Obstructive sleep apnea (OSA) has a high incidence in patients with morbid obesity (MO) who are candidates for bariatric surgery (BS). Adequate screening would decrease the number of respiratory polygraphies (RPs).
ObjectiveTo analyze the value of a sequential model consisting of a questionnaire (modified Dixon (DXM VS STOP-Bang) and nocturnal pulse oximetry (POX) in patients who were candidates for BS.
MethodsA prospective study was conducted from July 1, 2014 to July 1, 2015 on candidates for BS, excluding those who have already undergone RP. Variables: questionnaires (Epworth, STOP-Bang, and DXM), anthropometric measurements, RP, and blood and gas tests. The sample was divided into patients with no or mild OSA (no OSA) and those with moderate to severe OSA (AHI>15).
ResultsA total of 70 patients were analyzed, 46 (65.7%) of them females. Moderate to severe OSA was diagnosed in 26 (37.1%) patients. STOP-Bang and DXM were compared using ROC curves, and greater area under the curve (AUC) was found for the latter (0.873(0.74−0.930) vs 0.781(0.673−0.888)). STOP-Bang had greater sensitivity, 100%, as compared to 73.1% for DXM. ODI3% showed greater diagnostic yield (AUC=0.982 (0.970-1). Use of the sequential model with STOP-Bang>3, DXM>5, and DXM>3 would have avoided 41 (58.5%), 50 (71.4%), and 41 (58.5%) RPs and 0, 7 (10%), and 0 false negatives respectively.
ConclusionUse of a sequential model based on the STOP-Bang and nocturnal POX is a useful tool for screening OSA in patients with MO candidates for BS, decreasing the number of RPs.
La apnea obstructiva del sueño (AOS) tiene una elevada incidencia en obesos móribos (OM) candidatos a cirugía bariátrica (CB). Un screening adecuado reduciría el número de poligrafías (PR).
ObjetivoAnalizar la utilidad de un modelo secuencial con un cuestionario (Dixon modificado (DXM VS STOP-Bang) y pulsioximetría nocturna (POX) en pacientes candidatos a CB.
MetodologíaEstudio prospectivo, desde el 1 de Julio de 2014 hasta el 1 de Julio de 2015. Se incluyeron candidatos a CB, excluyéndose aquellos que ya se habían sometido a una poligrafía. Variables: cuestionarios (Epworth, STOP-Bang y DXM), medidas antropométricas, poligrafía y analítica de sangre y gases. Se dividió la muestra entre los que no tenían AOS o era leve (No AOS) y los que tuvieron una AOS moderada - grave (IAH>15).
ResultadosSe analizaron 70 pacientes, de los cuales 46 (65,7%) mujeres. Se diagnosticaron 26 (37,1%) de AOS moderada - grave. Comparamos STOP-Bang y DXM mediante curvas ROC con una mayor área bajo la curva (AUC) para este último (0,873(0,74 -0,930) VS 0,781(0,673-0,888)). La sensibilidad fue superior para el STOP-Bang con un 100% VS 73,1% de DXM. El IDO3% presentó mayor rentabilidad diagnóstica AUC=0,982(0,970-1). La aplicación del modelo secuencial con STOP-Bang>3, DXM>5 y DXM>3 hubiese evitado 41(58,5%), 50(71,4%) y 41(58,5%) poligrafías y 0, 7(10%) y 0 falsos negativos respectivamente.
ConclusiónLa aplicación de un modelo secuencial basado en el STOP-Bang y POX nocturna es una herramienta útil para el screening de AOS en OM candidatos a CB, reduciendo el número de PR.
Morbid obesity (MO), defined as a body mass index (BMI) of >35kg/m2, is a chronic disorder characterized by disproportionate fat accumulation. There are a number of therapeutic options for MO, with bariatric surgery (BS) being reserved for the most severe cases (BMI>40kg/m2 or >35kg/m2 with one or more associated comorbidities).1 According to the world Health Organization (WHO), the prevalence of obesity has tripled worldwide since 1975, reaching 13% (11% in men and 15% in women) in 2016.2 In Spain, 18.2% of all males and 16.7% of all females suffer obesity according to the Spanish National Health Survey of 2017.3
The incidence of obstructive sleep apnea (OSA) in patients with MO can reach 90%.4 The coexistence of OSA and MO in patients who are candidates for BS not only increases the cardiovascular risk but also the perioperative risk of cardiorespiratory complications.5 Positive pressure therapy reduces the risk of such complications6 without increasing the surgical risk.7 A diagnosis of OSA is therefore needed for the benefit of patients eligible for BS.
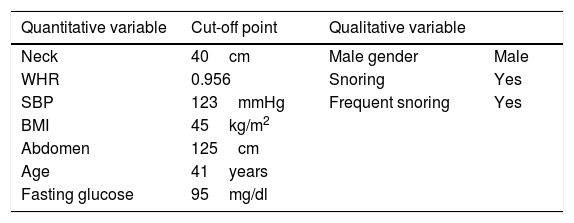
Multicomponent indices have been described for predicting the risk of developing OSA. The best known of these is STOP-Bang,8,9 which analyzes the presence of symptoms (snoring, apnea and excessive daytime sleepiness), associated diseases (arterial hypertension [AHT]) and other related factors (the BMI, neck circumference, age and gender). This questionnaire has been validated in individuals with obesity and with MO, exhibiting 100% sensitivity for severe OSA (sleep apnea-hypopnea index [AHI]>30).10 Its handicap is its low specificity: less than 20% in reference to obesity and 7% in MO. Anthropometric measurements play a key role in OSA, even in MO, where the location of the accumulated adipose tissue determines AHI.11 The Dixon model is the instrument that includes the most variables related to central obesity. This model was introduced in 200312 and was modified in 201113; it includes 9 variables (systolic blood pressure, waist-hip ratio, neck and abdominal circumference, the BMI, gender, age, snoring and fasting glucose) (Table 1).
Variables of the modified Dixon model.
| Quantitative variable | Cut-off point | Qualitative variable | |
|---|---|---|---|
| Neck | 40cm | Male gender | Male |
| WHR | 0.956 | Snoring | Yes |
| SBP | 123mmHg | Frequent snoring | Yes |
| BMI | 45kg/m2 | ||
| Abdomen | 125cm | ||
| Age | 41years | ||
| Fasting glucose | 95mg/dl |
WHR: waist-hip ratio; BMI: body mass index; SBP: systolic blood pressure.
Laboratory-based polysomnography (PSG) is the gold standard for the diagnosis of OSA. A less complex choice for diagnosis is home respiratory polygraphy (RP).14 Nocturnal pulse oximetry (POX), which only analyzes SatO2 and nocturnal heart rate, has also been studied and proposed as a screening option in candidates for BS, resulting in a reduction of up to 40% in the need for more complex sleep studies.15 The parameter exhibiting the greatest agreement with AHI is the >3% oxygen desaturation index (ODI). The sensitivity of devices of this kind reaches 100%, with a specificity of close to 70%.15 On the basis of the above, we designed a prospective study with the aim of assessing sequential screening in patients with MO and eligible for BS. To this effect, we first compared the performance of the STOP-Bang, modified Dixon model (DXM) and nocturnal POX parameters. Secondly, we applied the sequential model (first questionnaire and then POX) to our cohort of patients to determine the number of RP studies that finally would have been needed.
Material and methodsStudy designA longitudinal, prospective, non-interventional cohort study was made of patients referred from the Bariatric Surgery Unit (BCU) to the Sleep Respiratory Disorders Unit (SRDU) of Royo Villanova Hospital in Zaragoza (Spain) between 1 July 2014 and 1 July 2015. The study was approved by the Instituto Aragonés de Ciencias de la Salud (IACS) (CEICA number: 23/2014). The results reported herein were obtained following the protocol of the Epigenetics dysfunction in Morbid Obesity with or without obstructive sleep apnea: the EPIMOOSA study (ClinicalTrials.gov identifier: NCT03995836).16 Informed consent was obtained before subject entry to the study, in accordance with the recommendations of the IACS and the Declaration of Helsinki.
Study populationPatients between 18−60years of age and eligible for BS were referred under the screening criteria indicated in Table 2. The patients were managed in the BCU clinic as usual, without this collateral study altering the therapeutic decisions in relation to the target disease (MO).
Selection criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Age 18−60years | Previously diagnosed sleep respiratory disorder and/or treatment with positive pressure devices |
| BMI>40kg/m2, maintained for 3−5 years, after more than one year of unsuccessful protocolized medical treatment. | Diagnosis of systemic inflammatory disease |
| BMI 35−40kg/m2, with comorbidities associated with MO that could improve with weight loss (AHT, DM, dyslipidemia, OSA, etc.), after more than one year of unsuccessful protocolized medical treatment. | Neoplastic diseases in the previous 5years |
| Signing of informed consent | Cardiovascular event in the previous 6months |
| Pregnancy |
OSA: obstructive sleep apnea; DM: diabetes mellitus; AHT: arterial hypertension; BMI: body mass index; MO: morbid obesity.
Clinical evaluation and questionnaires. The following clinical variables and complementary tests were recorded on the first visit: a) sociodemographic data, medical-surgical history and regular medication use; b) level of daytime sleepiness based on the Epworth sleepiness scale (ESS); c) body weight (in kg), height (in cm), the BMI (weight [kg]/height [m]2), neck circumference (cm), abdominal circumference (cm) and hip circumference (cm); d) blood pressure according to the protocol of the European clinical practice guide; and e) post-bronchodilator spirometry. The following questionnaires were used to assess the probability of OSA: STOP-Bang8 and DXM,13 unifying the variables referring to snoring, and reducing the variables to 9.
Blood tests. Standard blood tests were performed on the baseline visit, including general biochemistry, total cholesterol, triglycerides, high-density lipoprotein-cholesterol (HDL) and low-density lipoprotein-cholesterol (LDL), glycosylated hemoglobin (HbG) and high-sensitivity C-reactive protein (CRP). Aliquots of serum, plasma and whole blood from each patient were stored at −80°C for future tests.
Arterial blood gases. The day after RP, arterial blood gases were measured breathing room air. In all cases this test was performed by the SRDU nurse. The samples were analyzed on the same day, recording the following: pH, PaCO2, PaO2 (mmHg) and bicarbonate (mmol/l).
Sleep study. Use was made of the ApneaLink vs10.20 polygraph (ResMed®, Sydney, Australia) of the SRDU, which includes: continuous recording of airflow via nasal cannula, chest motion, SatO2, snoring and body position. A trained nurse explained the use of the system and retrieved it the following day. The reading of the recording was performed manually by a trained technician blinded to the patient disease. Studies with a duration of less than 180min were repeated for both pulse wave and respiratory events. We defined apnea as the absence of flow for > 10s and hypopnea as a discernible decrease (>30% and <90%) in respiratory signal amplitude with a duration of >10s, or a marked decrease in the thoraco-abdominal sum associated with desaturation (≥3%). The AHI was calculated as the sum of apnea and hypopnea episodes per hour of the recorded time period. The automatic nocturnal oximetry analysis included: ODI, mean SatO2 and the percentage of time with SatO2 < 90% (CT90%).
Statistical analysisThe study sample was divided into two groups: no OSA or mild OSA (AHI<15) and moderate-severe OSA (AHI>15). The data are reported as the mean (standard deviation [SD]) or percentage. Normal data distribution was not assumed; nonparametric testing therefore was used for the comparison of means: the Mann-Whitney U-test. Linear and logistic regression models were used for the relationship between the different variables and AHI (quantitative) and the diagnosis of OSA. Diagnostic performance was assessed based on the receiver operating characteristic (ROC) curve, and the results were expressed using the area under the curve (AUC) and the corresponding confidence intervals. The Youden index was used to select the optimum cut-off points for sensitivity and specificity. Comparison of the ROC curves was made using the Epidata 3.1 statistical package. The analysis was performed using the SPSS version 20.0 statistical package, Microsoft Excel 2016 and GraphPad Prism 7. The recommendations of the STARD 2015 guide for diagnostic tests were followed.17
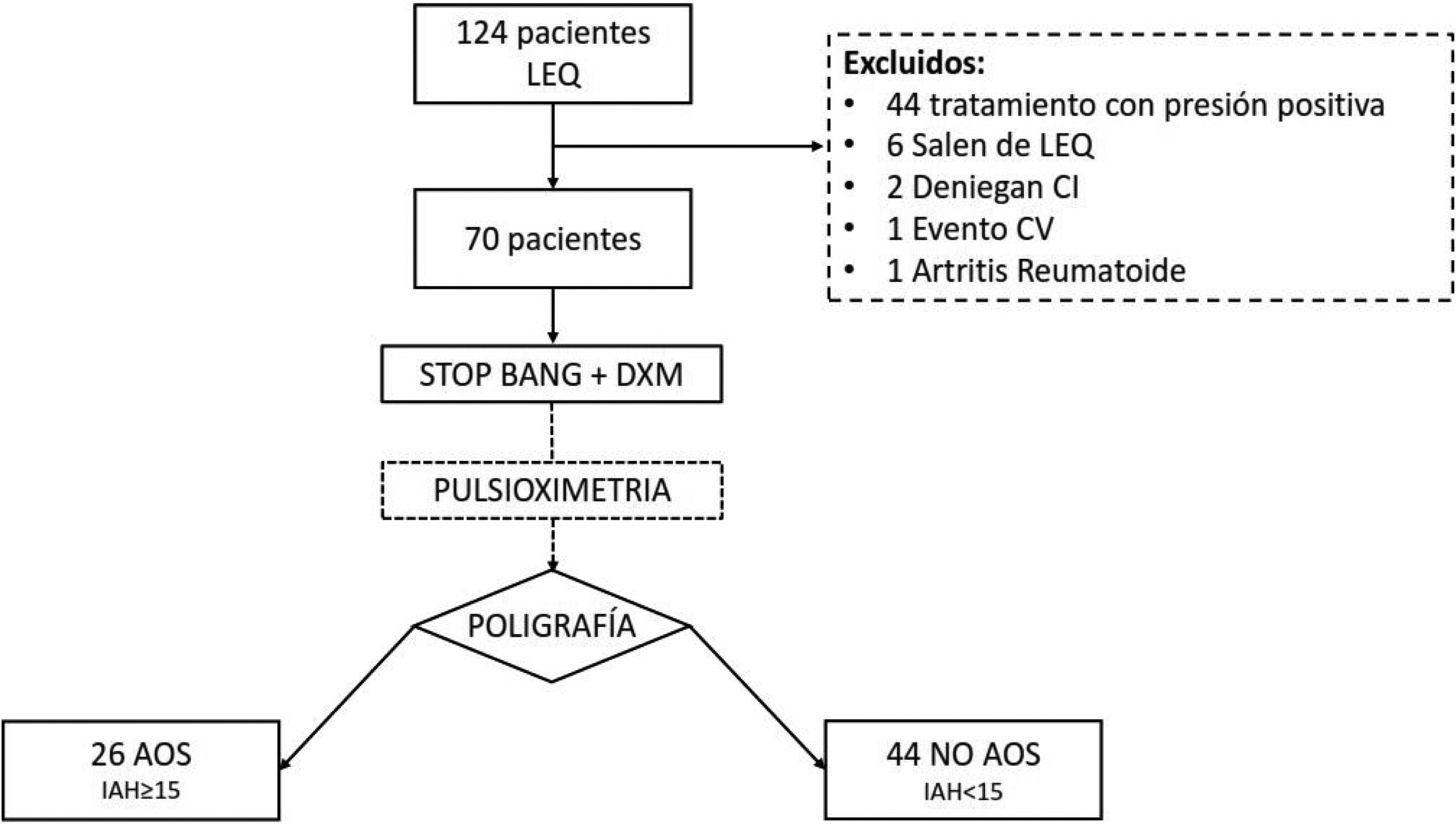
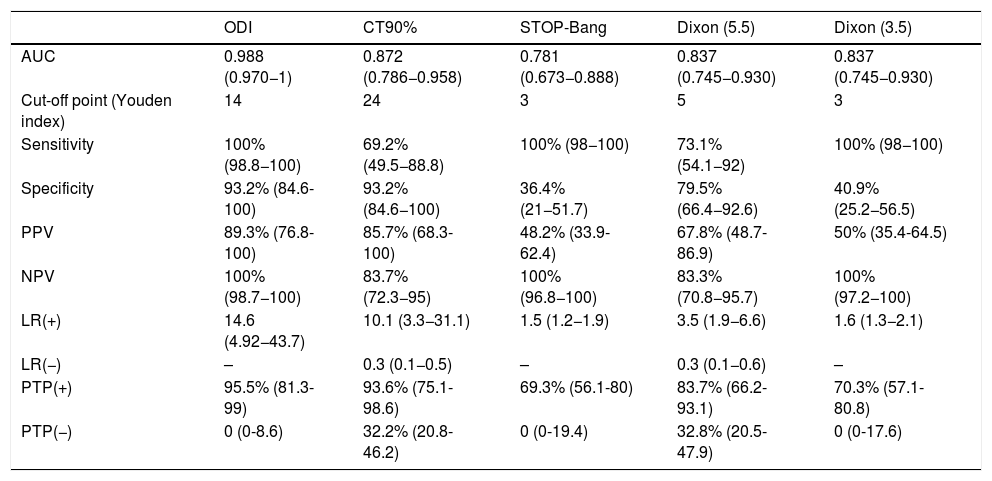
ResultsA total of 124 patients were referred to the SRDU, of whom 70 were finally analyzed (Fig. 1). The characteristics of the group are shown in Table 3. A total of 42 patients (60%) presented AHI>5. Moderate-severe OSA (AHI>15) was diagnosed in 26 patients (37.1%). Of the 70 studies made, 8 had to be repeated: 7 because of error in the nasal cannula recordings, and one due to POX failure.
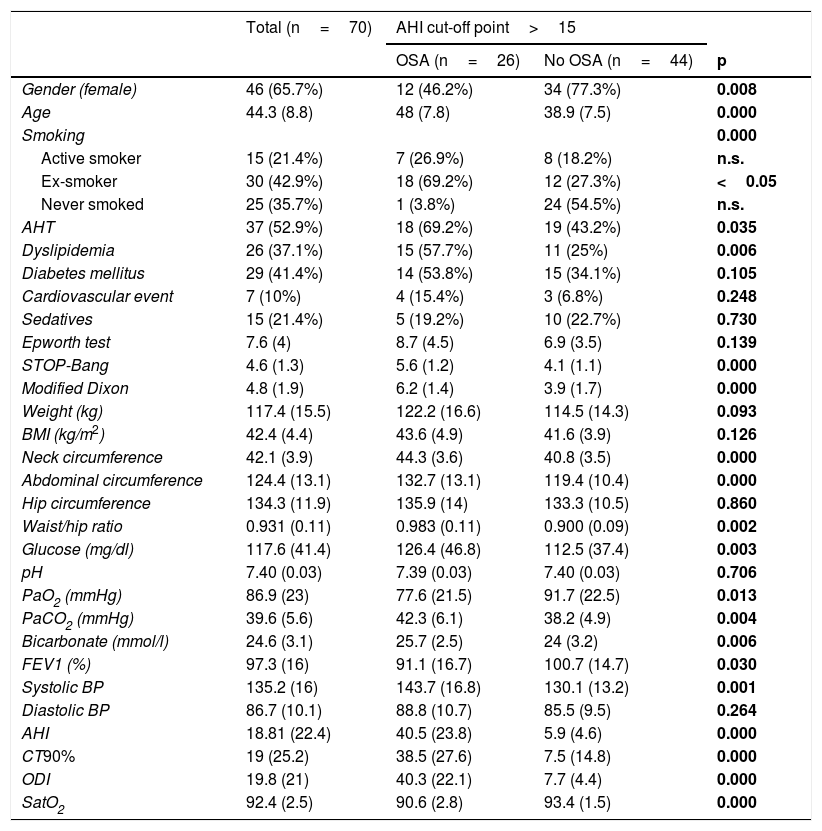
Description of the study sample.
| Total (n=70) | AHI cut-off point>15 | |||
|---|---|---|---|---|
| OSA (n=26) | No OSA (n=44) | p | ||
| Gender (female) | 46 (65.7%) | 12 (46.2%) | 34 (77.3%) | 0.008 |
| Age | 44.3 (8.8) | 48 (7.8) | 38.9 (7.5) | 0.000 |
| Smoking | 0.000 | |||
| Active smoker | 15 (21.4%) | 7 (26.9%) | 8 (18.2%) | n.s. |
| Ex-smoker | 30 (42.9%) | 18 (69.2%) | 12 (27.3%) | <0.05 |
| Never smoked | 25 (35.7%) | 1 (3.8%) | 24 (54.5%) | n.s. |
| AHT | 37 (52.9%) | 18 (69.2%) | 19 (43.2%) | 0.035 |
| Dyslipidemia | 26 (37.1%) | 15 (57.7%) | 11 (25%) | 0.006 |
| Diabetes mellitus | 29 (41.4%) | 14 (53.8%) | 15 (34.1%) | 0.105 |
| Cardiovascular event | 7 (10%) | 4 (15.4%) | 3 (6.8%) | 0.248 |
| Sedatives | 15 (21.4%) | 5 (19.2%) | 10 (22.7%) | 0.730 |
| Epworth test | 7.6 (4) | 8.7 (4.5) | 6.9 (3.5) | 0.139 |
| STOP-Bang | 4.6 (1.3) | 5.6 (1.2) | 4.1 (1.1) | 0.000 |
| Modified Dixon | 4.8 (1.9) | 6.2 (1.4) | 3.9 (1.7) | 0.000 |
| Weight (kg) | 117.4 (15.5) | 122.2 (16.6) | 114.5 (14.3) | 0.093 |
| BMI (kg/m2) | 42.4 (4.4) | 43.6 (4.9) | 41.6 (3.9) | 0.126 |
| Neck circumference | 42.1 (3.9) | 44.3 (3.6) | 40.8 (3.5) | 0.000 |
| Abdominal circumference | 124.4 (13.1) | 132.7 (13.1) | 119.4 (10.4) | 0.000 |
| Hip circumference | 134.3 (11.9) | 135.9 (14) | 133.3 (10.5) | 0.860 |
| Waist/hip ratio | 0.931 (0.11) | 0.983 (0.11) | 0.900 (0.09) | 0.002 |
| Glucose (mg/dl) | 117.6 (41.4) | 126.4 (46.8) | 112.5 (37.4) | 0.003 |
| pH | 7.40 (0.03) | 7.39 (0.03) | 7.40 (0.03) | 0.706 |
| PaO2 (mmHg) | 86.9 (23) | 77.6 (21.5) | 91.7 (22.5) | 0.013 |
| PaCO2 (mmHg) | 39.6 (5.6) | 42.3 (6.1) | 38.2 (4.9) | 0.004 |
| Bicarbonate (mmol/l) | 24.6 (3.1) | 25.7 (2.5) | 24 (3.2) | 0.006 |
| FEV1 (%) | 97.3 (16) | 91.1 (16.7) | 100.7 (14.7) | 0.030 |
| Systolic BP | 135.2 (16) | 143.7 (16.8) | 130.1 (13.2) | 0.001 |
| Diastolic BP | 86.7 (10.1) | 88.8 (10.7) | 85.5 (9.5) | 0.264 |
| AHI | 18.81 (22.4) | 40.5 (23.8) | 5.9 (4.6) | 0.000 |
| CT90% | 19 (25.2) | 38.5 (27.6) | 7.5 (14.8) | 0.000 |
| ODI | 19.8 (21) | 40.3 (22.1) | 7.7 (4.4) | 0.000 |
| SatO2 | 92.4 (2.5) | 90.6 (2.8) | 93.4 (1.5) | 0.000 |
Values are reported as the mean (standard deviation) for quantitative variables and number (percentage) for qualitative variables. Differences explored with the chi-square test for qualitative variables and the Mann-Whitney U-test for the comparison of means.
OSA: obstructive sleep apnea; CT90%: percentage of time with SatO2 < 90%; AHT: arterial hypertension; AHI: sleep apnea/hypopnea index; ODI: oxygen desaturation index; BMI: body mass index; n.s.: nonsignificant; BP: blood pressure.
The differences between the groups with/without OSA are shown in Table 3. There was a similar incidence in both genders in the OSA group, with a slight male predominance (14 patients; 53.8%). Females predominated in the group without OSA. The incidence of cardiovascular risk factors (CVRFs) was higher for patients with OSA, except as regards diabetes mellitus (DM). With regard to the anthropometric variables, the group with OSA presented a higher BMI, though without statistically significant differences. The parameters referring to central fat distribution (abdominal circumference, neck circumference and waist-hip ratio) were higher in the group with OSA. Blood gas recordings were obtained in 63 patients (in 7 cases the sample could not be analyzed). Seven patients presented PaCO2 > 45mmHg and were diagnosed with obesity hypoventilation syndrome (OHS) (Fig. 2).
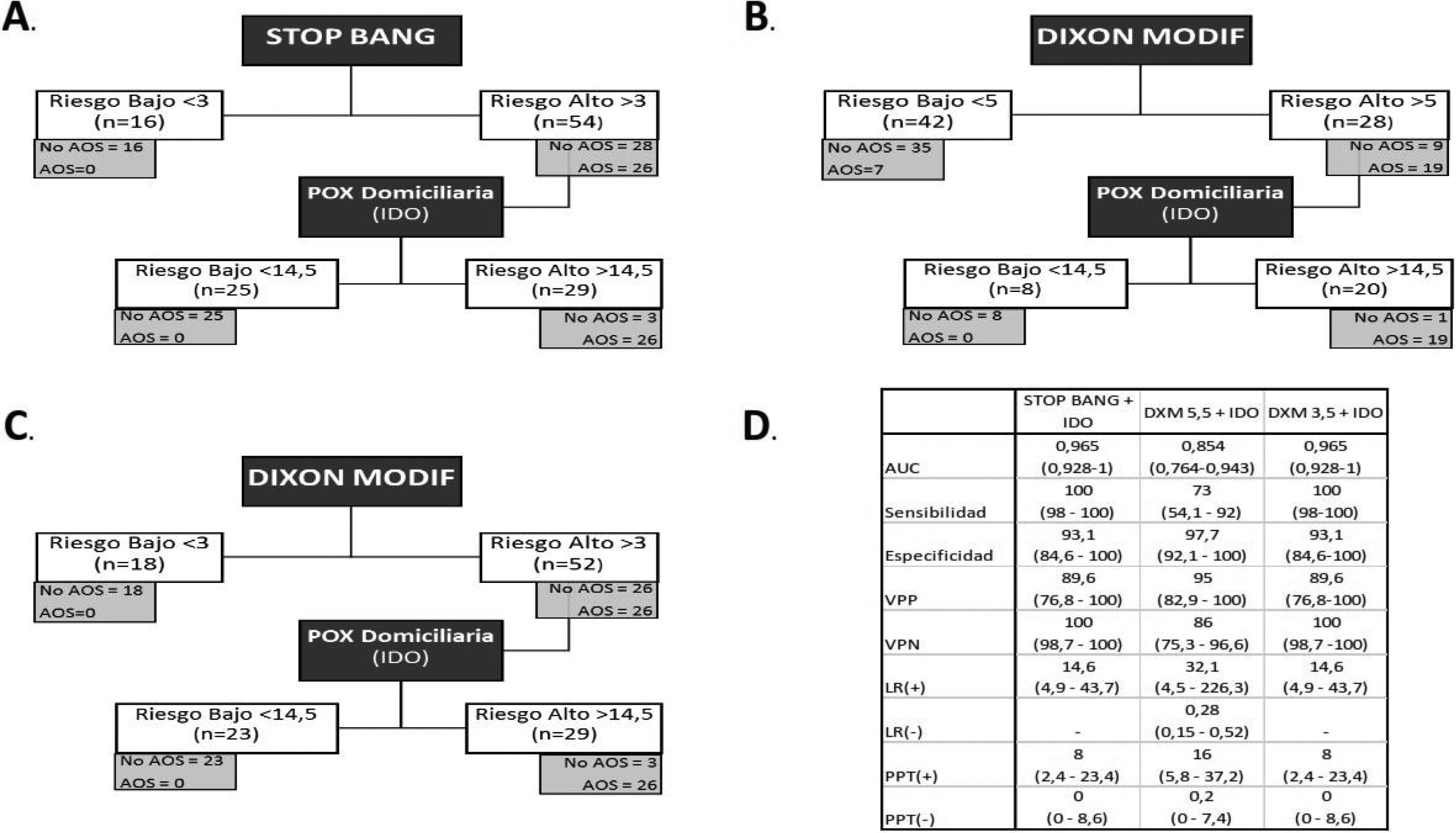
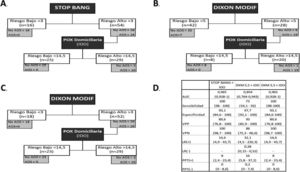
Analysis of the sequential model. A) Sequential model with STOP-Bang and pulse oximetry. B) Sequential model with the modified Dixon tool (cut-off point 5) and pulse oximetry. C) Sequential model with the modified Dixon tool (cut-off point 3) and pulse oximetry. D) Analysis of diagnostic tests of the different sequential models.
Table 4 shows the analysis of the diagnostic capacity of each of the POX parameters and of the questionnaires. The nocturnal oximetry parameters showed a greater AUC, with statistically significant differences in favor of ODI versus CT90% (p=0.0041), and the STOP-Bang (p=0.0000) and DXM questionnaires (p=0.0004). The DXM showed a better AUC than STOP-Bang, though the differences were not statistically significant (p=0.212). The optimum cut-off point for STOP-Bang (>3) yielded a sensitivity of 100%, while in the case of DXM (>5) the sensitivity was 73%. With DXM, the cut-off point had to be reduced to 3 in order to reach 100% sensitivity.
Individualized analysis of the diagnostic tests.
| ODI | CT90% | STOP-Bang | Dixon (5.5) | Dixon (3.5) | |
|---|---|---|---|---|---|
| AUC | 0.988 (0.970−1) | 0.872 (0.786−0.958) | 0.781 (0.673−0.888) | 0.837 (0.745−0.930) | 0.837 (0.745−0.930) |
| Cut-off point (Youden index) | 14 | 24 | 3 | 5 | 3 |
| Sensitivity | 100% (98.8−100) | 69.2% (49.5−88.8) | 100% (98−100) | 73.1% (54.1−92) | 100% (98−100) |
| Specificity | 93.2% (84.6-100) | 93.2% (84.6−100) | 36.4% (21−51.7) | 79.5% (66.4−92.6) | 40.9% (25.2−56.5) |
| PPV | 89.3% (76.8-100) | 85.7% (68.3-100) | 48.2% (33.9-62.4) | 67.8% (48.7-86.9) | 50% (35.4-64.5) |
| NPV | 100% (98.7−100) | 83.7% (72.3−95) | 100% (96.8−100) | 83.3% (70.8−95.7) | 100% (97.2−100) |
| LR(+) | 14.6 (4.92−43.7) | 10.1 (3.3−31.1) | 1.5 (1.2−1.9) | 3.5 (1.9−6.6) | 1.6 (1.3−2.1) |
| LR(−) | – | 0.3 (0.1−0.5) | – | 0.3 (0.1−0.6) | – |
| PTP(+) | 95.5% (81.3-99) | 93.6% (75.1-98.6) | 69.3% (56.1-80) | 83.7% (66.2-93.1) | 70.3% (57.1-80.8) |
| PTP(−) | 0 (0-8.6) | 32.2% (20.8-46.2) | 0 (0-19.4) | 32.8% (20.5-47.9) | 0 (0-17.6) |
Confidence intervals are shown in parentheses.
AUC: area under the curve; CT90%: percentage of time with SatO2 < 90%; ODI: oxygen desaturation index; LR(+): positive likelihood ratio; LR(−): negative likelihood ratio; PTP(+): positive post-test probability; PTP(−): negative post-test probability; PPV: positive predictive value; NPV: negative predictive value.
The sequential model starting with STOP-Bang >3 would have correctly excluded 16 patients (22.8%). The remaining 54 patients would have undergone POX, and of these 25 (46.2%) would have been classified as low risk. The remaining 29 (41.4%) of the first 70 subjects would have been the final candidates for RP/PSG, with 41 cases being avoided (58.5%) (Fig. 2).
The sequential model starting with DXM was analyzed based on the optimum cut-off point calculated from the Youden index (>5) and achieving a sensitivity of 100% (>3.5). In the former case, 42 patients (60%) were classified as low risk after the model was applied. Of these, 7 (16.6%) were erroneously classified as low risk, since they actually suffered OSA. After applying the nocturnal POX cut-off point, 20 patients (28.5%) of the first 70 would have required RP/PSG, with 50 cases being avoided (71.4%).
Using the cut-off point of 3 for the DXM model, in the first phase 18 patients (25.7%) would have been excluded, as they were of low risk, thus leaving 52 patients who would have undergone POX. This test would have excluded 23 patients (44.2%), classifying them as low risk, and leaving 29 patients (41.4%) to ultimately undergo RP/PSG, with 41 cases being avoided (58.5%).
DiscussionIn our study cohort, the application of a sequential model with a questionnaire followed by POX would have considerably reduced the number of RP recordings. This is the first study to date analyzing a sequential model for the screening of OSA in a population with a high prevalence of the latter disorder. The DXM model, with a greater number of anthropometric variables, obtained a better AUC, though from the statistical perspective it was not superior to the STOP-Bang questionnaire as a screening tool. The ODI afforded the greatest diagnostic performance and thus should be included in the screening protocol of patients with MO eligible for BS.
The prevalence of OSA in our patient cohort was 60%, which is similar to that reported in other studies,18,19 with a clear predominance of females, though the incidence of OSA is higher in males. Among the CVRFs, arterial hypertension and dyslipidemia were more prevalent in the moderate-severe OSA group. Diabetes mellitus and cardiovascular antecedents, as in other studies,20 showed no significant differences between the two groups, possibly because of the strong cardiovascular dimension of obesity. The incidence of OHS in our study was higher than reported in other studies,21 though it is not common for them to include blood gas measurements to confirm this.
The symptoms of OSA are easily recognizable in the form of snoring, apnea and excessive daytime sleepiness.22 This symptom is closely related to MO, independently of the presence of OSA.23 Nocturnal symptoms, particularly snoring, are nonspecific, and their underlying etiology is variable. From the anthropometric perspective, close relationships have also been found between the BMI and abdominal or cervical circumference (among others), and AHI.24 In addition, patients with OSA are at an increased risk of presenting other CVRFs such as AHT, dyslipidemia or DM.4
The questionnaires showed a relationship with AHI and the diagnosis of OSA. The DXM tool showed greater predictive capacity than STOP-Bang, as well as a greater AUC, though without significant differences. The sensitivity of STOP-Bang was greater than that of the DXM model for the optimal cut-off points indicated by the Youden index. In order to obtain the same sensitivity with DXM, the cut-off point would have to be reduced to 3, resulting in a considerable decrease in specificity. The STOP-Bang questionnaire is recommended for OSA screening in BS.7 Its sensitivity in discriminating moderate to severe OSA ranges from 85 to 100%, depending on the study,10,25 though this tool has also been questioned by other authors who have recorded lower sensitivities.26 In our comparative analysis of STOP-Bang and DXM, the AUC was found to be greater using the latter, though the differences were not significant.
Other questionnaires and mathematical models used for screening purposes have been analyzed. We ruled out the ESS due to the high incidence of daytime sleepiness in patients with MO. The formula proposed by Palla et al.20 incorporates the ESS together with three other variables (gender, age and minimum SatO2), reaching sensitivities of close to 100%. An analysis of four mathematical models including variables such as the BMI and neck circumference showed the diagnostic precision to increase, but with poor specificity.27 Other questionnaires such as the multivariate apnea predictor (MAP) score offer acceptable sensitivity, but were discarded because of their low negative predictive value.28
All these questionnaires, mainly targeted to obese populations, do not include determining factors for the development of OSA in patients with MO. Anthropometric measurements are able to explain 20% of AHI, and such measurements should include those referring to central obesity: neck and abdominal circumference, together with the waist-hip ratio.11 The only model found that takes these three parameters into account is that developed by Dixon in 2003 and modified by Kolotkin. The Kolotkin model obtained an AUC=0.821 with a sensitivity of 77% and 85% for AHI>15 and AHI>30, respectively. The sensitivity of the Dixon model was greater than that of the Kolotkin model, reaching 89% for AHI>15 and 96% for AHI>30.
The oximetric variables used in our study were ODI, CT90% and mean SatO2. The ODI showed the best correlation and diagnostic precision. Its usefulness as a screening tool in a cohort with MO has already been demonstrated,15 reaching AUC=0.950 for ODI 3% and AUC=0.910 for CT90%, with a sensitivity in both cases of 100%, but with very low specificity. Pulse oximeters are easy to read and interpret and do not require an exhaustive analysis of the parameters recorded, which facilitates their use in screening. The sequential use of POX followed by PSG was studied in a cohort of patients with clinically suspected OSA, and demonstrated its usefulness,29 though the disadvantage of this strategy is that all patients need to pass through the sleep laboratory.
We therefore propose a model with two levels: at the clinic with the questionnaire and at the home of the patient with nocturnal POX. The application of this model would have avoided 59% of the RP recordings, which is higher than reported by Vries et al.15 This model has been studied in the general population with the OSA-50 and Philips questionnaires, reaching sensitivities of 91% and 100%, and significantly reducing the number of RP studies required.30,31 A similar analysis was made in a cohort of 141 candidates for BS,21 where the selected cut-off point for STOP-Bang was >4. Patients at high risk underwent POX, which directly yielded a diagnosis of OSA. This study has a significant methodological bias, because the incidence of OSA was not confirmed with the gold standard or with RP. In our study, the application of the sequential model solved the problem of low specificity, establishing it at > 95% and maintaining 100% sensitivity. According to the reviewed literature, this is the first study to analyze a sequential model for OSA screening in morbidly obese candidates for BS.
Our study has limitations, such as its single-center nature, which affects its external validity. The POX data were obtained from the RP registry and not with a separate device. The gold standard for the diagnosis of sleep respiratory disorders (PSG) was not available. There is another limitation regarding the exclusion criteria, since patients who had already undergone a sleep study were removed from the study. On making comparisons with other studies, the incidence of OSA was lower, which suggests that the results may have been overestimated.
ConclusionsThe sequential model combining STOP-Bang first and followed by POX offers the best results in terms of the differentiation of morbidly obese patients with OSA (AHI>15). Individually, no questionnaire proved superior to POX in screening for OSA. Sequential screening improves specificity while maintaining high sensitivity and lessening the need for RP/PSG. A multicenter study is required to validate this sequential model, including the STOP-Bang questionnaire and POX, as a screening tool for OSA in morbidly obese candidates for bariatric surgery.
Financial supportThis research project has been supported by FIS (15/01940), the Sociedad Española de Patología del Aparato Respiratorio (SEPAR-2017) and the Sociedad Aragonesa de Aparato Respiratorio (SADAR-2016).
AuthorshipJL and JMM designed the study; JL, PC, SS, CC and JF recorded the data and analyzed the complementary tests; JL and JMM performed the final analysis of the data; JL and JMM wrote the manuscript, which has been reviewed and approved by the other authors.
Conflicts of interestNeither the principal author nor the other authors have any conflicts of interest in relation to this article.
Thanks are due to our colleagues of the Pneumology Department of Hospital Royo Villanova.
Please cite this article as: Lázaro J, Clavería P, Cabrejas C, Fernando J, Segura S, Marín JM. Sensibilidad de un modelo secuencial basado en cuestionario (STOP-Bang vs. Dixon) y pulsioximetría nocturna para el screening de apnea obstructiva del sueño en pacientes obesos mórbidos candidatos a cirugía bariátrica. Endocrinol Diabetes Nutr. 2020;67:509–516.