The study of genetic mutations in thyroid nodules makes it possible to improve the preoperative diagnosis of and reduce unnecessary surgeries on benign nodules. In this study, we analysed the impact of implementing a 7-gene mutation panel that enables mutations to be detected in BRAF and RAS (H/N/K) and the gene fusions PAX8/PPARG, RET/PTC1 and RET/PTC2, in a population in northern Argentina.
MethodsWe performed a prospective analysis of 112 fine needle aspirations diagnosed as having indeterminate cytology according to the Bethesda classification system. These include the Bethesda III or atypia of unknown significance/follicular lesion of unknown significance and Bethesda IV or follicular neoplasm/suspicious for follicular neoplasm categories. The mutations of the 7-gene panel were analysed and this information was linked to the available histology and ultrasound monitoring.
ResultsThe BRAF V600E and RET/PTC1 mutations were associated with carcinoma in 100% of cases (n = 8), whereas only 37.5% (n = 3) of the nodules with RAS and 17% (n = 1) with PAX8/PPARG mutations were associated with carcinoma. From the histological diagnosis and ultrasound monitoring of patients, we can estimate that this panel has a sensitivity of 86% in detecting malignant carcinoma, a specificity of 77%, a positive predictive value (PPV) of 54% and a negative predictive value (NPV) of 94%. In this study, it was possible to reduce the number of surgeries by 48% in the patients analysed.
ConclusionThe implementation of the mutation panel allowed the appropriate surgical strategy to be selected for each patient, the number of two-step surgeries to be reduced, and active follow-up to be established in low-risk patients.
El estudio de mutaciones presentes en nódulos tiroideos permite mejorar el diagnóstico preoperatorio y reducir la realización de cirugías innecesarias de nódulos benignos. En este estudio analizamos el impacto de la implementación de un panel de 7 genes que permite detectar mutaciones en los genes BRAF, RAS (H/N/K) y las fusiones PAX8/PPARG, RET/PTC1 y RET/PTC2, en una población del norte de Argentina.
MétodoSe analizaron prospectivamente 112 punciones aspirativas con aguja fina diagnosticadas citológicamente como indeterminadas según la clasificación del sistema Bethesda. Estas incluyen las categorías Bethesda III o atipia/lesión folicular de significado incierto y Bethesda IV o neoplasia folicular/sospechoso de neoplasia folicular. Se analizó el panel de mutaciones de 7 genes, luego esa información se vinculó con la histología disponible y el seguimiento ecográfico.
ResultadosLas mutaciones BRAF V600E y RET/PTC1 estuvieron asociadas en un 100% a carcinomas (n = 8), en cambio las mutaciones en RAS y PAX8/PPARG estuvieron asociados en un 37,5% (n = 3) y 17% (n = 1) a carcinomas, respectivamente. El diagnóstico histológico y el seguimiento ecográfico de pacientes permitió determinar una sensibilidad del 86%, una especificidad del 77%, un valor predictivo positivo (VPP) del 54% y un valor predictivo negativo (VPN) del 94%. En este estudio, se logró disminuir las cirugías en un 48% en los pacientes analizados.
ConclusiónLa implementación del test del panel de mutaciones permitió seleccionar la estrategia quirúrgica adecuada para cada paciente, reducir las cirugías en dos pasos, y establecer un seguimiento activo en pacientes de bajo riesgo.
Differentiated thyroid cancer is the most common endocrine neoplasm. The incidence of thyroid cancer has increased worldwide over the last three decades. This may be due, in part, to increased detection of small or subclinical thyroid nodules by ultrasound or other imaging techniques1,2 and to a true increase in the incidence of thyroid cancer.3,4 However, similar to what occurs in other countries, studies carried out in the Autonomous City of Buenos Aires and Greater Buenos Aires indicate that in the period 2012-2016 the incidence of thyroid cancer increased by 62.5%, reaching 11.83 new cases/100,000 inhabitants/year5 in relation to the period 2003-2011, where it was 6.51 cases/100,000 inhabitants/year according to the age-standardised rate.6
Fine needle aspiration (FNA) followed by cytological analysis is one of the key tools for the initial diagnosis of thyroid nodules.7,8 This methodology is very useful for the surgical management of the patient9 since it can define with high precision whether a nodule is benign or malignant.8 However, 20-30% of aspirations are indeterminate, or in grey areas, according to the Bethesda system classification,8,10–12 and correspond to the following categories: Bethesda III, Atypia of Uncertain Significance/Follicular Lesion of Undetermined Significance (AUS/FLUS), and Bethesda IV, Follicular Neoplasm/Suspicious for Follicular Neoplasm (FN/SFN).8,13,14 The malignancy rate observed in these categories varies in the different centres and institutions, ranging from 6 to 48% for Bethesda III and from 14 to 34% for Bethesda IV.10 The frequency of malignancy in these groups generates diagnostic uncertainty that leads to repeated FNAs or unnecessary surgeries.10,15 Furthermore, it is known that in 10-40% of these surgeries these turn out to be benign nodules.8,16
During the last decade, numerous molecular tests have been developed in thyroid nodules that have improved the diagnostic precision of cytology, implementing the search for somatic mutations that would help discriminate thyroid cancers with greater sensitivity and specificity.14–16
Papillary thyroid carcinoma frequently presents mutations in the BRAF and RAS genes or RET/PTC gene fusions. These mutually exclusive somatic mutations are found in more than 70% of papillary carcinomas and are generally associated with more aggressive tumour behaviour.17,18 Follicular thyroid carcinoma can also present mutations in RAS and the PAX8/PPARG gene fusion, which are found in 80% of these tumours.17
The feasibility of studying the most common somatic mutations of differentiated thyroid cancers in thyroid FNA and providing useful information for diagnosis has been previously described.7,19,20 Since its review in 2009, the American Thyroid Association recommends and highlights the importance of molecular studies in nodules with indeterminate cytology (AUS/FLUS and FN/SFN) to guide the physician in patient management.21 However, a consensus has not yet been reached on what would be the best strategy for the study of mutations in thyroid nodule aspirations.22
In the present work we evaluate the usefulness of a molecular panel that includes the study of point mutations in the BRAF (exon 15), NRAS, HRAS and KRAS (exons 2 and 3) genes, and the RET/PTC1, RET/PTC3 and PAX8/PPARG gene fusions from FNAs of indeterminate cytology for the AUS/FLUS and FN/SFN categories. At the same time, we analyse the results of this molecular panel in relation to the cytological and histological diagnosis, determining its usefulness to define the treatment of patients at the Hospital de Endocrinología y Metabolismo Dr. Arturo Oñativia [Dr. Arturo Oñativia Hospital of Endocrinology and Metabolism] in the city of Salta, Argentina.
Materials and methodsPatient samplesWe prospectively analysed 112 FNA samples diagnosed as AUS/FLUS or FN/SFN corresponding to patients at Hospital Dr. Arturo Oñativia in the city of Salta. The samples included in this study correspond to the period from December 2014 to December 2018. FNA was the standard method for collecting samples for molecular study.8,12 The FNAs were performed under ultrasound guidance with physicians specialised in imaging, minimally invasive surgery, and anatomical pathology. In the case of multiple nodules in a patient, the nodule with the highest ultrasound criteria for malignancy was selected for the study. The FNA material was smeared on slides with ground edges and immediately fixed in 95% alcohol for Papanicolaou staining and dry for May-Grunwald-Giemsa staining. The smears were analysed by two specialists in anatomical pathology. The inclusion criterion of a smear for subsequent mutation analysis was the presence of at least 6 groups of 10 or more cells, according to the Bethesda system.8 The patients included in this study had two consecutive FNAs diagnosed as AUS or FLUS or one diagnosed as FN/SFN. This work was approved by the Teaching and Research Commission of the Hospital Dr. Arturo Oñativia. All the patients signed a consent form prior to the study.
Nucleic acid extractionTotal RNA extraction was performed from the remaining material obtained from the FNA, after performing the cytological smear, applying the TRIzol protocol (TRIPure Isolation Reagent, Roche). Cell scrapings from cytological smears were used to obtain genomic DNA,23 and purified with the High Pure PCR Template Preparation Kit (Roche) according to the manufacturer's instructions. The concentration of RNA and DNA was determined with the Quibit 2.0 Fluorometer v3.11. The presence of thyroid cells, the integrity of the RNA and the efficiency of the reverse transcription by conventional PCR amplification of gene expression for GUS®, PAX8 and TSH genes were confirmed in each sample. The quality of the extracted DNA was evaluated by qPCR amplification of the BRAF and RAS genes.
Gene fusion studyPAX8/PPARG, RET/PTC1 and RET/PTC3 gene fusions were studied by RT-PCR followed by Nested-PCR,20,24 with self-designed primers flanking the fusion points. Reverse transcription of total RNA to complementary DNA (cDNA) was performed with Random Hexamers plus dT oligos and the reverse transcriptase enzyme RevertAid RT (Thermo Scientific) according to the manufacturer's protocol. For reverse transcription, 500 ng of RNA was used. Conventional PCR and Nested-PCR were performed with the enzyme Hot FIREPol DNA polymerase (SolisBioDyne). The PCR reactions were carried out in a thermal cycler from Applied Biosystems (Brand: Veriti, 96-Well Thermo Cycler). The PCR products were detected by electrophoresis on a 2% (w/v) agarose gel stained with GelGreen (Biotium. Nucleic Acid Gel Stain, 10,000 X in water) in 1X TAE, at 150 V, for 40 minutes at room temperature. Fragments were visualised under blue light on an Invitrogen Safe Imager 2.0 transilluminator. The size of the DNA fragments was estimated by comparison with a 100 bp molecular weight marker (Genbiotech, 100 bp DNA Laeder).
Detection of point mutations in the BRAF and RAS genesThe detection of point mutations in exons 2 and 3 of H-, K- and N-RAS, plus exon 15 of BRAF were studied by High Resolution Melting (HRM) and Sanger sequencing. In parallel, the Thyroid Cancer Mutation Analysis Kit (Entrogen, Inc) was used to validate the HRM results.
High Resolution Melting analysisA real-time PCR was performed with specific primers of our own design (oligonucleotide sequences are available upon request) for each exon and protocols designed according to the conditions of each amplicon, using the HOT FIREPol EvaGreen HRM Master Mix (Solis BioDyne). A real-time thermocycler was used LightCycler 96 (Roche). In each round of amplification, in addition to the samples, normal controls, positive controls previously sequenced by Sanger, and negative controls (H2O), all in duplicate, were included. Analysis was then performed with the Light Cycler 96 Roche software.
Sanger sequencingAccording to the results of the HRM study, the samples that presented curves different from normal curves were sent to Macrogen, Inc. (Seoul, South Korea) for sequencing. Each amplicon was sequenced in both directions and the sequences obtained were analysed with the Sequencher™ programme (version 4.1.4). The Ensembl Genome Browser 96 (www.ensembl.org) and ClinVar (www.ncbi.nlm.nih.gov/clinvar/) platforms were used for the analysis of changes found in the sequences to identify them.
Confirmation by commercial kitThe Thyroid Cancer Mutation Analysis kit specifically amplifies mutated sequences in samples containing a mixture of DNA with and without mutations. It uses dual-labelled hydrolysis probes for the detection of amplification products. The kit enables the study of the following mutations. BRAF: V600E; KRAS: G12D, G12A, G12V, G12S, G12R, G12C, G13D, Q61E, Q61K, Q61L, Q61R, Q61P, Q61H, Q61H; NRAS: G12D, G12S, G12C, G13R, G13V, Q61K, Q61L, Q61R, Q61H, A146T, and HRAS: G12V, G13R, Q61R. The presence of mutations is detected with a FAM-BHQ labelled probe, while internal control gene amplification is detected with a Cal-Fluor Orange 560–BHQ probe (VIC/HEX equivalent).
Statistical analysisTo determine sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), calculations were performed as follows:
- •
Sensitivity = true positives / (true positives + false negatives)
- •
Specificity = true negatives / (true negatives + false positives)
- •
PPV = true positives / (true positives + false positives)
- •
NPV = true negatives / (true negatives + false negatives)
Histology was considered to be the gold standard for defining malignancy. In the case of follicular adenomas, a negative molecular result was considered a true negative, while a positive result was considered a false positive. On the other hand, if the histological diagnosis was carcinoma, a negative molecular result was considered a false negative. In the case of patients who did not undergo surgery, with nodules negative for the molecular panel, they were considered true negatives.
The risk of malignancy (ROM) was calculated at two different times. First, the so-called pre-test ROM was calculated in the 2012-2014 period, prior to the implementation of the molecular biology test in our institution. The pre-test ROM was calculated for the AUS/FLUS and FN/SFN categories on the total number of patients operated on in each category. After applying the mutation test, we calculated the post-test ROM, taking into account the patients with nodules that presented a mutation and malignant histology in relation to the total number of mutations found in each category.
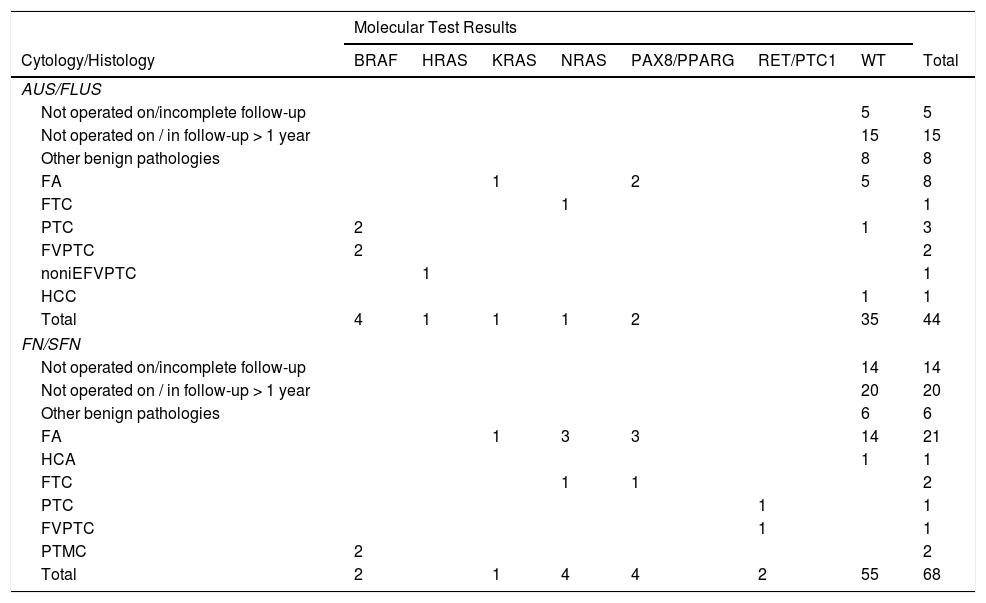
ResultsEvaluation of samples for molecular analysisAmong the 112 indeterminate FNA samples, 44 were diagnosed as AUS/FLUS (39%) and 68 as FN/SFN (61%) (Table 1). It was possible to obtain DNA and RNA of sufficient quality in 95 samples and a second aspiration was requested in the remaining 17 patients, which made it possible to obtain better quality nucleic acids, enabling studies of gene mutations and fusions to be completed. The implementation of tissue-specific control genes, such as PAX8 and TSH, and constitutional ones such as GUS®, allowed us not only to verify the quality of the RNA but also to confirm the thyroid cell origin.
Detail of mutations found with the molecular test.
| Molecular Test Results | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cytology/Histology | BRAF | HRAS | KRAS | NRAS | PAX8/PPARG | RET/PTC1 | WT | Total |
| AUS/FLUS | ||||||||
| Not operated on/incomplete follow-up | 5 | 5 | ||||||
| Not operated on / in follow-up > 1 year | 15 | 15 | ||||||
| Other benign pathologies | 8 | 8 | ||||||
| FA | 1 | 2 | 5 | 8 | ||||
| FTC | 1 | 1 | ||||||
| PTC | 2 | 1 | 3 | |||||
| FVPTC | 2 | 2 | ||||||
| noniEFVPTC | 1 | 1 | ||||||
| HCC | 1 | 1 | ||||||
| Total | 4 | 1 | 1 | 1 | 2 | 35 | 44 | |
| FN/SFN | ||||||||
| Not operated on/incomplete follow-up | 14 | 14 | ||||||
| Not operated on / in follow-up > 1 year | 20 | 20 | ||||||
| Other benign pathologies | 6 | 6 | ||||||
| FA | 1 | 3 | 3 | 14 | 21 | |||
| HCA | 1 | 1 | ||||||
| FTC | 1 | 1 | 2 | |||||
| PTC | 1 | 1 | ||||||
| FVPTC | 1 | 1 | ||||||
| PTMC | 2 | 2 | ||||||
| Total | 2 | 1 | 4 | 4 | 2 | 55 | 68 | |
Other benign pathologies include: thyroiditis, goitres and adenomatous nodules.
HCA: Hürthle cell follicular adenoma; FA: follicular adenoma; FTC: follicular thyroid carcinoma; PTC: papillary thyroid carcinoma; FVPTC: follicular variant papillary thyroid carcinoma; noniEFVPTC: non-invasive encapsulated follicular variant of papillary thyroid carcinoma; PTMC: papillary thyroid micro carcinoma; HCC: Hürthle cell carcinoma; WT: wild type gene version.
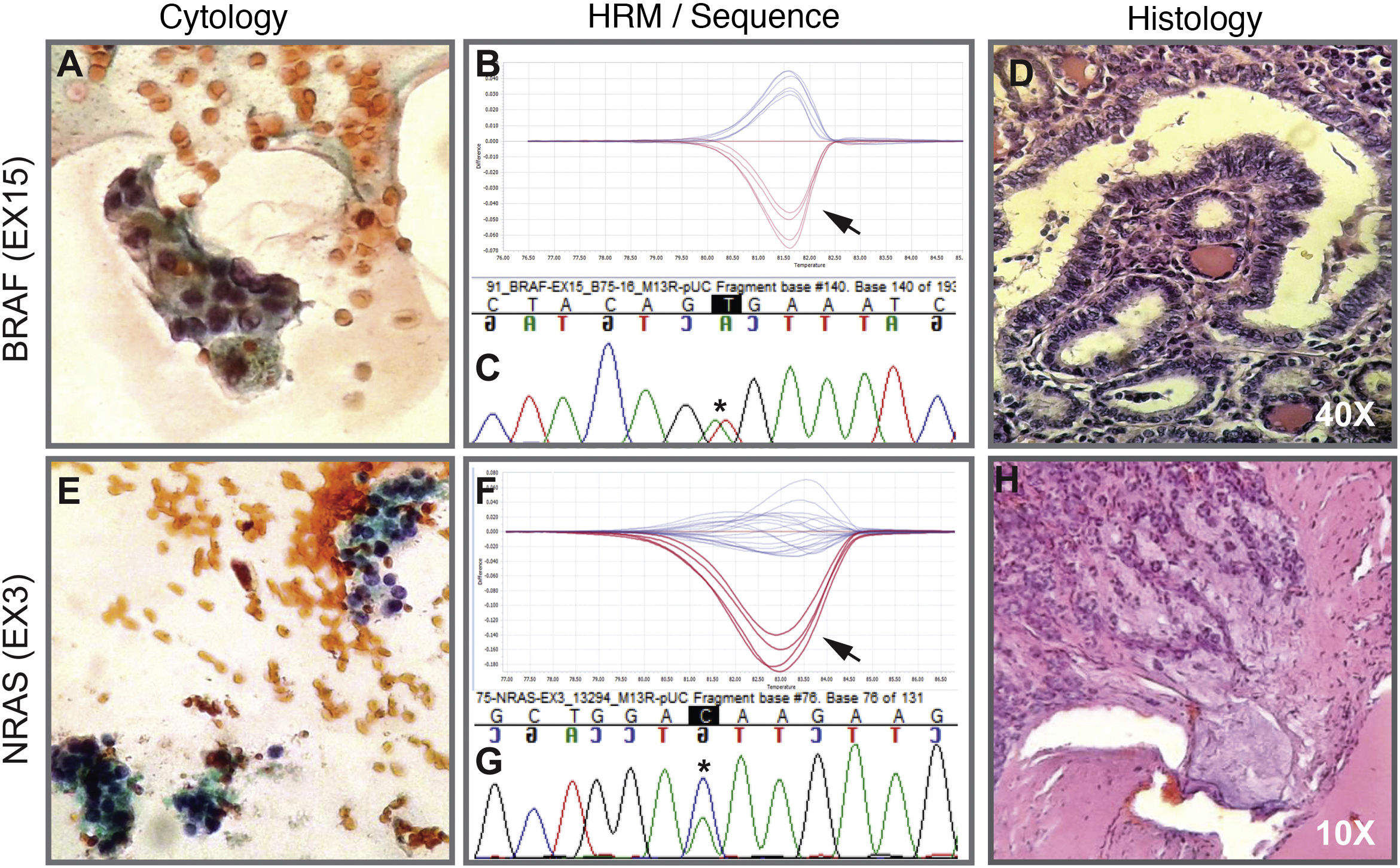
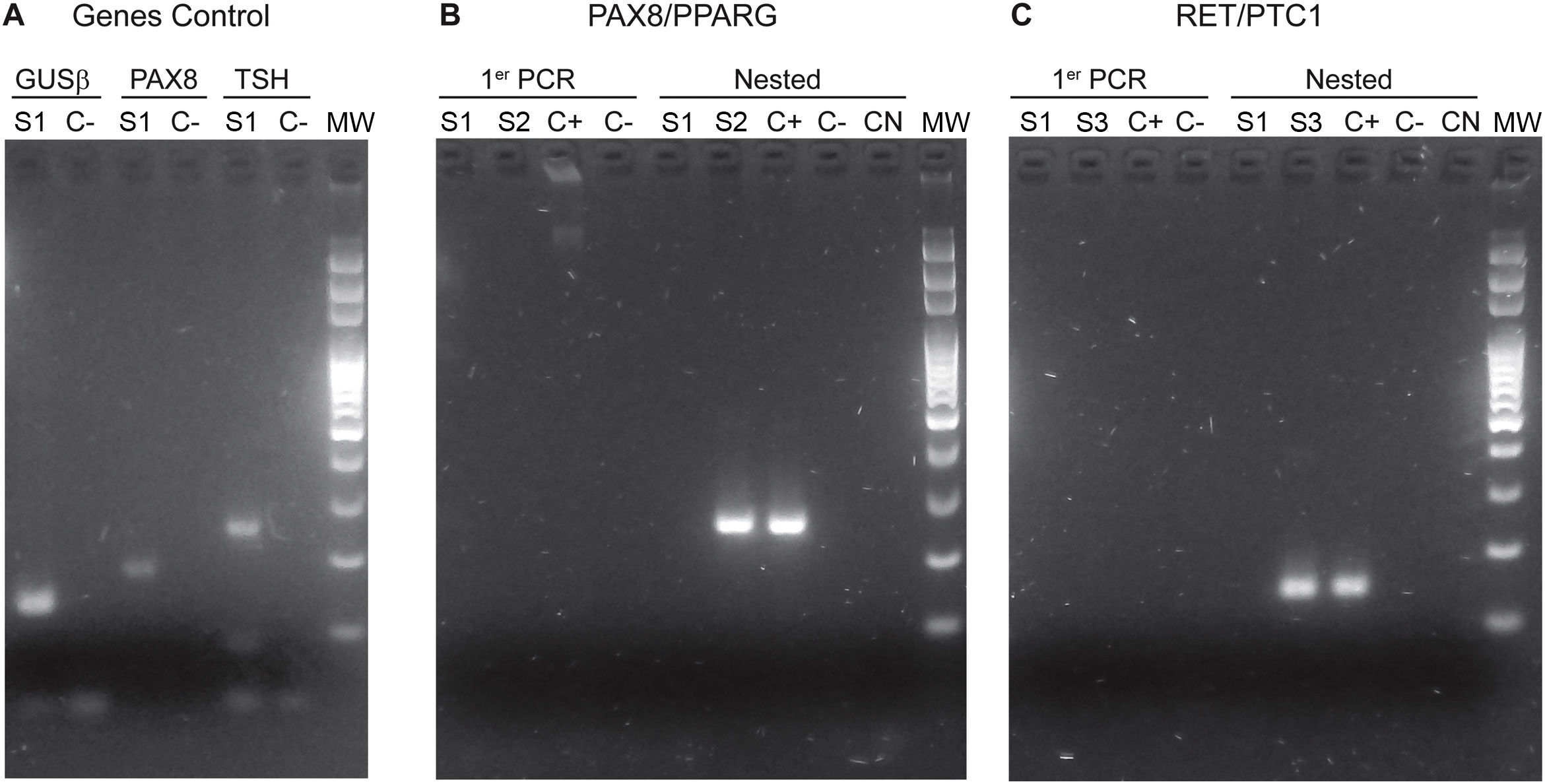
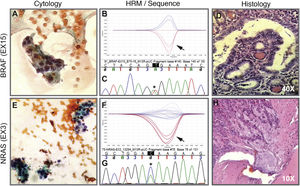
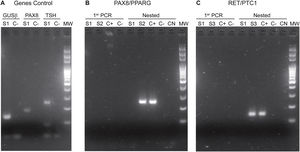
The implementation of the panel that includes point mutations in exon 15 of the BRAF gene, exons 2 and 3 of the NRAS, HRAS and KRAS genes and the RET/PTC1, RET/PTC3 and PAX8/PPARG gene fusions enabled the identification of 22 mutations in the 112 samples (20%). Among the most common are eight samples with mutations in RAS (8%), distributed among the three members of the family: NRAS, HRAS and KRAS (Table 1). This is followed in frequency by six samples with the V600E mutation in the BRAF gene (5%). Fig. 1 shows representative examples of cytology, HRM mutation analysis, sequencing, and histopathology of patient samples with NRAS and BRAF mutations. Then, when analysing gene fusions, it was observed that the most common was PAX8/PPARG found in six samples (5%) and RET/PTC1 fusion was found in only two samples (2%). Representative examples of how PAX8/PPARG and RET/PTC1 fusions are detected are shown in Fig. 2.
Cytology image, HRM, sequence and histology. Above. Example of a cytological smear of an AUS/FLUS category indeterminate follicular proliferation (A) that was positive for the BRAF V600E mutation indicated by the arrow on the HRM curve (B) confirmed by Sanger sequencing (C). Then, the histology in D indicates that it is an invasive non-encapsulated follicular variant of papillary thyroid carcinoma. Below. Example of cytology of a nodule diagnosed as suspicious for follicular neoplasm (E) with the presence of the NRAS-Ex3 mutation detected by HRM (F) and confirmed by Sanger sequencing (G). Histology H indicates that it is a minimally invasive encapsulated follicular thyroid carcinoma. A and E correspond to cytology stained with the Papanicolaou technique. Biopsies D and H are stained with haematoxylin-eosin. The black arrows indicate the curve of the positive sample plus the positive control. Asterisks in black indicate the base change associated with the mutation.
Photos of electrophoretic PCR runs performed for the identification of fusions. A) Genes Control: GUSβ in lines 1 and 2. PAX8 in lines 3 and 4. TSH in lines 5 and 6. The positive bands of the S1 sample are observed. B) PAX8/PPARG fusion: The primer PCR of samples S1 and S2 is observed in lines 1, 2, 3 and 4. The nested PCR is in lines 5, 6, 7, 8 and 9; the band corresponding to the presence of the PAX8/PPARG fusion can be observed in the S2 sample and the corresponding positive control. C) RET/PTC1 fusion. The primer PCR of samples S1 and S3 is observed in lines 1, 2, 3 and 4. The nested PCR is in lines 5, 6, 7, 8 and 9 The bands corresponding to the presence of the RET/PTC1 fusion can be observed in the S3 sample and the positive control. MW: molecular weight marker. C(+): positive control. C(−): negative control. NC: negative control of the nested PCRs. S1, S2 and S3 are samples from different patients.
Of the 112 FNA samples analysed, 58 patients (51%) underwent surgery (24 with AUS/FLUS cytology and 34 with FN/SFN), a relationship between cytology, molecular biology and histology was established, and 54 patients were followed up. The results of the cytology, molecular studies and histology of the patients who underwent surgery are detailed in Table 1.
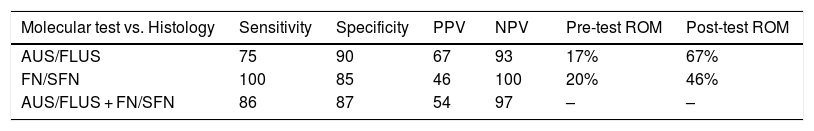
Samples with AUS/FLUS cytologyIn the samples with AUS/FLUS cytology, nine samples with mutations (20%) were identified, of which six turned out to be nodules with malignant histology and three with benign histology. Four malignant nodules carried the BRAF V600E mutation, while two had mutations in the RAS genes. In turn, three positive benign nodules were determined (two with PAX8/PPARG fusion and an RAS mutation) that were considered false positives due to their histological diagnosis of follicular adenoma. These results allowed us to calculate a risk of malignancy (post-test ROM) of 67% for nodules with AUS/FLUS cytology, positive for a mutation (Table 2). All the nodules that presented mutations in BRAF V600E were diagnosed postoperatively as papillary carcinomas, two were classic papillary carcinomas and two were follicular variant of papillary thyroid carcinomas. The three nodules with mutations in the RAS genes were diagnosed as follicular thyroid carcinoma, follicular adenoma, and non-invasive encapsulated follicular variant of papillary thyroid carcinoma. The latter was reclassified as non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). By subdividing the nodules into those with AUS (n = 8) and FLUS (n = 16) characteristics, we can see that AUS cytologies have a higher frequency of mutations (62.5%) and a higher risk of malignancy in the presence of a mutation (80%) than FLUS cytologies (25% mutations and 25% malignancy in the presence of mutation). This may be associated with the higher frequency of the BRAF V600E mutation in samples with AUS cytology (three out of five mutations found) compared to those with FLUS cytology (one out of four mutations found).
Diagnostic performance of the molecular test analysis in the AUS/FLUS, FN/SFN categories.
| Molecular test vs. Histology | Sensitivity | Specificity | PPV | NPV | Pre-test ROM | Post-test ROM |
|---|---|---|---|---|---|---|
| AUS/FLUS | 75 | 90 | 67 | 93 | 17% | 67% |
| FN/SFN | 100 | 85 | 46 | 100 | 20% | 46% |
| AUS/FLUS + FN/SFN | 86 | 87 | 54 | 97 | – | – |
Risk of malignancy (ROM) before the seven-gene panel (pre-test ROM), and after the application of the molecular test (post-test ROM).
PPV: positive predictive value; NPV: negative predictive value.
In relation to the 15 nodules in which no mutations were found, five follicular adenomas were detected, eight with other benign pathologies and two carcinomas, which were a classic papillary carcinoma and the other a Hurthle cell carcinoma. In the case of classic papillary carcinoma, the panel of seven genes was able to be repeated on the surgical specimen, confirming the absence of the mutations investigated with the panel used. In this category, 20 patients with nodules negative for the seven-gene panel mutations and not undergoing surgery were followed up with ultrasound for longer than one year (in five of these patients follow up could not be completed). These results allowed us to calculate a NPV of 93% and a specificity of 90%. Finally, when calculating the risk of malignancy for the AUS/FLUS category before and after the implementation of the mutation panel, we observed an increase from 17% to 67% (Table 2).
Samples with FN/SFN cytologyIn the nodules with FN/SFN cytological diagnosis, 13 samples with mutations (19%) were detected, of which five were mutations in the RAS gene, two mutations in BRAF, 4 PAX8/PPARG fusions and two RET/PTC1 fusions. Histological characterisation of the two nodules with BRAF V600E mutation and the two nodules with RET/PTC1 fusion revealed that they were associated with papillary microcarcinoma and follicular variant of papillary thyroid carcinoma, respectively (Table 1). It should be noted that the microcarcinomas were not incidental findings since the patients underwent biopsy due to the ultrasound characteristics of the nodules. In addition, four follicular adenomas and one follicular thyroid carcinoma associated with mutations in the RAS gene family were diagnosed. The PAX8/PPARG fusion was the second most common mutation in this cytological group, of which three were found in follicular adenomas and one in encapsulated follicular thyroid carcinoma with angioinvasion. In this category, six of the 13 positive nodules were confirmed as malignant post-surgery, that is, a post-test ROM of 46%. The remaining seven nodules were benign (54%) but with PAX8/PPARG and RAS mutations. Of the 21 nodules in which no mutations were found, 14 follicular adenomas were identified, one Hurthle cell adenoma, and six corresponded to other benign pathologies. In this category, 34 patients did not undergo surgery and were followed up by ultrasound for a period longer than one year. Of these patients, 14 did not complete the follow-up period and were not considered true negatives. With the results obtained in this category, a NPV of 100% and a specificity of 85% were calculated. When calculating the risk of malignancy for the FN/SFN category before and after the implementation of the molecular test, we observed an increase from 20% to 46% (Table 2).
Patients in follow-upThe group of 54 patients who did not present with mutations in their nodules was followed up. This consisted of follow-up ultrasounds from 12 to 48 months. Of the 54 patients, 20 had cytologies diagnosed as AUS/FLUS (37%) and 34 had FN/SFN cytologies (63%). It was possible to carry out the corresponding follow-up in 35 patients (65%), 15 with AUS/FLUS cytology and 20 with FN/SFN, which did not present an increase in nodular size in the analysed period and are considered true negatives for the purposes of calculating the NPV (Table 1).
General analysis of all samplesWhen analysing the total group of patients who underwent surgery and those who were followed up together, it was possible to observe that, of 112 thyroid nodules, 14 were diagnosed as malignant in the histological analysis. Of the 98 remaining samples, 44 were confirmed as benign by histology and 35 were considered benign by ultrasound follow-up. Information could not be obtained for 19 patients whose nodules were negative for the seven-gene panel and who did not complete follow-up. The presence of a mutation was indicative of malignancy in 55% of nodules (12 of 22). The 10 nodules with mutations, but with benign histology, were considered false positives and therefore the specificity of the test was 87% considering both categories together. Of all the malignant nodules, mutations were not identified in only two, therefore a sensitivity of 86% was calculated (Table 2).
DiscussionPrevious studies have demonstrated the importance of screening for these seven genes in FNA with indeterminate cytology,16,19,20,25 but this is the first time that the results obtained with the implementation of the molecular biology test in a population from northern Argentina are presented. The results of the seven-gene panel on 112 samples, along with the histology and follow-up of the patients, have made it possible to estimate a sensitivity for the AUS/FLUS and FN/SFN categories of 75% and 100%, respectively, comparable to that obtained by Nikiforov et al.16 and Bellevicine et al.22 With regard to specificity, in the AUS/FLUS category (90%) it is between the values reported in other works that range between 82% and 100%. In the FN/SFN category, the specificity obtained was 85% and the PPV 46%, which may be due to the greater number of RAS and PAX8/PPARG mutations detected in nodules with benign histology. Therefore, we observed that the RAS mutation and the PAX8/PPARG fusion are associated with a low risk of thyroid malignancy of 37.5% and 17%, respectively.
Mutations in RAS and PAX8/PPARG can be found in thyroid carcinomas, follicular adenomas and hyperplastic nodules.19 Several studies suggest that mutations in the RAS gene are involved in the first phase of tumour transformation (adenoma to carcinoma) and in tumour differentiation (differentiated to poorly differentiated and undifferentiated carcinoma).23 In addition, transgenic mice carrying RAS mutations have been shown to develop thyroid carcinomas.23 Therefore, it could be postulated that RAS is an oncogene that contributes to the gradual transformation of thyroid cells and the progression from benign disease to malignant tumour.26 It is also possible to find mutations in the RAS gene in poorly differentiated thyroid carcinomas and in anaplastic carcinoma of the thyroid, increasing the evidence that mutations in this gene collaborate with the progression towards more aggressive forms.27
In our study, both the BRAF V600E mutation and the RET/PTC1 fusion were 100% associated with malignancy. Considering these results, patients with indeterminate cytologies that present one of these two mutations would be candidates for total thyroidectomies. It is important to note that most of the published works include cytology suspicious for malignancy in their molecular studies, contributing to increasing the probability of finding positive nodules with malignant histology, overestimating the sensitivity and specificity of the tests.
There are discrepancies regarding the follow-up of those nodules that are negative for molecular markers and their consideration as benign pathology. This may result in inaccuracies in diagnostic value calculations.3 For this reason, in the statistical analysis of this study, patients with negative results for the seven-gene panel and with ultrasound follow-up for more than one year, without nodular enlargement, were included as true negatives, in the same way as performed by Eszlinger et al.28 and Bellevicine et al.,22 and Nikiforov et al. in 2009,19 in their studies. Some authors indicate that to include these nodules that have not undergone surgery and to be considered "true negatives", they must remain without suspicious changes during a follow-up period.29 In a five-year prospective study of 1,567 thyroid nodules diagnosed as benign on ultrasound or cytologically, growth was observed in 11.1% of nodules.30 In our experience, a nodule with a negative molecular test was associated with a residual risk of malignancy of less than 3%. Therefore, ultrasound study was the clinical choice for the follow-up of these patients, thus avoiding unnecessary surgeries.
The implementation of the seven-gene panel had an important impact in our institution. The pre-test ROM calculated by the institution's anatomical pathology programme prior to the implementation of the mutation detection test was 17% for the AUS/FLUS category and 20% for FN/SFN. The Bethesda system indicates an increase in ROM ranging from 10 to 30%8 for the AUS/FLUS category; in our institution, the increase in ROM during the period covered by this study was from 17% to 67% post-test, for this category. In the FN/SFN category, according to Bethesda, the increase could be from 25 to 40%8; in our experience, the ROM increased from 20 to 46% with the implementation of the seven-gene panel (post-test ROM). The higher risk of malignancy with molecular testing in the AUS/FLUS category would be explained by the fact that the mutations found include, in greater numbers, BRAF mutations and correlate with the undetermined atypical papillary/follicular cellular changes found in these categories. In this context, we were able to reduce surgeries in 48% of the patients analysed in this study who, without the use of the molecular test, would have undergone diagnostic surgeries.
However, our study has certain limitations. First is the number of samples included. Although it was a population of 112 patients, only 22 mutations were detected (six in BRAF, eight in RAS, six PAX8/PPARG fusions and two RET/PTC1 fusions). Secondly, only the BRAF mutation and the RET/PTC1 fusion were 100% associated with malignancy. Mutations in RAS and the PAX8/PPARG fusion were only associated with malignancy in 37.5% and 17% of cases, respectively, so they would have a lower predictive value for the clinical management of the patient. However, despite the low association with malignancy of RAS mutations in this study, the literature indicates that they may represent preinvasive forms of carcinomas or minimally invasive carcinomas.31 Therefore, the incorporation of new patients will allow the number of mutations detected to increase and the impact of each mutation on the prediction of malignancy in our region to be more accurately evaluated.
Our study demonstrates that patients with a thyroid nodule whose cytology is indeterminate (Bethesda categories III and IV) can benefit from testing using a panel of seven mutations, reducing the number of unnecessary surgeries by 48% given the high negative predictive value of the test. BRAF mutations and RET/PTC1 fusions in this panel were the most important predictors of malignancy in the Bethesda III and Bethesda IV categories, respectively. In contrast, mutations in the RAS genes have low specificity as they are present in both adenomas and carcinomas. Therefore, we believe that in the near future it will be necessary to implement new technologies such as next-generation sequencing (NGS) to simultaneously study mutations in numerous genes such as TP53, TERT and PIK3CA31 to achieve greater accuracy in the diagnosis of malignancy in thyroid aspirations.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We would like to thank the members of the endocrinology programme, the area of percutaneous surgery, cardiac techniques and imaging, and the anatomical pathology sector of Hospital Dr. Arturo Oñativia for their collaboration in carrying out this work. This research has been carried out with the resources of the Hospital Dr. Arturo Oñativia and has not received specific funding from public sector agencies, the commercial sector or non-profit organisations.
Please cite this article as: Tolaba N, Spedalletti Y, Bazzoni P, Galindez M, Cerioni V, Santillan C, et al. Testeo de mutaciones en nódulos tiroideos con citología indeterminada: estudio prospectivo de 112 pacientes en Argentina. Endocrinol Diabetes Nutr. 2022;69:122–130.