Sensor-augmented insulin pump therapy (SAPT) with low-glucose suspend (LGS) is an effective and safe alternative for treating patients with type 1 diabetes mellitus (T1DM). New predictive low-glucose management (PLGM) systems decrease the severity and duration of hypoglycemic events. However, evidence of benefits in patients previously treated with SAPT-LGS is limited.
MethodsA prospective before-after study was conducted in patients with T1DM treated with SAPT-LGS, who were switched to the Minimed® 640G system with SmartGuard® to assess the impact on A1c levels, severe hypoglycemia (SH), hypoglycemia unawareness (HU), and area under the curve (AUC) <70mg/dL after 3 months of follow-up.
ResultsFifty-five patients with T1DM with a mean age of 37.9 (IQR 6, 79) years and a mean baseline A1c level of 7.52±1.11% were enrolled. After 3 months under PLGM, A1c levels significantly decreased to 7.18±0.91% (p=0.004). SH rate decreased from 2.47 (CI 0.44, 4.90) to 0.87 (CI 0.22, 1.52) events/patient-year (Incidence rate ratio 0.353, 95% CI 0.178, 0.637), AUC <70mg/dL decreased from 0.59±0.76 to 0.35±0.65mg/dL×min (p=0.030). HU determined by Clarke questionnaire resolved in 23 out of 30 patients (p=0.002).
ConclusionsThis study suggests that SAPT with PLGM decreases the frequency of SH, HU, exposure to glucose levels below 70mg/dL, and A1c levels. Based on these results, this therapy should be considered in T1DM patients previously treated with SAPT-LGS with persistent SH and HU. Further clinical trials comparing the efficacy and safety of these features are required.
La terapia con bomba de insulina integrada a sistema de monitoreo continuo con suspensión en hipoglucemia (SAPT-LGS) es una alternativa efectiva y segura para el tratamiento en pacientes con diabetes tipo 1 (DM1). La función de suspensión antes del límite bajo (PLGM) reduce la gravedad y la duración de los eventos hipoglucémicos. Sin embargo, la evidencia del beneficio en pacientes tratados previamente con SAPT-LGS es limitada.
MétodosSe realizó un estudio longitudinal antes y después con pacientes DM1 tratados con SAPT-LGS que se cambiaron al sistema Minimed® 640G con SmartGuard®, con el fin de evaluar el impacto en los niveles de A1c, hipoglucemia severa (HS), hipoglucemia asintomática (HA) y área bajo la curva (AUC) <70mg/dl después de tres meses de seguimiento.
ResultadosSe incluyeron 55 pacientes con DM1, de 37.9 (IQR 6, 79) años, A1c basal de 7.52±1.11%. A los 3 meses bajo PLGM, la A1c se redujo significativamente a 7.18%±0.91% (p=0.004). La tasa de HS se redujo de 2.47 (CI 0.44,4.90) a 0.87 (CI 0.22,1.52) eventos/año del paciente (índice de incidencia 0.353 IC 95%, 0.178, 0.637), el AUC <70mg/dl se redujo de 0,59±0,76 a 0,35±0,65mg/dl x minuto (p = 0,030). HA determinado por el cuestionario Clarke resolvió en 23 de 30 pacientes (p=0,002).
ConclusionesEste estudio sugiere que PLGM reduce la frecuencia de HS, HA, la exposición a niveles de glucosa por debajo de 70mg/dl y A1c. Con base a estos resultados, esta terapia debería considerarse en pacientes con DM1 tratados previamente con SAPT-LGS que persisten con HS e HA. Se requieren ensayos clínicos adicionales que comparen la eficacia y la seguridad de estas características.
In patients with type 1 diabetes (T1D), hypoglycemia persists as a limiting factor for optimal glycemic control. The relationship between hypoglycemia, increased morbidity, mortality and increased risk of cardiovascular death have been described.1–3
Sensor-augmented insulin pump therapy (SAPT) with low-glucose suspend (LGS) feature allows the automatic suspension of insulin delivery when hypoglycemia threshold is reached, this suspension could last for up to 2h; and is an effective alternative for the improvement of metabolic control with reduction of nocturnal hypoglycemia and minimal risk of diabetic ketoacidosis (DKA).2–4 However, in a percentage of this population, severe hypoglycemia (SH) and hypoglycemia unawareness (HU) persisted in 2.7% and 10.8% respectively, in long-term studies.5
Predictive Low-Glucose Management (PLGM) is a different algorithm. It is activated when the glucose level is predicted to drop ≥20mg/dL above the preset limit in the next 30min and automatically resumes if the glucose level increases by 20mg/dL above the “threshold” within 30min after the beginning of the suspension, or if the suspension is greater than 120min Once the system is restarted it enters a refractory period where it cannot be suspended for 30min6
Descriptive studies using SAPT with PLGM activated have documented a high detection rate of episodes, avoiding hypoglycemia by more than 80% of the events,7 although no differences were described in terms of exposure to hyperglycemia.8,9
Evidence regarding “real-life” safety and efficacy of this device is limited. The short-term impact on A1c levels and additional potential benefits has not been formally evaluated in high-risk of hypoglycemia populations who are already being treated with SAPT-LGS. The objective of this study is to describe the use of SAPT with PLGM in terms of efficacy and reduction of hypoglycemia in patients diagnosed with T1D previously treated with SAPT-LGS.
MethodsA prospective before-after study was conducted. Patients diagnosed with T1D on treatment with SAPT-LGS using Paradigm® Veo™ (Medtronic, Northridge, CA) and continuous glucose monitoring (CGM) system (Enlite, Medtronic, Northridge, CA) with LGS function active at least for 3 months were identified. The inclusion criteria were patients who used the sensor more than 90% of the time, use of the bolus wizard function 100% of the time and 3 or more self-monitoring blood glucose (SMBG) per day. We excluded patients who were in pregnancy. The ethics committee of the Hospital Universitario San Ignacio approved the study, following the parameters of good clinical practice and the Helsinki Declaration.
Prior to admission to the study, informed consent was signed. The demographic information and baseline characteristics of the population, including the incidence of SH under SAPT-LGS were extracted from the systematic medical records and complemented with an interview. Clarke questionnaire was filled up to assess the presence of HU.10,11 The information of the interstitial monitoring data for the last 2 weeks on SAPT-LGS was downloaded using CareLink Personal software version 3.0 (Medtronic, Minneapolis, MN), and imported into a MATLAB® calculation software for analysis.
Then we proceeded to install MiniMed 640G® insulin pump with PLGM function, associated to Enhanced Enlite sensor and Guardian 2 Link transmitter (Medtronic, Northridge, CA). Each patient's insurance company delivered all components and accessories such as infusion sets, insulin reservoirs, glucometers and test strips.
At the beginning of therapy, the PLGM function was activated with a threshold of 60mg/dL, until the end of the follow-up. All patients were instructed to avoid carbohydrate intake during PLGM activation to reduce hyperglycemia. Adjustments were made at 24h, 72h, 7, 15 days and then monthly for 12 weeks. At the end of follow-up, the CGM information was downloaded and the Clarke questionnaire was filled up again. Serious adverse events, such as SH, DKA, hospitalization, infection at the cannula insertion site, or device dysfunction were recorded at each visit.
The last 2 weeks of CGM of each patient under PLGM therapy were downloaded with the same software previously described and considered for the analysis. These data were pre-processed from the records to discard monitoring days with consecutive losses greater than 50 samples,12 lower losses were linearly interpolated. The data of each patient were organized by calendar days (00:00 to 23:59h). Based on these data, we calculated the metrics of glycemic variability including standard deviation (SD) and coefficient of variation (CV). In addition, metrics such as mean, area under the curve (AUC) in hyperglycemia and hypoglycemia, percentage of time in hyper and hypoglycemia, and the number of events of hyper and hypoglycemia with a minimum duration of 20min each were calculated. The confidentiality and privacy of the data was protected by maintaining the collection formats under secure access.
SH was defined as an episode of hypoglycemia in which the subject requires assistance by a third party for the administration of carbohydrates, glucagon or other measures.13 DKA was defined as central glucose ≥250mg/dL associated with low pH (<7.3) or low bicarbonate levels (<15mEq/L), and presence of positive serum or urine ketones. The hypoglycemia rate was assessed as number of episodes with glucose levels below 54 and 70mg/dL for at least 20 consecutive minutes.14 The altered perception of hypoglycemia was determined according to the Clarke questionnaire score and was classified as abnormal perception of hypoglycemia in individuals who answered 4 or more responses designated with the letter “R”.10,11
The primary end point was the efficacy and safety of SAPT with PLGM in terms of A1c, number of events and rate of hypoglycemia. The secondary end points were changes in baseline glycemic variability measured by SD, CV%, incidence of hypoglycemia events (<70 and <54mg/dL), exposure time in hypo and hyperglycemia (>180mg/dL) and change in hypoglycemia perception assessed with the Clarke questionnaire at 3 months follow up.
Statistical analysisThe sample studied was described with means and standard deviations, or medians and interquartile ranges as appropriate. Comparisons between both treatment groups were made under the hypothesis that SAPT with PLGM was better than SAPT-LGS in reduction of hypoglycemia. The expected percentage of reduction of hypoglycemia events of 20% was taken into account as a clinically significant reduction to calculate the sample size. The sample size was calculated, with a margin of error of 5%, confidence level of 95% and a power of 0.95 with a result of 56 patients. Student's paired t test or Wilcoxon signed rank test were used for quantitative variables and McNemar or Stuart Maxwell test for qualitative variables, with a level of significance of 0.05. The analysis was performed with STATA 15®.
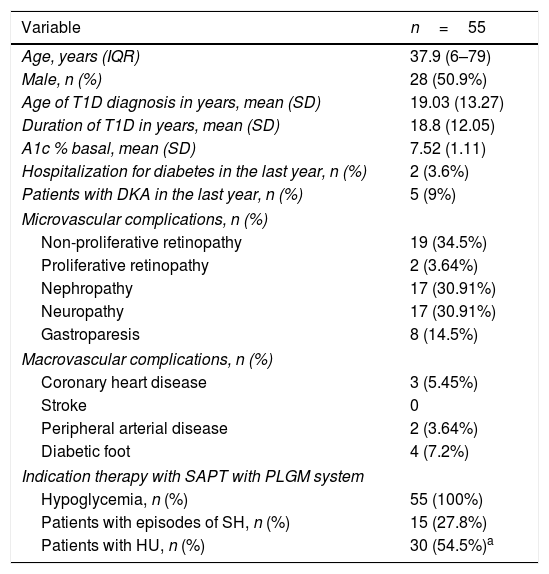
ResultsThe study included 55 patients between April 2016 and May 2017, including pediatric population. Clinical and demographic characteristics are described in Table 1. The mean age was 37.9 years. Average time of disease was 18.8±12.05 years. Baseline A1c was 7.52±1.11%; and the main indication for initiation of therapy with SAPT with PLGM was hypoglycemia.
Baseline characteristics of patients.
| Variable | n=55 |
|---|---|
| Age, years (IQR) | 37.9 (6–79) |
| Male, n (%) | 28 (50.9%) |
| Age of T1D diagnosis in years, mean (SD) | 19.03 (13.27) |
| Duration of T1D in years, mean (SD) | 18.8 (12.05) |
| A1c % basal, mean (SD) | 7.52 (1.11) |
| Hospitalization for diabetes in the last year, n (%) | 2 (3.6%) |
| Patients with DKA in the last year, n (%) | 5 (9%) |
| Microvascular complications, n (%) | |
| Non-proliferative retinopathy | 19 (34.5%) |
| Proliferative retinopathy | 2 (3.64%) |
| Nephropathy | 17 (30.91%) |
| Neuropathy | 17 (30.91%) |
| Gastroparesis | 8 (14.5%) |
| Macrovascular complications, n (%) | |
| Coronary heart disease | 3 (5.45%) |
| Stroke | 0 |
| Peripheral arterial disease | 2 (3.64%) |
| Diabetic foot | 4 (7.2%) |
| Indication therapy with SAPT with PLGM system | |
| Hypoglycemia, n (%) | 55 (100%) |
| Patients with episodes of SH, n (%) | 15 (27.8%) |
| Patients with HU, n (%) | 30 (54.5%)a |
27.8% of the patients presented at least one event of SH in the year before switch from SAPT-LGS to SAPT-PLGM and 56.3% presented HU determined by Clarke questionnaire.
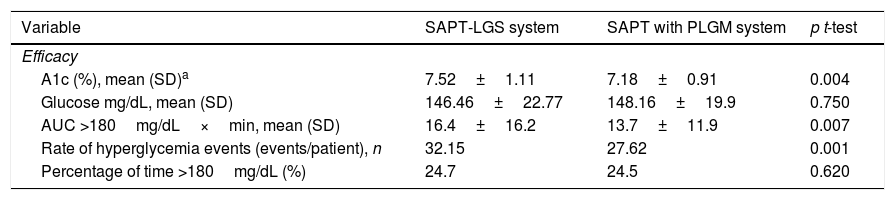
EfficacyTable 2 summarizes the results. A1c was 7.18%±0.91% at 3 months follow-up after initiation of SAPT-LGS (mean difference: −0.34 95% CI, −0.9, −0.59, p=0.004). 15 patients (27.8%) achieved A1c less than 7% using SAPT-LGS; 12 weeks after the beginning of SAPT with PLGM, this number increased to 21 (40.5%) (p=0.05). 14 patients (26.9%) reached A1c less than 6.5% (p=0.05).
Efficacy of SAPT-LGS and SAPT with PLGM.
| Variable | SAPT-LGS system | SAPT with PLGM system | p t-test |
|---|---|---|---|
| Efficacy | |||
| A1c (%), mean (SD)a | 7.52±1.11 | 7.18±0.91 | 0.004 |
| Glucose mg/dL, mean (SD) | 146.46±22.77 | 148.16±19.9 | 0.750 |
| AUC >180mg/dL×min, mean (SD) | 16.4±16.2 | 13.7±11.9 | 0.007 |
| Rate of hyperglycemia events (events/patient), n | 32.15 | 27.62 | 0.001 |
| Percentage of time >180mg/dL (%) | 24.7 | 24.5 | 0.620 |
The percentage of time >180mg/dL defined by CGM was similar for both therapies (p=0.620). The area under the curve >180mg/dL was significantly reduced from 16.4±16.2 to 13.7±11.9mg/dL×min after switching to SAPT with PLGM (p=0.007). The events of hyperglycemia/patient in 2 weeks of CGM were reduced from 32.15 to 27.62 (rate ratio: 0.86, 95% CI, 0.78, 0.93).
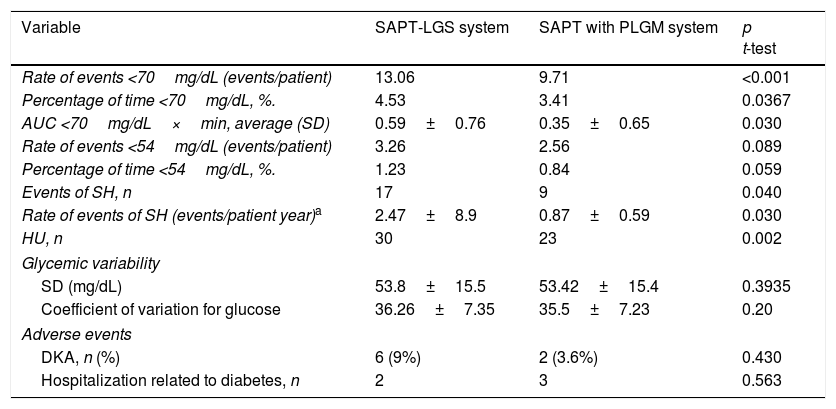
SafetyThe rate of SH reduced from 2.47 (CI 0.44, 4.90) to 0.87 (CI 0.22, 1.52) events/patient year (Incidence rate ratio 0.353 95% CI, 0.178, 0.637). HU assessed by the Clarke questionnaire improved in 7 patients at 3 months follow up, going from 30 to 23 patients (p=0.002).
A significant reduction was documented in the AUC <70mg/dL evaluated by CGM (Table 3). The percentage of time <70mg/dL was reduced from 4.53% to 3.41%, p=0.0367 (Table 3). The rate of hypoglycemia events (<70mg/dL) was reduced from 13.06 to 9.71 events/patient, which corresponds to a rate ratio of 0.74 (95% CI, 0.64, 0.86).
Safety of SAPT-LGS and SAPT with PLGM.
| Variable | SAPT-LGS system | SAPT with PLGM system | p t-test |
|---|---|---|---|
| Rate of events <70mg/dL (events/patient) | 13.06 | 9.71 | <0.001 |
| Percentage of time <70mg/dL, %. | 4.53 | 3.41 | 0.0367 |
| AUC <70mg/dL×min, average (SD) | 0.59±0.76 | 0.35±0.65 | 0.030 |
| Rate of events <54mg/dL (events/patient) | 3.26 | 2.56 | 0.089 |
| Percentage of time <54mg/dL, %. | 1.23 | 0.84 | 0.059 |
| Events of SH, n | 17 | 9 | 0.040 |
| Rate of events of SH (events/patient year)a | 2.47±8.9 | 0.87±0.59 | 0.030 |
| HU, n | 30 | 23 | 0.002 |
| Glycemic variability | |||
| SD (mg/dL) | 53.8±15.5 | 53.42±15.4 | 0.3935 |
| Coefficient of variation for glucose | 36.26±7.35 | 35.5±7.23 | 0.20 |
| Adverse events | |||
| DKA, n (%) | 6 (9%) | 2 (3.6%) | 0.430 |
| Hospitalization related to diabetes, n | 2 | 3 | 0.563 |
The rate of events ≤54mg/dL was reduced from 3.26 to 2.56 events/patient. The percentage of time ≤54mg/dL was reduced after initiation of therapy with SAPT, but these changes did not reach statistical significance (Table 3).
Composite outcomeAt the end of the study 12 of 14 patients achieved A1c ≤6.5% without SH and 6 of 11 patients achieved this A1c goal without episodes of clinically significant hypoglycemia (<54mg/dL). 18 of 21 achieved A1c ≤7% goal without SH, and 6 of 15 patients achieved this goal of A1c without clinically significant hypoglycemia.
Glycemic variabilityMetrics were analyzed to evaluate glycemic variability. However, no significant differences were found (Table 3).
Adverse eventsThere was no statistically significant difference neither in the number of DKA events (p=0.43), nor in the number of hospitalizations between the two therapies (p=0.563) (Table 3).
DiscussionDiabetes Control and Complications trial (DCCT) and its extension study changed the approach in the management of patients with T1D. Following the publication of these studies, strict glycemic control became the main tool to decrease the occurrence of microvascular complications. However, the intensification of insulin therapy has been associated with an increase in the rate of SH; of these episodes, more than 50% occur during the night period making it difficult to optimize metabolic control, increasing the number of emergency visits and hospitalizations related to diabetes.15
Advances in technology have shown a reduction in the rate of hypoglycemia; even though, the data on the impact on glycemic control is limited due to the short follow-up time of clinical trials. A long-term study showed significant A1c reduction which was maintained during follow-up in patients treated with SAP-LGS.5 The data about the impact of SAPT with PLGM on glycemic control is limited. However the increase of A1c related to the suspension of the insulin infusion, rise in the mean glucose and decreasing the time in goals,9 has become one of the main concerns regarding this therapy.
In a previous study using SAPT with PLGM with a short follow up time, Battelino et al. reported an increase in time spent >140mg/dL without changes in A1c or increase of DKA.9 Our study is the first “real-life” data study, which documented a significant reduction of A1c levels after switching to SAPT with PLGM in patients previously treated with SAPT-LGS. This result may be associated with several factors: (1) significant reduction in the number of hyperglycemia events with significant decrease of AUC >180mg/dL, (2) patients were instructed to “rely on the algorithm” and avoid carbohydrate-loaded corrections during PLGM activation and (3) the use of a lower suspension threshold compared to other studies which could prevent the rebound hyperglycemia.
We described a significant decrease of AUC >180mg/dL and a 14% reduction in the event rate of hyperglycemia using SAPT-PLGM, probably because all our patients were instructed to avoid carbohydrate intake during PLGM activation in order to reduce hyperglycemia following resumption of basal infusion. The carbohydrate intake during PLGM activation is a factor associated with hyperglycemia following resumption of basal infusion, interstitial glucose levels 1 hour after automatic restart (without carbohydrate intake) have been reported to be 30–40mg/dL higher, without rebound hyperglycemia.8 Other studies have documented a significant decrease in the need for correction with simple carbohydrates when comparing SAP-LGS vs SAPT with PLGM.16
In this study, the majority of the patients were adults; the threshold to suspend was 60mg/dl during all the follow-up. The threshold suspension is another determinant factor in the effectiveness of the therapy, the use of a lower threshold could reduce the rebound hyperglycemia.8 Different threshold between 65 and 70mg/dL have been used according the age of the population.8,9,16 In this studies the episodes of hypoglycemia without hyperglycemia after resumption of insulin delivery were prevented in 60%.17
The development of SAPT-LGS has reduced by 45% the AUC <70mg/dL at night the severity and the duration of these events in retrospective studies and clinical trials.2,4,18 However, this technology does not prevent all episodes of clinically significant hypoglycemia, an average of 24min per day of interstitial glucose less than 55mg/dL was recorded.2 The use of SAPT-LGS allows the suspension of the insulin infusion when the pre-established threshold is reached,17 showing a reduction in 31.8% of hypoglycemia events (1.5±1.0 vs. 2.2±1.3 per patient/week, p<0.001) and a 37.5% reduction in the mean AUC in nocturnal hypoglycemia (980±1200 vs. 1568±1995mg/dL×min, p<0.001), without deterioration in metabolic control, and no increase in DKA.14 The reduction in duration and severity of exercise-associated hypoglycemia using this function has also been described.16 However, SH and HU persist in 2.7% and 10.8% in the patients receiving this therapy in long-term studies.5 We found a significant reduction in the number of hypoglycemic alert events (<70mg/dl), AUC <70mg/dL, the SH events per patient and the HU assessed by the Clarke questionnaire improved in high-risk population which means that the probability of a hypoglycemic alert event is reduced by 24% in SAPT with PLGM users.
A prospective multicenter study in the pediatric population with a 3-month follow-up found a reduction in the number of glucose measurements <70mg/dL (1.02±0.52 at 0.72±0.36, p=0.027), AUC <70mg/dL (73±56 at 31±22min), with no significant changes in mean glucose (171±26 to 180±19mg/dL, p=0.111) or A1c (7.5%±0.5% to 7.6%±0.7%, p=0.329) in patients who used SAPT with PLGM with a suspension threshold of 70mg/dl.8 We showed a reduction of episodes of hypoglycemia with A1c reduction and a decrease of HU after 3 months of follow-up, this could be one of the mechanism that reduces the incidence of SH.19
There was a significant clinical reduction on rate of events <54mg/dL, however it was not statistically significant, due to the relatively low number of events. Similar findings were described by Thomas Dane in an analysis of 5 patients who showed that SAPT with PLGM was superior to SAP-LGS in terms of percentage reduction in time <70mg/dL, with a discrete increase in the mean interstitial glucose of 10mg/dL approximately.6 In a parallel clinical study including 2 centers, reported a significant reduction in hypoglycemic alert events (<65mg/dl) in the group with activated PLGM function (4.46±4.5 vs 7.4±6.3, p=0.008) was reported; although, the number of hypoglycemia events less than 50mg/dL was reduced, this reduction was not statistically significant in relation to the short follow-up time and the low number of events.9
This is the first “real life” data study designed to evaluate changes in A1c in very high-risk patients previously treated with SAPT-LGS, which suggests additional benefits to the reduction of hypoglycemia events associated with a statistically significant reduction of time of hyperglycemia and A1c. However, this study has some limitations.
The lack of a control group makes it difficult to assess whether the improvement in the clinical outcomes is associated with the PLGM function or additional factors such as the educational program. The included population consisted of patients with a good glycemic control using SAPT followed in a highly specialized center, with systematic and intensive patients training program and the low number of hypoglycemia events <54mg/dl. Our design did not let us to differentiate the effect of each component of the therapy, the avoidance carbohydrate-loaded corrections during PLGM activation or the use of a lower suspension threshold. New clinical trials are necessary to solve these questions.
ConclusionThis study suggests that SAPT with PLGM system reduces the frequency of SH and decrease of HU after 3 months of follow-up with a significant reduction of A1c. Based on these results the use of PLGM function should be considered in T1D patients treated previously with SAPT-LGS who persist with SH and HU. However, further analysis with long-term clinical trials comparing the efficacy and safety of these features are required.
Funding sourceNone.
Conflict of interestAMG reports speaker fees from Novo Nordisk, Elli Lilly, MSD, Novartis, Abbott and Medtronic and research grants from, Novartis, Novo Nordisk and Abbott. DCH reports speaker fees from Novo Nordisk, Medtronic and research grants from Novo Nordisk. No other potential conflicts are reported.
We are grateful to all integrant from the diabetes center at Hospital Universitario San Ignacio.
Please cite this article as: Gómez AM, Henao DC, Imitola A, Muñoz OM, Sepúlveda MAR, Kattah L, et al. Eficacia y seguridad del tratamiento con bomba de insulina con sensor (SAPT) con gestión predictiva de la hipoglucemia en pacientes con diagnóstico de diabetes mellitus tipo 1 tratados previamente con SAPT y suspensión por hipoglucemia. Endocrinol Diabetes Nutr. 2018;65:451–457.
Chief of Endocrinology Unit, Pontificia Universidad Javeriana, Hospital Universitario San Ignacio, Carrera 7 No. 40-62, Bogotá, Colombia.
Endocrinology Unit, Pontificia Universidad Javeriana, Hospital Universitario San Ignacio, Carrera 7 No. 40-62, Bogotá, Colombia.
Department of Internal Medicine, Department of Clinical Epidemiology and Biostatistics, Pontificia Universidad Javeriana, Hospital Universitario San Ignacio, Carrera 7 No. 40-62, Bogotá, Colombia.
Department of Clinical Epidemiology and Biostatistics, Pontificia Universidad Javeriana, Carrera 7 No. 40-62, Bogotá, Colombia.
Endocrinology Unit, Pontificia Universidad Javeriana, Hospital Universitario San Ignacio, Carrera 7 No. 40-62, Bogotá, Colombia).
Pontificia Universidad Javeriana, Carrera 7 No. 40-62, Bogotá, Colombia.