition, ever since 1993 when all Spain was shown to have low ioduria and be endemic for goiter, actions to promote the use of IS have mostly been undertaken by committed professionals. Programs from regional governments or the central government have been only sporadic. Furthermore, it is important to note that all experts agree that this improvement in iodine nutrition with respect to previous decades is only partly due to increased IS consumption. The high amount of iodine in cow's milk and dairy products has been the true “silent prophylaxis” in many countries, and probably also in Spain.48 Both in the Tirokid study and in another study conducted in 2013 in Madrid among children 3–14 years of age, UIC was found to be very consistently related to both the use of IS and to the consumption of at least two glasses of milk a day.24,49 Children not receiving IS or drinking at least two glasses of milk a day were found to have a median urinary iodine level of 90 (interquartile range [IQR] 48.00–141.00) μg/l, and four times the risk of iodine deficiency as compared to the group of children who did receive IS and drank no less than two glasses of milk a day.49
Some groups have suggested that milk is the alternative to salt iodization in countries with low salt intake, but the problem is that legal regulations on the required iodine levels in milk are currently lacking. There are countries, such as Australia, in which the amount of iodine in milk has decreased drastically in the last decade as a result of the use of non-iodinated antiseptics for processing. In consequence, the median UIC in the general population has decreased from 200μg/l to less than 100μg/l in 10 years.50 In recent years, other countries such as the United Kingdom have seen a decrease in UIC due to lesser milk consumption on the part of the population.48 This shows that milk constitutes “vulnerable and fluctuating” prophylaxis, and that IS should therefore be the true iodization agent in the Spanish population. With regard to salt, there has always been widespread concern about the cardiovascular risk associated with moderate-high salt consumption. According to the NAOS (nutrition, physical activity and prevention of obesity) strategy of the Spanish Ministry of Health, 75% of the total salt consumed in Spain comes from processed foods, and these are usually not elaborated with IS. Knowing this, the logical strategy is to use IS in industrial food preparation and to reduce the total amount of salt used. Patients in which salt is contraindicated, such as, for example, children with nephrotic syndrome, should receive oral iodine supplementation.
A final point to note is that an increasing number of studies show that urinary iodine levels in schoolchildren (a group classically evaluated in population-based studies) are not representative of all age groups, and particularly not of women who are pregnant or breastfeeding, which are groups particularly vulnerable to IDD. Comparative studies in Thailand and China showed that on examining a group of schoolchildren and their pregnant mothers, the former had optimum iodine levels while the mothers were mostly iodine deficient.51,52 The urinary iodine levels within the normal range recently reported in Spanish adults and children may therefore not be applicable to Spanish pregnant women, with the fetal risk that this implies. In our study of children between 3 and 14 years of age, we found the 11–14 year-old group (pubertal period) to have a median UIC of 100μg/l (IQR 58.00–145.00), i.e., at the limit of iodine deficiency, and this value was much lower than in younger children between 3–6 years of age, where the median UIC was 148.50 (IQR 95.00–230.75) μg/l (p<0.001). Although no differences were found between the use of IS and the consumption of iodine-rich food in these age groups, and we therefore do not know the exact cause of the above finding, the fact that pubertal girls present borderline urinary iodine levels – exposing them to a serious risk of fetal brain damage in the event of potential pregnancy – is a considerable cause for concern.49
Authorship/collaboratorsAll the authors participated in the design and conduction of the study. Dr. García-Ascaso drafted the manuscript, and Drs. Ros and Ares Segura reviewed the intellectual content.
All the authors reviewed and approved the final version for publication and its submission for publication. All the authors state that there have been no sources of funding and that the article has not been submitted to any other journal. Likewise, the authors state that the intellectual property of the work is transferred to Endocrinology, Diabetes, and Nutrition, along with the right to allow the reproduction of data or illustrations in other publications.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: García-Ascaso MT, Ares-Segura S, Ros-Pérez P. ¿Es suficiente la nutrición de yodo en la población infantil española? Revisión histórica y situación actual. Endocrinol Diabetes Nutr. 2018;65:458–467.