Identify presurgical factors associated with surgical remission in Cushing's disease (CD).
MethodsAll the patients with ACTH-dependent Cushing's Syndrome in follow-up at our centre between 2014–2021 (n=40) were identified. Those patients with CD diagnosis who underwent transsphenoidal surgery by the same neurosurgeon (n=32) were included. Surgical remission was defined as plasma cortisol <1.8μg/dl and normal or low urinary free cortisol (UFC) after surgery.
ResultsSixty-three per cent (n=20) were women, and the mean age at diagnosis was 42.3±17.9 years. Six patients had macroadenomas, 17 had microadenomas, and in the other 9 patients, no pituitary lesion was identified on the MRI. Seven patients were previously operated on in another centre. Surgical remission was achieved in 75% (n=24). Only three patients experienced recurrence. No association between pre-surgical demographic (age, sex, comorbidities) or hormonal (UFC, ACTH, late-night salivary cortisol levels) characteristics and the probability of surgical remission was observed. The only variable associated with a greater chance of remission was the presurgical visualisation of the adenoma on MRI (OR 8.3, P=0.02). It was also observed that patients with a history of a previous pituitary surgery had a lower tendency to achieve remission, although statistical significance was not reached (OR 0.17, P=0.09).
ConclusionsIn our experience, 75% of patients with CD achieved biochemical cure after the intervention. Surgical remission was up to eight times more frequent in those patients in whom the adenoma was visualised before the intervention, but no other presurgical predictive factors of cure were identified.
Identificar factores prequirúrgicos asociados a curación en la enfermedad de Cushing.
MétodosDe todos los pacientes con síndrome de Cushing dependiente de ACTH en seguimiento en nuestro centro entre 2014-2021 (n=40), se estudiaron aquellos diagnosticados de enfermedad de Cushing que fueron intervenidos por vía transesfenoidal por un mismo neurocirujano (n=32). Se definió curación bioquímica como un cortisol plasmático<1,8μg/dl y un cortisol libre urinario normal o bajo tras la cirugía.
ResultadosEl 63% (n=20) fueron mujeres y la edad media al diagnóstico de 42,3±17,9 años. Seis pacientes tenían macroadenomas, 17 microadenomas y en el resto no se visualizaba lesión hipofisaria. En 7 pacientes existían antecedentes de una cirugía hipofisaria previa en otro centro. Se alcanzó la remisión quirúrgica en el 75% (n=24). Entre ellos, 3 presentaron recidiva durante el seguimiento. No se observó asociación entre las características demográficas (edad, sexo, comorbilidades) ni hormonales (niveles de cortisol libre urinario, ACTH, cortisol salival nocturno) prequirúrgicas y la probabilidad de curación. La única variable asociada a una mayor probabilidad de curación fue la visualización prequirúrgica del adenoma en la RMN (OR 8,3; p=0,02). También se observó que los pacientes con antecedentes de cirugía previa presentaban una menor tendencia a la curación, aunque no se alcanzó la significación estadística (OR 0,17; p=0,09).
ConclusionesEn nuestra experiencia, el 75% de los pacientes con enfermedad de Cushing alcanzó la curación bioquímica tras la intervención. La curación fue hasta 8 veces más frecuente en aquellos en los que se visualizaba el adenoma antes de la intervención, pero no se identificaron otros factores prequirúrgicos predictivos de curación.
Cushing's disease (CD) is usually caused by an overproduction of adrenocorticotropin hormone (ACTH) arising from pituitary corticotroph adenomas.1 Hypercortisolism state in patients with CD is associated with an increased risk of cardiometabolic comorbidities and mortality.2 Thus, treatment is mandatory in these patients, being transsphenoidal surgery (TSS) the first-line therapeutic option.3 However, surgical remission rates are frequently below desired, primarily when surgery is performed in centres and surgeons with low experience.4 In this way, surgical remission is reported from 40 to 50% in some series5,6 to up to 90–96% in others.7,8
Identifying presurgical predictors of surgical remission would allow us to plan better surgery in patients with CD. Several studies have identified postoperative predictors of surgical remission, such as immediate postoperative morning cortisol, ACTH, or urinary free cortisol (UFC).6,7 However, information about presurgical predictive factors of remission is scarce. A recent study found that the number of operations, duration of disease, tumour invasion, tumour size, and preoperative ACTH concentration could be predictors of surgical remission,9 but others have found similar rates of remission between patients with microadenomas and no visible pituitary tumours10; no differences based on preoperative ACTH levels have been shown,11 and several studies have not analysed the impact of disease duration on surgical outcomes.
The aim of our study was to identify presurgical factors associated with surgical remission in CD. This information would be helpful in planning surgery more carefully, submit the patient to a pituitary reference centre and/or consider complementary promising intraoperative techniques like intraoperative fluorescence imaging12 in those cases with a lower probability of surgical remission
MethodsPatientsData were collected from a retrospective database of all patients with ACTH dependent Cushing's Syndrome in follow-up at our centre between 2014 and 2021 (Registry of patients with Neuroendocrine Neoplasms, approved by the local Ethical Committee, approval date: 10th November 2020 ACTA 402). Out of 40 patients with ACTH-dependent Cushing's syndrome, 32 presented CD and were operated by endoscopic transsphenoidal approach by the same neurosurgeon and were included. CD diagnosis was based on patient history and results of a physical examination, laboratory tests, magnetic resonance imaging (MRI), and histopathology.13
Laboratory determinations and assaysAll anterior pituitary hormones were measured pre-and postoperatively following our protocol.14 Presurgical measurements were performed at the time of diagnosis before any medical or surgical treatment. Other determinations included two determinations of 24h UFC, 1-mg cortisol post dexamethasone suppression test (DST) and late-night salivary cortisol (LNSC) or 23pm-serum cortisol. Moreover, low (LDDST) and high-dose dexamethasone suppression test (HDDST) in 6 and 5 patients, and CRH test in 3 patients.
Serum and urine cortisol were measured by immunochemiluminescence assays in an Architect i2000 systems Abbott Diagnostics platform, with an intra-assay coefficient of variation (CV)<10%; the reference range was 102.1–535.2nmol/L (3.7–19.4μg/dl) for serum cortisol and less than 3862.1nmol/24h (140μg/24h) for 24-h urine cortisol (UFC). ACTH was measured by immunochemiluminescence assays (Immulite 2000 Siemens before 2019 and Liaison XL Diasorin after that), with an intra-assay CVs<10%. The reference range for ACTH was 2.0–10.1pmol/L (9–46pg/ml) for the Immulite assay and 1.0–10.7pmol/L (4.7–48.8)pg/ml for the Liaison XL assay. LNSC was measured by electroimmunochemiluminescence in a Cobas 6000 Roche autoanalyser, with an intra-assay coefficient of variation (CV)<10% and a reference range lower than 157nmol/L.
Clinical definitionsThe diagnosis of endogenous hypercortisolism was confirmed by increased UFC, loss of circadian rhythm (evidenced by high serum or salivary midnight cortisol levels) and lack of suppression of cortisol secretion after either a single 1mg dose or 0.5mg/6h/48h dexamethasone. Diagnosis of ACTH-dependent Cushing's syndrome was based on ACTH levels>20pg/ml and of CD based on MRI combined with HDDST and CRH test, including bilateral petrosal sinus sampling (BPSS) in 5 patients. A visual evaluation was performed using our protocol,10 and visual involvement was defined as the presence of any degree of visual acuity compromise, from mild visual acuity involvement to severe and from partial to complete field conditions. To diagnose hypopituitarism, we have employed the same definition as we have previously reported in previous studies.15
Surgical remission was defined as morning serum cortisol values<1.8μg/dL and normal or low UFC within 4–5 days after surgery, as described previously in our protocol.14 Long-term surgical remission was defined as normal UFC at least six months after surgery. Recurrence was defined as high UFC after at least 3 months of normocortisolemia.16
Radiological assessment and other studiesMagnetic resonance imaging (MRI) studies were performed with 1.5T, GE 450w. MRI, sagittal and coronal T1, T2-weighted and dynamic sequences, with gadolinium contrast being performed preoperatively and before any medical or surgical treatment, and 3–6 months postoperatively. Based on the largest diameter of the adenoma, pituitary adenomas were categorised into microadenoma (<10mm) and macroadenoma (≥10mm). BPSS was performed in 5 patients. ACTH secretion was stimulated by administering 100μg of CRH. If the inferior petrosal sinus/peripheral plasma ACTH ratio was ≥2.0 at baseline, or the post-CRH stimulation ratio was ≥3.0, the diagnosis of CD was established.
Surgical procedureSurgeries were performed by an experienced endoscopic pituitary surgeon (VRB), with more than 300 endoscopic pituitary surgeries performed and an average of 35 pituitary surgeries/year during the last ten years. A conventional endoscopic endonasal approach was used in all surgeries. In the case of the cavernous sinus, invasion expanded transcavernous approach was added. Following Oldfield surgical description,17 an extracapsular dissection was attempted whenever possible. If no clear pseudocapsule was found, a thin portion of pituitary tissue surrounding the lesion was additionally removed (enlarged adenomectomy).18 The entire gland was explored with several (1–2mm interval) vertical incisions (from one internal carotid artery to the other) if no tumour was clearly visible in preoperative MRI, mainly guided by the lateralisation of inferior petrosal sinus sampling. If no tumour could be identified during surgery, hemihypophysectomy was performed according to preoperative inferior petrosal sinus sampling lateralisation. If the IPSS did not lateralise, subtotal hypophysectomy was performed.
Statistical analysisThe statistical analysis was performed with STATA.15. In the descriptive analysis, categorical variables were expressed as absolute and relative (%) frequencies; quantitative variables were expressed as mean±standard deviation or median and range if the normal assumption was not fulfilled. The normality assumption was studied with the Shapiro–Wilk test and the variance homogeneity assumption with the Levene test. For comparing differences in continuous parameters, Student's t-tests and linear regression analysis were performed, and for the comparison of categorical variables between independent samples, the chi-squared test and the logistic regression analysis were performed. In all cases, a two-tailed P value<0.05 was considered statistically significant.
ResultsBaseline characteristicsA total of 32 patients with CD were included. Six patients had macroadenomas, 17 had microadenomas, and in the other 9 patients, no pituitary lesion was identified on the MRI. Three macroadenomas had cavernous sinus invasion (two patients Knosp III and one patient Knosp IV). Moreover, three macroadenomas had suprasellar extension (two of the macroadenomas with cavernous sinus invasion). Seven patients were previously operated in another centre. 13 patients received treatment with ketoconazole and 4 metopirone before surgery. The baseline characteristics of the patients are described in Table 1.
Population study, baseline characteristics.
| Variable | Global (n=32) |
|---|---|
| Age (years) | 42.3±17.9 |
| Female | 62.5% (n=20) |
| Diabetes | 25.0% (n=8) |
| Hypertension | 34.4% (n=11) |
| Obesity | 40.0% (n=12) |
| Visual involvement | 9.7% (n=3) |
| Headache | 20.7% (n=6) |
| Hypopituitarism | 6.3% (n=2) |
| 8am serum cortisol (μg/dL) | 36.8±77.85 |
| Urinary free cortisol (μg/24h) | 256 (range 74.4–1903) |
| Late-night salivary cortisol (μg/dL) | 20.2 (range 3.2–32.61) |
| 23pm-serum cortisol (μg/dL) | 18.2 (range 8.7–21.2) |
| ACTH (pg/mL) | 74.9 (range 20.9–444) |
| 1mg-dexamethasone suppression test (μg/dL) | 13.3 (range 5.4–28.5) |
| Glucose (mg/dL) | 113.3±47.93 |
| Serum potassium (mEq/mL) | 4.1±0.46 |
Reference ranges: serum cortisol (3.7–19.4μg/dl); urinary -free cortisol (<140μg/24h); ACTH (9–46pg/ml); late-night salivary cortisol (<4.2μg/dL), serum glucose (70–110mg/dL), serum potassium (3.5–5.5mEq/mL).
Immediate surgical remission was achieved in 75% (n=24), 100% in macroadenomas, 82.4% in microadenomas and 44.4% in patients with no visible tumour. Patients with previous surgery achieved remission in 42.9% % and patients operated on for the first time in 81.8%. After a median follow-up of 4.9 [1.86–11.72] years, only three patients experienced recurrence during follow-up. The other patients remained with normal UFC. Two patients experienced surgical complications (1 patient cerebrospinal (CSF) leakage, 1 CSF leakage and meningitis). There were eight cases of diabetes insipidus (DI), only 1 case of permanent DI. Five patients with normal presurgical pituitary function developed new anterior pituitary deficiencies (different from ACTH deficit) after surgery. TSH deficiency was the most common (4/5), followed by FSH/LH deficiency (3/5).
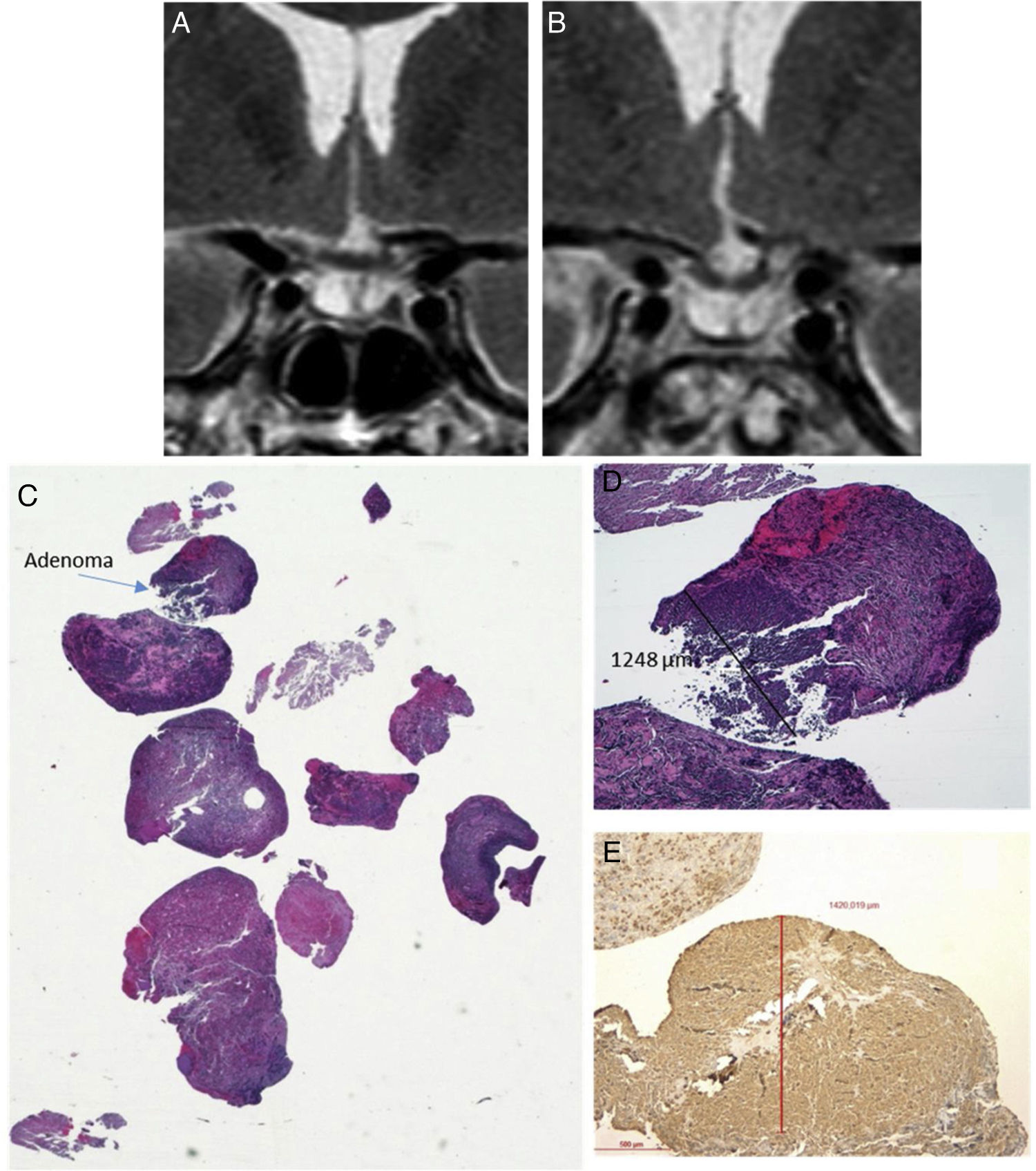
Predictors of surgical remissionNo association between presurgical demographic or hormonal characteristics and the probability of surgical remission were observed (Table 2). The only variable associated with a greater probability of remission was the visualisation of the adenoma on MRI (OR 8.3 95% CI 1.39–48.87, P=0.02). Only in 7 of the 9 patients with no visible tumour on MRI, pathological results confirmed the presence of a pituitary tumour. Positive immunostaining for ACTH was demonstrated in all patients with microadenomas and macroadenomas, but only in 4 patients with no visible tumour (Fig. 1). It was also observed that patients with a history of previous surgery had a lower tendency to achieve remission, although statistical significance was not reached (OR 0.17 [95% CI 0.02–1.42], P=0.09) (Table 2).
Predictors of surgical remission in Cushing disease.
| Variable | Cured (n=24) | Not cured (n=8) | Odds ratio, 95% CI | P value |
|---|---|---|---|---|
| Age, years | 42.3±17.69 | 42.3±19.66 | 1.00 [0.96–1.04] | 0.996 |
| Female sex | 62.5% (n=15) | 62.5% (n=5) | 1.00 [0.96–1.04] | 0.979 |
| ACTH | 98.1±89.84 | 74.6±41.5 | 1.01 [0.99–1.02]* | 0.469 |
| 8am serum cortisol | 41.7±88.07 | 19.7±6.18 | 1.02 [0.94–1.12]* | 0.269 |
| UFC | 510.6±638.3 | 329.7±123.01 | 1.00 [1.00–1.00]* | 0.482 |
| LNSC | 14.7±8.81 | 24.4±7.21 | 0.83 [0.61–1.11]* | 0.093 |
| DST | 15.7±7.60 | 11.6±3.59 | 1.13 [0.90–1.42]* | 0.285 |
| Visible tumour on MRI | 83.3% (n=20) | 37.5% (n=3) | 8.33 [1.39–49.87] | 0.016 |
| Previous surgery | 25.0% (n=3) | 66.7% (n=4) | 0.17 [0.02–1.42] | 0.087 |
| Presurgical medical treatment | 45.8% (n=11) | 50.0% (n=4) | 0.85 [0.17–4.20] | 0.838 |
DST, dexamethasone suppression test; LNSC, late-night salivary cortisol; MRI, magnetic resonance imaging; UFC, urinary free cortisol; * per each unit. Presurgical medical treatment makes reference to metopyrone or ketoconazole treatment before surgery. Categorical variables were compared with logistic regression model, considering the non-cured the reference group. Continuous variables were compared with Student's t-test.
Histopathological sample of ACTH-secreting pituitary adenoma not visible on preoperative MRI. (A) Coronal T2 preoperative MRI; (B) Coronal T2 postoperative MRI (reduction of glandular parenchyma was observed after surgery); (C) Material sent HE (macro) where the small fragment of 1mm in diameter corresponding to the tumour is observed); (D) Adenoma HE 4×; (E) Immunohistochemical staining with 4× ACTH antibody.
In this series of patients with CD operated by a single surgeon at one experienced tertiary centre over a 7-year period, we have demonstrated that TSS, as the initial treatment for CD, achieved cure in 75% of the patients. Only 2 out of 32 patients experienced complications (all of them mild complications). The most important finding of our study was that those patients with presurgical MRI that locates the pituitary adenoma had an 8-fold higher probability of curing after surgery than those with unlocated tumours.
The most commonly used remission criteria in recent publications have been defined as a post-surgical baseline plasma cortisol value<1.8μg/dL or <5μg/dL. In our study, we used the threshold of 1.8μg/dL to increase the sensitivity to detect recurrence. Surgical remission was achieved in 75% of our patients, similar to what was reported in other series describing rates between 70 and 80%.9,19 However, the reported remission rate is as low as 50–60% in other studies.20,21 Overall, as expected, the highest remission rates were seen when using the criterium of baseline post-surgical cortisol <5μg/dl, rather than <1.8μg/dl. Besides, remission was higher when surgical resection was performed by an experienced neurosurgeon with a high number of patients, as in our centre, where an average of 35 endoscopic pituitary surgeries is performed per year. The predominant postoperative complication was transient DI which occurred in 22% of the patients, a rate similar to reported in other series.19–21 Similarly, 16% developed adenohypophyseal deficiencies, with rates similar or lower than reported in other studies.19,20
We identified the location of the pituitary adenoma on the presurgical MRI as the unique variable associated with surgical remission. It is not surprising that MRI-visible adenoma is an independent predictor factor since it is easier to resect a microadenoma once it is located or visible, as confirmed previously by other authors.5,11,18 However, other studies have found no difference in remission rates between negative or positive preoperative MRI CD patients. Starker et al.22 found no significant difference in remission among patients with microadenoma, MRI-negative or macroadenoma, but these results may be subject to type II error due to inadequate sample size in each subgroup. Cebula et al.23 found a recurrence rate for negative preoperative MRI higher (71.41%) but not statistically significant compared to positive (OR 1.89, P=0.077). This may be explained by the fact that 77.1% of the negative-pituitary adenoma were identified during the hypophyseal exploration.
In relation to second surgeries, the remission rate after a second surgery is reported around 54–64%, with high variability between centres (38–90%) due to the lack of homogeneity in the definitions of remission/recurrence/persistence and the potentially different goals of repeat surgery.24–26 Our series also found a lower tendency to surgical remission rate in patients who have already undergone surgery, although no statistical significance was reached. The lower chance of cure after second surgery could be due to inherent qualities of a recurrent tumour (the lesions may present more aggressive characteristics in these cases like a greater degree of invasion of adjacent structures with a lower degree of resectability or a higher mitotic index in the pathological anatomy), and the distortion of the anatomy and the presence of scar tissue that makes surgery more difficult. Despite a lower tendency to remission in these patients and a higher rate of hypopituitarism due to the aggressiveness of the surgeries, a second transsphenoidal surgery remains an effective and safe treatment option, especially in cases where adenoma is evident on complementary imaging tests.24–26 The lack of statistical significance in our series could be related to the small sample size of our study.
No association was found between the presurgical hormonal status and surgical remission in our cohort. In contrast to our results, Dai et al.9 found that a higher preoperative ACTH level was associated with a lower biochemical remission rate. Similarly, Cannavó et al.27 demonstrated that mean preoperative ACTH values were significantly higher in the not cured patients. Kuo et al.28 showed that higher ACTH concentrations before treatment predicted a recurrence of CD. However, the predictive value of preoperative ACTH levels has not been widely accepted and remains controversial. In addition, no correlation between remission and preoperative 24-h UFC levels was detected in our study, consistent with previous studies’ results.9,29 Interestingly, Schernthaner-Reiter et al.30 reported that lower baseline UFC was associated with a higher number of long-term comorbidities, possibly due to the,more prolonged exposure to excess glucocorticoids in milder CD.
The main limitations of our study are its retrospective nature and the small sample size that could limit the formulation of a conclusion with high strength of recommendation. Moreover, the data on long-term follow-ups and further treatments of a large proportion of patients were not available. However, we consider that the information provided in our study could be helpful to orientate new multicentric prospective studies focused on surgical remission in CD.
ConclusionIn our experience, 75% of patients with CD achieved biochemical cure after endoscopic transsphenoidal surgery. Surgical remission was up to eight times more frequent in those patients whose pituitary adenoma was visualized before the intervention, but no other presurgical predictive factors of cure were identified.
Ethical approvalAll procedures performed on the study participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consentAbstention of consent has been requested to the Ethical Committee due to the study's retrospective nature.
Financial supportThis work has not received any financial support.
Conflict of interestThe authors have no conflict of interest.