Research in obesity has traditionally focused on prevention strategies and treatments aimed at changing lifestyle habits. However, recent research suggests that eating behavior is a habit regulated not only by homeostatic mechanisms, but also by the hedonic pathway that controls appetite and satiety processes. Cognitive, emotional, social, economic, and cultural factors, as well as organoleptic properties of food, are basic aspects to consider in order to understand eating behavior and its impact on health. This review presents a multisensory integrative view of food at both the homeostatic and non-homeostatic levels. This information will be of scientific interest to determine behavior drivers leading to overeating and, thus, to propose effective measures, at both the individual and population levels, for the prevention of obesity and associated metabolic diseases.
Las investigaciones sobre obesidad se centran fundamentalmente en buscar estrategias de prevención y tratamientos encaminados a los cambios de hábitos de estilos de vida. Sin embargo, con nuevas investigaciones, empieza a asumirse que el comportamiento alimentario es una conducta regulada no solo por mecanismos homeostáticos, sino que también es necesario valorar la vía hedónica que regula los procesos de apetito y saciedad. Los factores cognitivos, emocionales, sociales, económicos y culturales y las propiedades organolépticas de los alimentos son aspectos básicos a valorar para comprender la conducta alimentaria y su impacto sobre la salud. Esta revisión realiza una integración multisensorial en referencia a la percepción de los alimentos, tanto a nivel homeostático como no homeostático, y de esta manera poder interpretar científicamente las conductas que conducen a una sobrealimentación y a proponer medidas eficaces tanto a nivel individual como poblacional en la obesidad y enfermedades metabólicas asociadas.
Obesity is a multifactorial chronic disease characterized by an increase in body fat that poses a risk to the health of the individual.1 Few chronic disorders have progressed in such an alarming way over the last few decades as obesity. According to the World Health Organization (WHO), the presence of obesity throughout the world has more than doubled since the year 1980. In 2014, over 1900 million adults were overweight, and of these more than 600 million were obese.2 These worrisome data are reason enough for adopting measures and implementing prevention and treatment strategies targeted at both the general population and the population at risk, with a view to achieving effective weight loss and securing its subsequent maintenance.3
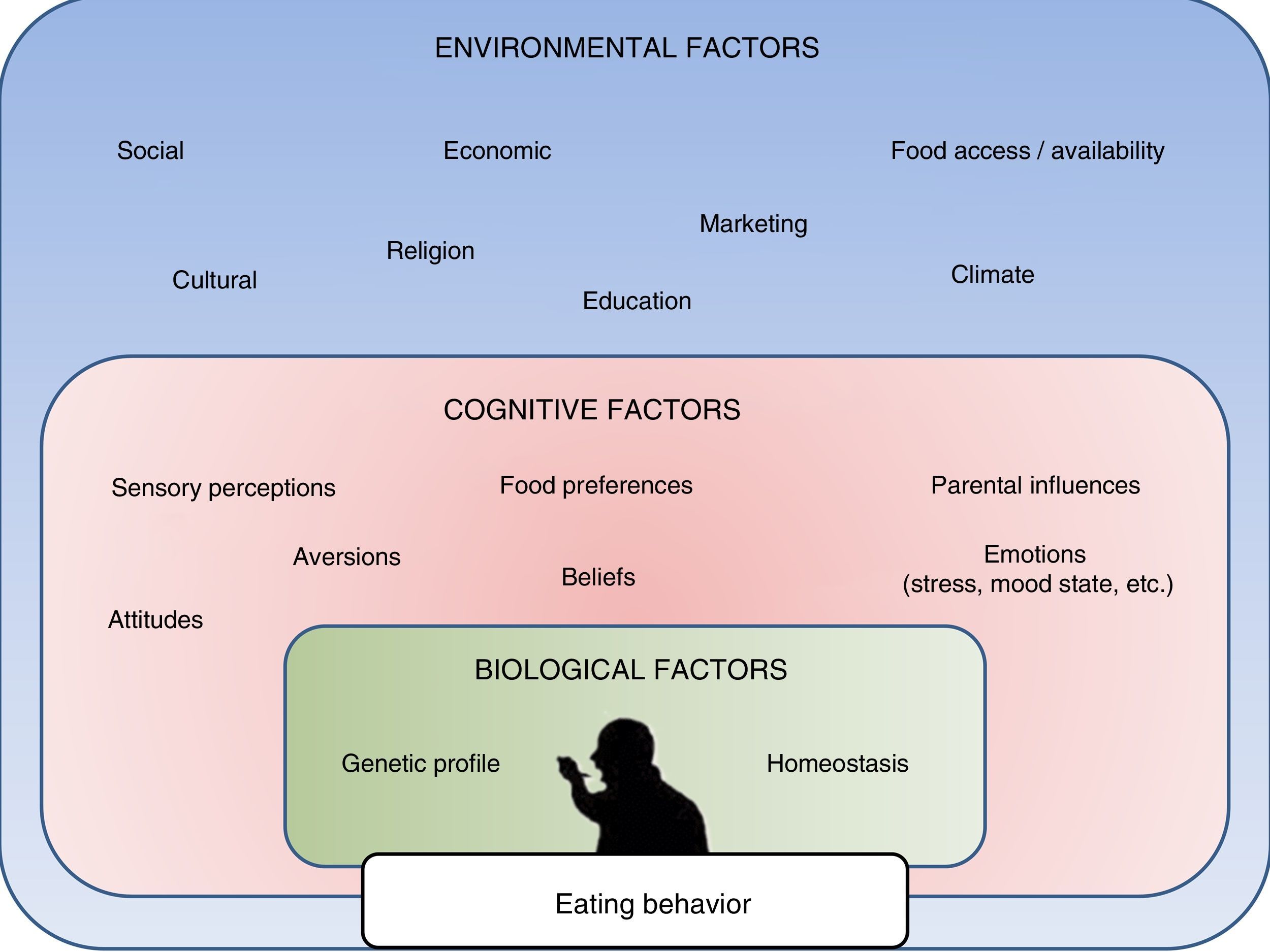
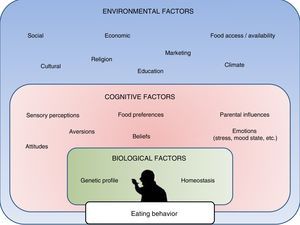
In this regard, research in recent years has made it possible to create and develop different methods, programs and treatments fundamentally focused on favoring changes in lifestyle (diet and physical activity).3 However, it is increasingly apparent that other types of factors must also be taken into account in relation to this public health problem. Recent studies have demonstrated that apart from lifestyle changes and underlying homeostatic mechanisms, obesity is also markedly influenced by cognitive, social, emotional, economic and even religious factors (Fig. 1).4,5
Hunger, satiety and the energy balance are regulated by a neuroendocrine system located within the hypothalamus.6 This system, based on a network of neurohormonal circuits, also includes molecular signals of peripheral and central origin (known as the homeostatic system), as well as other factors of a sensory, mechanical and cognitive nature.7 This system is also referred to as the hedonic system, and is associated with activation of the neuronal reward system in response to any highly palatable food, i.e., any food which independently of its nutritional value produces a pleasurable sensation.8
The factors that regulate hedonic consumption include the senses, which detect flavors, smells, textures and even sounds, and play a decisive role in causing an individual to choose one food or another.9 Considering that this sensory component is very important in terms of energy intake, and therefore relevant to the development of obesity and its associated diseases, the present article offers a review of the different associations detected to date regarding the senses and eating behavior.
Firstly, in order to place the subject in context, a brief description will be provided of the homeostatic component implicated in the regulation of eating behavior.
Homeostatic regulation of eating behaviorKnowledge of the homeostatic regulation of eating behavior has improved greatly in the last 20 years thanks to brain imaging techniques such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). These techniques have afforded improved understanding of the way in which different brain regions respond to food and control the host homeostatic and hedonic responses.10 The hypothalamus is the main brain center containing a complex network of neuronal mechanisms in charge of regulating hunger, satiety and energy balance.6
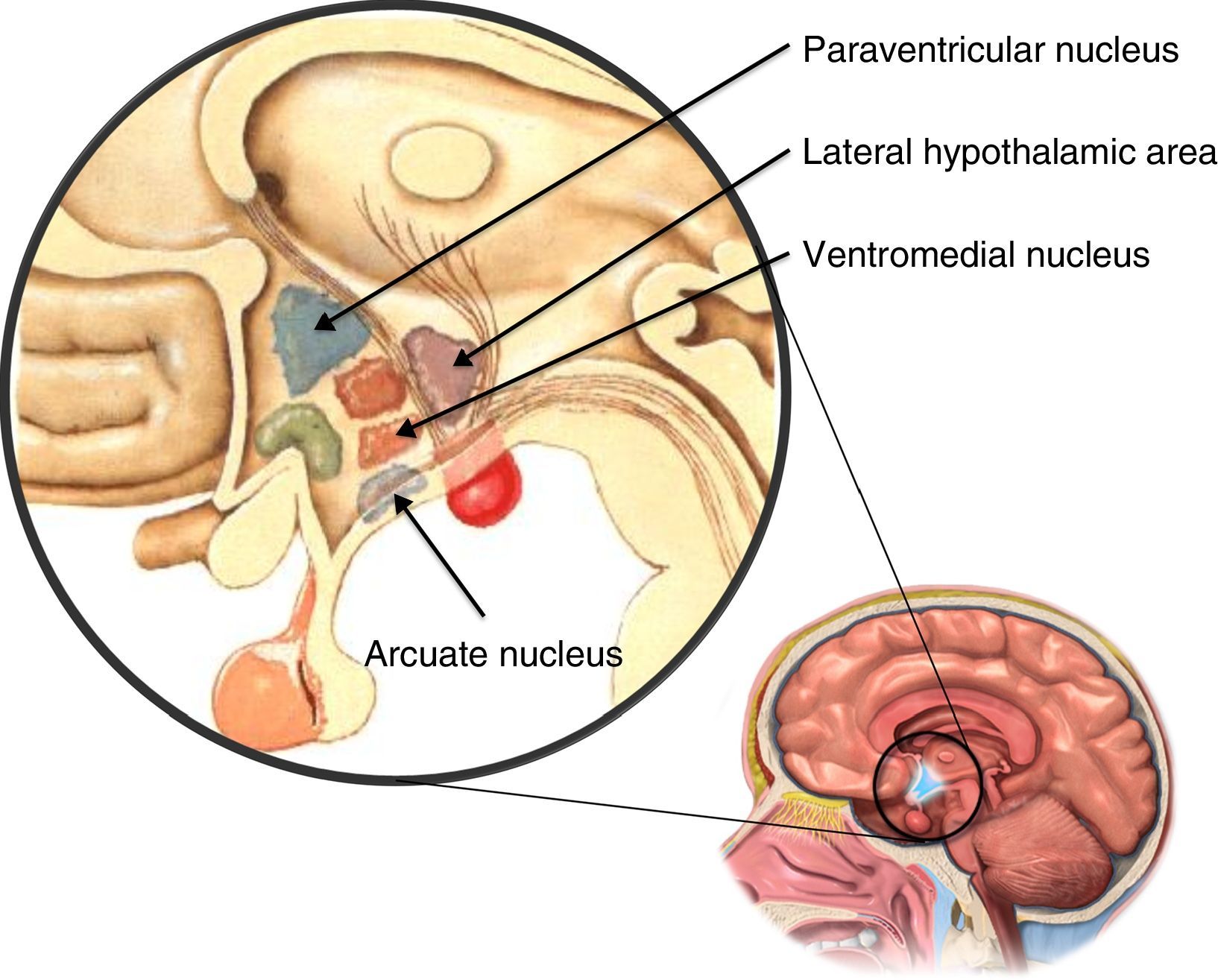
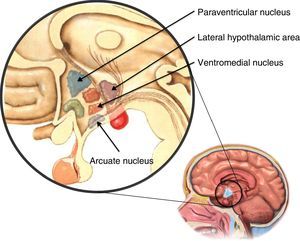
The hypothalamus is composed of different nuclei (Fig. 2) in charge of regulating a series of body functions such as, for example, food intake. These food consumption-regulating nuclei include the ventromedial nucleus, which when damaged results in increased appetite and obesity; the lateral hypothalamic area, which when damaged induces a decrease in food intake and weight loss; the paraventricular nucleus, which is in charge of receiving information regarding food intake from other brain nuclei; and lastly the arcuate nucleus, which integrates the main appetite-regulating peptide secretory neurons.11 Recent studies indicate that this latter nucleus is essential for the regulation of appetite, and damage to the arcuate nucleus in mice has been shown to produce hyperphagia and obesity.7
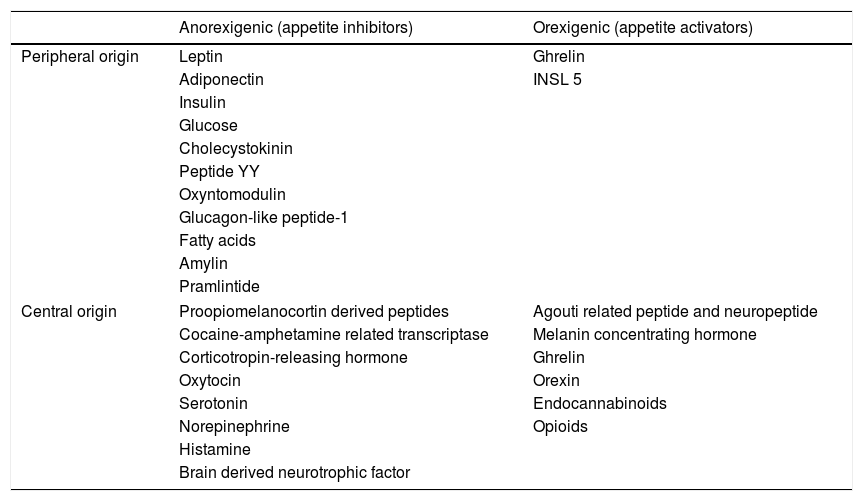
All these nuclei are interconnected, and in turn receive information from the central nervous system (e.g., the vagus nerve), hormone stimuli (insulin, leptin, cholecystokinin and glucocorticoids), as well as signals from the digestive system such as ghrelin and peptide YY14 (Table 1).
Principal food intake regulating hormones.
| Anorexigenic (appetite inhibitors) | Orexigenic (appetite activators) | |
|---|---|---|
| Peripheral origin | Leptin | Ghrelin |
| Adiponectin | INSL 5 | |
| Insulin | ||
| Glucose | ||
| Cholecystokinin | ||
| Peptide YY | ||
| Oxyntomodulin | ||
| Glucagon-like peptide-1 | ||
| Fatty acids | ||
| Amylin | ||
| Pramlintide | ||
| Central origin | Proopiomelanocortin derived peptides | Agouti related peptide and neuropeptide |
| Cocaine-amphetamine related transcriptase | Melanin concentrating hormone | |
| Corticotropin-releasing hormone | Ghrelin | |
| Oxytocin | Orexin | |
| Serotonin | Endocannabinoids | |
| Norepinephrine | Opioids | |
| Histamine | ||
| Brain derived neurotrophic factor | ||
The different signals that regulate this neuroendocrine system can be of central origin (originating in the central nervous system) or peripheral origin (originating in peripheral tissues and organs). In terms of the duration of their action, these signals may be short-acting, i.e., activity is limited to during and just after a meal (e.g., cholecystokinin) or long-acting (e.g., leptin).
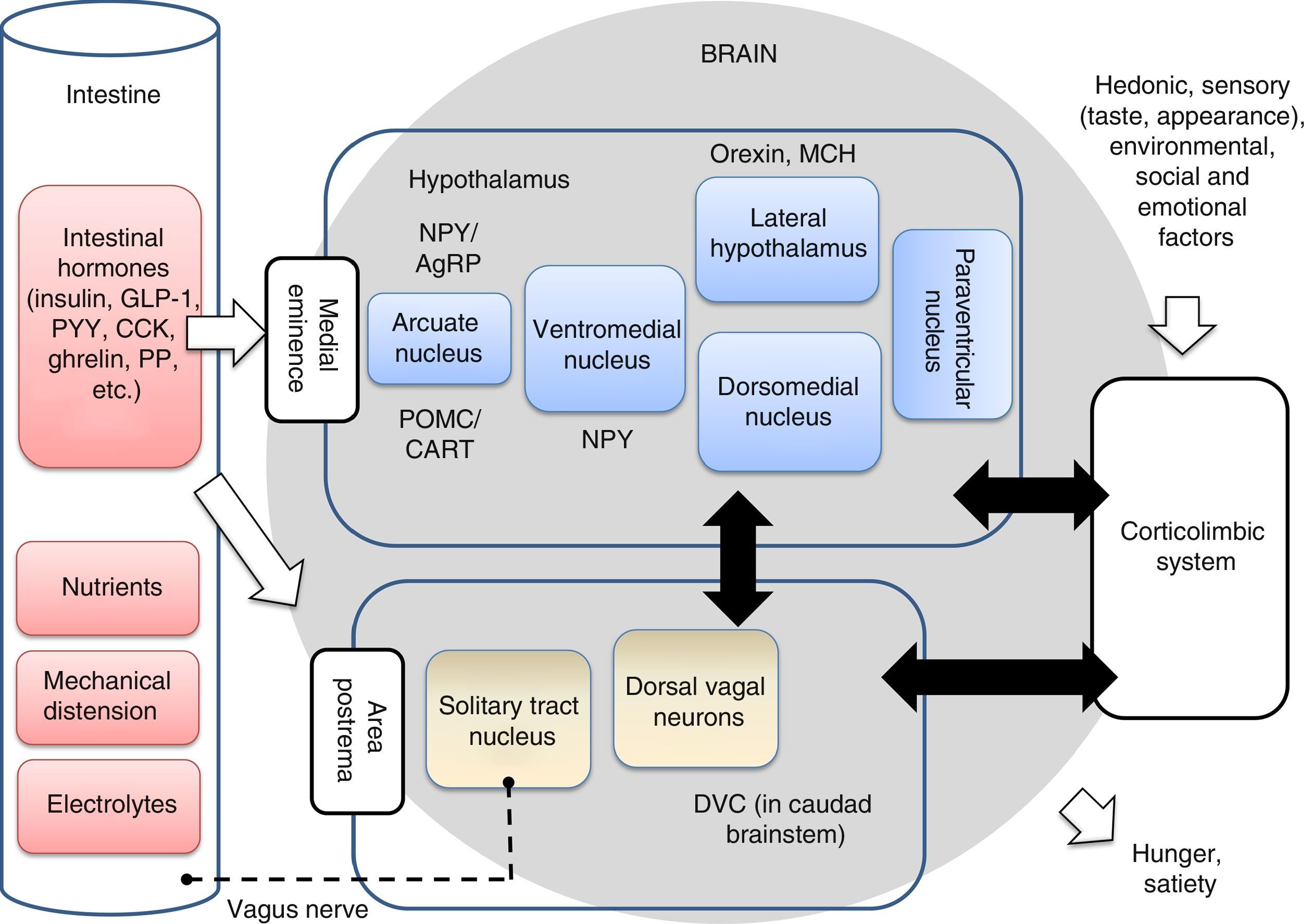
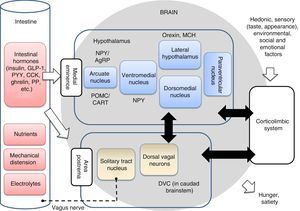
The anabolic (orexigenic) system is in charge of regulating the maintenance or increase in body weight through the stimulation of food intake; the hunger- and appetite-inducing mechanisms; and activation of the mechanisms that inhibit energy expenditure. By contrast, the catabolic (anorexigenic) system regulates the maintenance or lowering of body weight, stimulating mechanisms that increase energy expenditure and reduce food intake.15 Both systems are integrated within the hypothalamus, though their activity is complemented with those of other brain centers, as will be further explained below. The arcuate nucleus of the hypothalamus contains hormones with a great affinity for the MC3R and MC4R receptors (POMC/CART), which stimulate the reduction of food intake and body weight (anorexigenic action). The arcuate nucleus of the hypothalamus contains hormones with a great affinity for the MC3R and MC4R receptors (POMC/CART), which stimulate the reduction of food intake and body weight (anorexigenic action).16 Together with the corticolimbic system and dorsoventral complex in the caudad brainstem, the hypothalamus controls the homeostatic and non-homeostatic regulation of appetite (Fig. 3). Of note is the fact that the worldwide prevalence of obesity associated with MC4R mutations currently reaches 2.5%.17 Different studies have shown that single nucleotide polymorphisms (SNPs) of this gene are strongly correlated to an increased body mass index (BMI) and to an increase in adipose tissue.18 The caudad brainstem in particular plays an important role in eating behavior, and can be regarded as the second homeostatic integrator in the control of food intake. The dorsal vagal complex (DVC), located in the caudad brainstem, establishes a connection between the periphery and the hypothalamus, thereby controlling food intake. The neuronal, hormonal and nutrient signals from the gastrointestinal tract are detected in the brainstem through mechanisms similar to those found in the hypothalamus. These and other signals are integrated in the hypothalamus to generate an efferent signal that is transmitted through the brainstem, thereby modulating appetite and gastrointestinal function. The vagus nerve plays an important role in the transmission of afferent and efferent neural signals between the gastrointestinal system and the solitary tract nucleus within the DVC. The cessation of these signals leads to altered eating behavior.19
Intestinal-brain axis implicated in the regulation of eating behavior. The main homeostatic center of this axis is the hypothalamus, followed by the caudad brainstem (which contains the dorsal vagal complex), and the corticolimbic system, which integrates the hedonic, emotional and environmental influences with the physiological aspects. The neuron signals are sent from the intestine to the brainstem through the vagus nerve in response to chemical, mechanical, hormonal and nutritional changes. The intestinal hormones can also reach the hypothalamus and brainstem directly through the medial eminence and area postrema. The taste, environmental and social stimuli are processed in the corticolimbic system and modulate the appetite centers of the hypothalamus. The integration of all these signals in the brain results in general hunger and satiety sensations. Some neuron populations in the hypothalamic nuclei are also indicated, such as neuropeptide Y (NPY/AgRP), proopiomelanocortin (POMC/CART), orexin and melanin concentrating hormone (MCH). CCK: cholecystokinin; DVC: dorsal vagal complex; GLP-1: glucagon-like peptide 1; PYY: peptide YY; PP: pancreatic polypeptide. Adapted from Hussain and Bloom19 and Escobar et al.20
On the other hand, the homeostatic control of food intake is strongly influenced by hedonistic impulses, the reward system and eating experiences.21 These non-homeostatic factors in turn are affected by the environment and are processed through the corticolimbic system.
The prefrontal cortex and nucleus accumbens, the ventral striate nucleus and the amygdala are structures forming part of the corticolimbic system. Studies of both this system and the hypothalamus have revealed the importance of the link between the homeostatic and non-homeostatic centers in food intake. These studies provide evidence that the non-homeostatic systems can be influenced by homeostatic signals such as those generated by the intestinal hormones. This suggests that it may be possible to modulate both the homeostatic and the non-homeostatic systems through the use of intestinal hormones. However, these aspects are still the subject of research, and further studies are needed in order to improve our understanding of this relationship between the homeostatic and non-homeostatic systems.19
As a summary of this complex regulatory system between orexigenic and anorexigenic factors, it can be concluded that there are two opposed neuronal circuits within the hypothalamus. One is in charge of stimulating food intake (orexigenic), while the other is in charge of inhibiting intake (anorexigenic). These two circuits send signals fundamentally to the paraventricular nucleus, though also to other hypothalamic nuclei that regulate eating behavior (Fig. 3). In turn, both circuits are affected by external hormones and other peripheral factors, and interact with other brain centers such as the corticolimbic system and the DVC, facilitating the integration of environmental, hedonic and homeostatic influences.14,19
Hedonic consumptionIf eating behavior were only regulated by homeostatic systems, food consumption would simply be a response to a purely physiological need, and the great majority of people would maintain a body weight considered normal.5 However, the regulation of appetite in humans is much more complex, since people experience subjective pleasure when eating, and enjoy the presentation of the meal, its aroma, texture and even the sound of chewing crunchy foods.22 All these stimuli act upon the reinforcement system, and the brain generates subjective pleasure sensations that can even lead an individual to eat compulsively. In this regard, the hedonic pathways can cancel the homeostatic system, increasing the desire to consume highly palatable food of great energy density, even when the individual already has important energy reserves and the hunger and appetite sensations are low.9,23 Different studies in children have shown that restrictive practices regarding food can lead to increased food intake and to the preference for highly palatable foods even in the absence of a feeling of hunger (intake in the absence of hunger). Furthermore, most of the above-mentioned studies show parental restriction to be associated with increased body weight in the child.24
Hunger, appetite and satietyIn order to correctly understand the physiology of food intake, we must define three basic concepts: hunger, appetite and satiety.
Hunger is defined as the physiological sensation generated in response to a biological need for energetic nutrients. The sensations associated with hunger can comprise the feeling of an empty stomach, gastric contractions and even headache and nausea. The appearance of a feeling of hunger gives rise to the need to eat, and the latter subsides as food is consumed.
In turn, appetite can be defined as the desire to eat a particular kind of food, or to eat in general, and this can largely condition intake depending on the choice of one type of food or other. This choice is fundamentally influenced by habits, fashion trends, prejudice, thoughts and hedonistic factors, in general.25,26
Lastly, satiety implies the inhibition of a feeling of hunger, and determines the time lapse between one meal and the next. The duration of a sense of satiety depends on the volume and composition of the food consumed, and is known as satiating efficacy. Mention should also be made here of the concept of satiation or fullness, which is often confused with satiety. Satiation is the control of the size of each meal and its duration, leading to the end of food intake. In sum, satiation occurs during the act of eating, while satiety intervenes between meals.25–27 These events have been conceptualized under the term “satiety cascade”, introduced by Blundell et al. in 1987, and which was the first idea to integrate behavioral and sensory elements with physiological factors. The sensory elements include smell, touch and sight which induce appetite before food intake as a preparatory act, thereby maintaining a degree of appetite inhibition for a certain period of time. Other post-ingestion and post-absorption events also occur after food intake, in a decreasing manner, while satiety gradually increases inversely.28 In addition to these factors, mention should be made of the behavioral elements, i.e., those related to learning, beliefs and practices such as eating restrictively or in an uninhibited manner. In line with the distinction between the physiological need to eat and the hedonistic approach to food, another interesting and determinant concept in food intake is the phenomenon known as sensory-specific satiety.25 This term refers to the tendency of organisms to consume a larger amount of food when its components have different sensory properties.29 In other words, although intake increases, satiety does not increase incrementally; in such cases the hunger and satiety signals do not seem to exert much of an influence.25
This latter concept is crucial in order to understand, for example, why a person is able to eat more food when visiting a buffet restaurant. When a single type of food is consumed, a point is reached where intake ceases because a sense of fullness has gradually developed, attributable to the sensory properties of this single type of food. However, if a new food with a form, color, texture or smell different from that of the previous food is then introduced, some of this second food will probably be consumed because its sensory qualities have not been linked to the satiation state of the first food.30
Thus, according to this concept, it can be understood that food intake involves both the sensory effects of the food and the motivational or post-ingestion factors that act at different points in time, as described above in reference to the satiety cascade.28,30
PalatabilityPalatability or the hedonic value of food refers to the pleasure experienced when a concrete food is consumed. This sensation depends on the organoleptic properties of the food, i.e., its taste, smell, color or texture, and significantly conditions the choice of food and its consumption.26,27 Palatability is believed to have a strong impact upon satiation, and possibly also exerts some influence on the regulation of satiety.31
Some studies32,33 have found that as palatability increases, appetite increases, and therefore also food intake. The effect of palatability upon appetite in the period after eating is not so clear.27
In general, humans tend to prefer sweet or salty foods and reject more bitter or acid foods. This can probably be explained in evolutive and survival terms, since sweet and salty foods are associated with high energy densities. For this reason, whenever food was scarce, humans tended to choose these types of foods. In contrast, a bitter taste can be associated with toxic alkaloids, while an acid flavor can be indicative of deterioration or immaturity, thereby inducing the innate human tendency to reject such foods.23,34,35
With regard to the impact of macronutrients upon palatability, it is known that fats are responsible for the flavor, texture and aroma of foods, which in turn represent aspects related to overeating and therefore can affect the energy balance and regulation of body weight over the long term.36 Sugar also has a strong impact upon palatability, encouraging the consumption of sweet foods.34 A number of studies have demonstrated that the combination sugar-fat in foods increases preference for the latter in comparison with the combination salt-fat.26,36 However, although the role of macronutrients in relation to palatability seems clear, this effect probably does not occur in relation to sensory-specific satiety, since the different studies published to date offer controversial results.27,29
Thus, it seems evident that the organoleptic properties of foods and their sensory aspects can modify and regulate food intake through their influence upon palatability, appetite and satiation, thereby possibly playing an important role in eating behavior.
Organoleptic aspectsThanks to the sensory systems, managed by the sense organs, we are able to perceive the nature of the range of substances contained in foods. The sensory properties of foods are responsible for causing us to choose one food or another, though learning and previous experience also influence this choice, as will be commented on below. Taste, color, aroma, texture and even sound exert a strong influence upon the control of food intake when they contribute to improving palatability, and may act as a vehicle favoring excessive consumption.37
From the moment of food perception to food intake, the sensory signals such as smell, taste, texture or appearance, among many other qualities, are transmitted through the cranial nerves toward the central nervous system. These signals trigger insulin secretion with the ingestion of food. The global signals associated with food intake are referred to as the brain or cephalic phase of eating. In compensation, the appetite-lowering mechanism is triggered through a series of oropharyngeal receptors that serve to control the total energy content consumed, generating signals for food intake to stop. When one or more meals are delayed, blood glucose levels decrease and a feeling of hunger increases with the aim of restoring the energy consumed during the fasting period (the so-called glucostatic theory).38,39
Another aspect that needs to be commented on is the influence of fasting upon the brain's response to different stimuli, demonstrating the interaction between the homeostatic and hedonic aspects of eating behavior. A study conducted in 2009 found that under fasting conditions, the brain reward systems focus on high caloric density foods. Furthermore, it was shown that fasting promotes a greater subjective attraction toward more energy-rich foods versus foods with a lower energy content.40 In accordance with these data, a previous study found that fasting also affects the perception of food volume, with portion size being viewed as smaller when fasting than under conditions of satiety.41
It is therefore very important to consider sensory experience beyond palatability alone, and to consider multisensory integration in the context of food perception.
Our senses of taste and smell are crucial for the perception of food flavor, though other types of stimuli must also be taken into account, such as cognitive, auditory, visual and tactile stimuli, since a global view of all of them may help us to generate new strategies in both the health setting and in the food industry.37,42
Taste sensationThe receptors corresponding to the 5 basic flavors (sweet, salty, acid, bitter and umami) are located throughout the oral cavity, but particularly in the tongue. This is where the taste buds are found, grouped within the taste papillae. The latter contain hundreds of taste buds, and each taste bud in turn has 50–150 gustatory cells. These cells possess receptors located in their apical region, exposed to the internal environment of the oral cavity. The chemical substances of food come into contact with the gustatory cells and interact with the taste receptors. Electrical changes in the gustatory cells are produced as an effect of this interaction, generating chemical signals that result in nerve impulses toward the brain.5,43
As mentioned above, taste sensation possibly evolved as a protective mechanism, helping humans to avoid eating poisonous substances and securing the calories and nutrients necessary for survival. In effect, many poisonous substances have a bitter or sour taste, which we tend to reject. By contrast, sweet and salty foods produce pleasure, thereby contributing to meeting our salt and carbohydrate needs. The flavor referred to as umami (“tasty” in Japanese) is associated with foods rich in glutamate, an amino acid present in meats, cheese and tomatoes. Umami increases the palatability of certain foods, and is therefore often used to improve the flavor of different products. Some authors have pointed to the existence of other flavors such as metallic taste and fatty flavor.44,45
Although taste sensation seems inherent to humans and a simple process, the truth is quite different, since it is a complex neurobiological process that is also influenced by many other factors such as genetics, age and experience.
Genetic factors influencing taste sensationThe studies carried out to date show that the diversity of preferences related to taste may be due to genetic differences in the taste receptors. This, in turn, may have relevant consequences affecting the choice of foods, nutrition and health.46
For example, there are certain variants of the TAS2R38 gene that can determine the capacity of an individual to identify bitter taste. This would explain why some people dislike the taste of certain green leaved plants such as broccoli.47
Another study concluded that certain individuals, known as “supertasters” (sensitive to 6-n-propylthiouracil [PROP]) inherit more taste receptors than other subjects, and find sweet taste to be less pleasant than non-PROP or non-taster individuals, who show a stronger tolerance of this flavor. Since a rational explanation for this has proved elusive, it may be postulated that a genetic predisposition to bitter taste interrelates with an affinity for sweet taste.48
Different sensory tests have been made in twins, showing that a preference for bitter food is influenced by the genes and is not determined by the eating habits of the family.49 In any case, this influence is not fully clear, since recent investigations suggest that environmental factors predominate over genetic influences in relation to PROP supertasters.50
Another investigation, the Toronto Nutrigenomics and Health Study, showed that a change of one nucleotide in the transient receptor potential and sodium channel beta-1 subunit (TRPV and SCNN1B) genes modifies salt taste perception, thereby largely accounting for the great inter-individual variability observed.51
It is noteworthy that genetic factors not only influence our sensation of taste, but also appear to have an impact upon eating behavior, appetite and satiety. A recent article (2017) has described how individuals with the MC4R rs7227255 A allele instead of the non-A allele experienced greater increases in appetite and craving on consuming a protein-rich diet for losing body weight.52 Another study published in 2015 found associations between genetic variants of the leptin gene and its receptor (LEP and LEPR) and certain dimensions of eating behavior in children.53 Recent studies have confirmed similar data in children, in particular for the FTO gene.54
Age and its influence upon tasteAs we grow older, all of our senses (taste, smell, touch, eyesight and hearing) decrease in intensity, and this may complicate perception of the food we eat.55 In relation to taste sensation, advancing age is associated with a loss of taste buds, and sensory acuity therefore decreases.56
A study conducted in 2013 compared young individuals (19–26 years of age) with older subjects (45–54 years of age) using functional magnetic resonance imaging. The results showed greater activation of the hedonic response among the younger individuals during sweet taste perception, though not so with bitter taste. These data may provide evidence of age-related differences in the central processing of taste sensation which occur before the appearance of cognitive functional defects in old age.57
On the other hand, elderly people may also suffer alterations in taste sensation and food intake secondary to different age-related processes and diseases, such as the loss of taste sensations, impaired chewing and swallowing function, gastrointestinal problems, loss of appetite, metabolic disorders and even social changes. All these factors can affect their choice of foods and their nutritional status.25
Previous taste experiences and choice of foodsOur first experiences with taste stimuli, first in the uterus and subsequently with breast milk, appear to be fundamental regarding the choice of the type of diet to be followed subsequently. When foods are first tasted, flavors are detected, preferences are established, and eating habits are formed. Infants quickly accept new flavors, though not so children of older ages. More or less around two years of age, infants tend to reject new foods, though studies indicate that repeated exposure to a flavor or food contributes to inducing a preference for it.25,44 Considering the above, early exposure to a variety of taste sensations is advised, starting even from pregnancy and lactation, since this can improve the diet followed in the future.
The setting in which infants are offered food also exerts an important influence, e.g., in nursery schools. Vegetable consumption increases if the infant sees that his or her companions also eat such food.25,58
Forbidding a tasty food can also affect its intake, since children tend to prefer it simply because it has been forbidden.59 It has been shown that obliging a child to eat a given food lessens his or her preference for that food, while, by contrast, when a food is offered as a reward for certain behavior, preference for that food tends to be reinforced.60,61
EyesightThe first sensory contact with a food before its consumption is through the eyes. Seeing a food close up is enough to trigger the eating process. This is the reason why the food industry, supermarkets and restaurants place special emphasis on the appearance of their products, with the aim of making them as attractive as possible to the consumer.37
Different experiments over the last few decades have shown that the appearance of food, including its shape, color, portion size and variety of presentation, has an impact upon consumption.62–64
For example, dividing foods such as cookies or chocolate bars, making them look smaller and more numerous, results in a decrease in the intake of those foods.65,66 This effect has been associated with the segmentation phenomenon (i.e., less is eaten when items, such as cookies, are divided into pieces). However, this does not prevent more in total being consumed than when presentation is in the form of a single item.67 Another study has shown that the colors present in foods and beverages can affect the identification of flavor, from both the perceptive and semantic perspectives.68
The size of the dishware is also a visual signal that conditions food portion size in some people. It has been reported that intake is greater when the plate or container is larger,37 though not all publications support this idea.69 Another important visual factor is the size of the packaging. In this regard, a larger package has been associated with a poorer capacity to estimate portion size41 and with greater potential consumption.70 The portion markings or guides on the products, packaging or utensils, such as images of plates and spoons, can offer a visual reference regarding recommended sizes for snacks, meals and beverages, and can help overweight people to control food portion size.71
The visual appraisal of food can also influence satiety. In a recent study, changes were observed in perceived intake and real intake through manipulation during the meal of the amount of soup added to a container. Immediately after the meal the subjects who had eaten the larger portion felt less hungry, irrespective of the size of the portion they had been shown beforehand. However, after 120 and 180min hunger was more influenced by the amount that had originally been shown than by what had actually been eaten. Thus, satiety was more influenced by what the individual saw and remembered having eaten than by what he or she actually ate.72 Furthermore, recent studies show that this effect can mediate how much food is picked up with the fork and the speed with which the person eats,73,74 these being factors associated with increased energy intake in general.75
In sum, it can be affirmed that visual signals also exert a marked influence upon food selection and intake; they can affect the identification of flavors, and may even modify satiety status.
Sense of smellSmell is also considered to be an important external signal that can affect food intake, since humans use their sense of smell to explore the palatability of food and thus select what to eat.76
A sense of smell, similar to that of taste, is a chemical phenomenon. Smell is mediated by specialized sensory cells called olfactory sensory neurons. These neurons are located in a small tissue zone in the upper part of the nose and are directly connected to the brain. Each olfactory sensory neuron has an olfactory receptor. The molecules released by substances in our surroundings stimulate these receptors, and the neurons send messages to the brain for identification of the smell.
Smells can reach the olfactory sensory neurons in two ways: through the nostrils or through a canal connecting the throat with the nasal spaces. Food in the mouth releases aromas that are picked up by the olfactory sensory neurons through this canal. In this regard, if the canal is obstructed, for example in situations of nasal congestion associated with the common cold, the smells are unable to reach and stimulate these neurons. In such situations a large part of the sensory perception of food is lost. It is clear that the senses of smell and taste are closely related.
If our sense of smell does not function, food can seem insipid. This is why our eating habits may be modified when this sense is altered, resulting in an increase or decrease in food intake. Moreover, since food becomes less tasty due to the loss of palatability, there is a risk of adding too much salt in an attempt to improve its taste.77
A number of studies have produced evidence that pleasant smells of food, such as for example warm pizza or cookies, can stimulate salivation, promote appetite and even increase intake among overweight individuals.78–80 These processes are usually mediated by hormones such as insulin and pancreatic polypeptide, and form part of the satiety brain response.32 Orexin and leptin have also been implicated as part of this response, particularly mediating the relationship among smell, eating behavior and the degree of obesity. Orexin is the hormone that stimulates energy intake and olfactory sensitivity, while leptin inhibits these two functions. It has also been shown that fasting in individuals with normal body weight induces an increase in olfactory sensitivity. By contrast, morbidly obese subjects appear to show diminished olfactory sensitivity.76
A recent article published in 2017 has shown for the first time that there is an inverse relationship between visceral fat and sensory signals such as smell and taste among individuals of highly diverse body weight, since the study sample ranged from low weight individuals to people with morbid obesity.81
Sense of touchFood texture can be explored through a sense of touch, though it is in fact multisensory, since texture can be defined as the sensory and functional manifestation of the structural, mechanical and surface properties of food, detected through sight, hearing, touch and kinesthesia.82 The assessment of texture may be less conscious than taste sensation in perceiving or appreciating food taste, but it is no less important.
The texture of food begins to be noted even before it is placed in the mouth, as when handling it with a knife and fork or upon inspection, though it is more fully appreciated during chewing and swallowing.83 Food firmness, softness, thickness and crunchiness are examples of the qualities of texture, and are very important parameters indicating the quality of food and its acceptance on the part of the consumer.37
Recent studies have underscored the role of texture in relation to food intake. Some studies show the food consumption rate to vary according to texture. In this regard, more viscous food and beverages, and harder foods, are eaten more slowly than those that have a softer texture. It has also been seen that the consumption of “hard” foods is associated with a greater energy intake than the consumption of “soft” foods. This indicates that changes in food texture can be useful as a means of reducing daily energy intake.84,85
Food shape and texture can also cause consumers to believe that a food or beverage has a greater satiating effect.86,87 Recent investigations indicate that foods that are seen as longer and thicker are perceived as being more abundant, and therefore more satiating.88 On the other hand, the effect of thickness or texture has been widely documented, with the implication of liquids as agents that “mask” the satiety signals generated by physiological and sensory pathways. This effect is particularly attributable to the rapid intake and gastric emptying of liquids following ingestion, compared with solids and semisolids (e.g., yoghurt), and to the excessive associated energy intake (particularly in the case of sugary beverages).86,87,89 Moreover, recent studies have shown that the sensory properties related to food texture can induce cognitive perceptions or effects that influence the degree of satiety experienced by the consumer. In this regard, a study carried out in the United States found that individuals that received a “smooth” beverage without additional information, or with information explaining that it quenches thirst, perceived the product as less satiating, and that this led them to greater subsequent energy intake versus those individuals that received a “thick” beverage.90
In general, it can be concluded that food texture is particularly important in relation to food consumption, and may be used as a tool to reduce daily energy intake.87
Sense of hearingThe sounds we hear when touching or using many of the objects around us in our daily life can provide very useful information about the nature of the stimuli we are interacting with. A sense of hearing, therefore, also has a great influence upon the perception of food products.91
Different studies have found that the sounds we hear affect both our choice of food and our taste perception; accordingly, hearing can have an impact upon eating behavior.92
A review conducted in 2012 analyzed different scientific studies published in previous years, and found a series of relationships between sounds and eating behavior. The sound of the food itself, of its packaging or preparation; the music or sounds we hear while shopping or eating; and the association between sounds and flavors or even the sound of the names of foods are auditory factors that have a certain impact upon our perception, and therefore condition food intake.92
In any case, and although many more studies are needed to understand the neurological bases of these aspects, it is clear that a sense of hearing plays a role in eating behavior in general.
ConclusionsThe appetite regulating processes in humans are complex and imply not only homeostatic, physiological and metabolic pathways but also a broad range of external factors related to perception (hedonic pathways), previous experience and personal eating behavior. Among the hedonic pathways, the most important factors include taste, visual, touch and smell stimuli, though other elements also play a relevant role. It is important to underscore that these processes are intimately related to hormonal phenomena through the effect of the stimuli upon the brain reinforcement system, resulting in the production of sensations of pleasure. In this regard, it is necessary to identify those cases in which the hedonic pathways can cancel the homeostatic system, even when the individual already has important energy reserves and the hunger and appetite sensations are low, leading to compulsive energy intake.
While the obesity epidemic continues its course, research focuses particularly on the development of prevention and treatment strategies seeking to induce changes in lifestyle, with particular regard to diet and physical activity. However, it must be remembered that eating behavior is not only regulated by homeostatic mechanisms, and that the hedonic pathway that regulates such behavior needs to be evaluated. Cognitive, emotional, social, economic and cultural factors, as well as the organoleptic properties of food, are some of the aspects that should be taken into account when trying to understand eating behavior.
In order to adopt useful measures which take all these factors into consideration, it is important to secure the commitment and collaboration of both public and private entities and organisms, thus making possible a multisector approach in which government, non-governmental associations and organizations, the food industry, the healthcare system, etc. all play a key role in the creation of programs and products designed to promote a healthy environment accessible to everyone. Only in this way will it be possible to apply strategies in relation to food, physical activity and changes in behavior that can help to prevent obesity and its associated diseases.
It is clear that humans seek to feed themselves far beyond their physiological needs. We enjoy food through its taste, smell, texture, color and even sound. All these aspects can cause us to overeat, even compulsively. Hence the need to take each of the five senses into account in order to understand eating behavior a little better.
It is extremely important to secure multisensory integration in the context of food perception, taking into account both the homeostatic and non-homeostatic factors that regulate food consumption. This will prove effective in increasing our understanding of the types of behavior that lead us to overeat and help us to establish the necessary measures at both the individual and the general population level.
AuthorshipJAM and SN-C participated in the conception of the idea and in the critical review of the manuscript. MHRE, EA-R and BMMA drafted the manuscript. SPD and RS-C made a critical review of the article and contributed substantially to improving the paper. All the authors have read and approved the final manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Hernández Ruiz de Eguilaz M, Martínez de Morentin Aldabe B, Almiron-Roig E, Pérez-Diez S, San Cristóbal Blanco R, Navas-Carretero S, et al. Influencia multisensorial sobre la conducta alimentaria: ingesta hedónica. Endocrinol Diabetes Nutr. 2018;65:114–125.