Thyroid nodule (TN) harbouring a thyroid carcinoma are more common in paediatric than adult patients. In paediatric population, the evaluation of a TN should require specific paediatric tools for its diagnostic and therapeutic management. High-resolution ultrasonography and cytological evaluation after fine-needle aspiration biopsy (FNAB) remain the cornerstones of evaluation of TN.
ObjectivesTo evaluate in paediatric TN for the first time the usefulness and precision of the ultrasound criteria defined by the “Thyroid Imaging Reporting and Data System (EU-TIRADS) 2017 in adults” to establish the ultrasound indication for the practice of FNAB and stratify the risk of malignancy.
Patients and methods24 paediatric patients under age 18 years with thyroid nodules were attended in the last 15 years, 24 of them (31 nodules; age: 15.2 ± 2.2 years; 18 women) met the inclusion criteria: FNAB with Bethesda classification and ultrasound with EU-TIRADS score. EU-TIRADS score were evaluated retrospectively. Fourteen patients underwent surgery and the definitive histological diagnosis was obtained, this allowed the calculations of sensitivity, specificity and positive and negative predictive values of the EU-TIRADS and Bethesda classification. Data on the largest diameters of the nodules were collected.
ResultsOf the overall 31 nodules, the distribution by EU-TIRADS (T) category was: T1 (3.2%), T2: 2 (6.4%), T3: 7 (22.6%), T4: 16 (51.6%) and T5: 5 (16.1%). All malignant nodules were included in EU-TIRADS category 4 or 5. By the other hand, 13 of the 25 benign nodules were also included in the EU-TIRADS 4 category, and one in the 5. The distribution by categories of Bethesda's classification (B): BI: 6 (19.4%), BII: 14 (45.2%), BIII: 5 (16.1%), BIV: 2 (6.5%), BV: 0 and BVI: 4 (12.9%). The pathological diagnosis of the 14 patients who underwent surgery was: 6 papillary carcinomas and 8 with benign lesions: 6 nodular hyperplasia and 2 follicular adenoma. The percentage of malignancy was 42%. The sensitivity of the EU-TIRADS classification to detect malignant nodules was 100%, the specificity was 25%, PPV 44% and NPV 100%. The sensitivity of the Bethesda classification to detect malignant nodules was 86%, the specificity was 75%, PPV 67% and NPV 90%. The analysis of the largest diameter of the nodules did not show statistically significant differences between benign and malignant lesions.
ConclusionsEU-TIRADS for ultrasonographic criteria classification in combination with the clinical history is an adequate and reproducible method to estimate suspicion of malignancy of paediatric TN. It is also a reliable diagnostic tool to decide which nodules will be candidates for FNAB.
Los nódulos tiroideos (NT) en la edad pediátrica requieren una evaluación diagnóstica completa porque el riesgo de malignidad es mayor con más frecuencia que en el adulto. La ecografía es la técnica de imagen clave para decidir la actitud a seguir.
ObjetivosEvaluar con carácter inédito la utilidad y precisión de los criterios ecográficos definidos por el «Thyroid Imaging Reporting and Data System (EU-TIRADS) 2017» en adultos para estratificar el riesgo de malignidad en pacientes pediátricos con NT y establecer la indicación de la práctica de una punción aspiración con aguja fina (PAAF).
Pacientes y métodosSe revisaron las historias clínicas de 24 pacientes con NT atendidos en los últimos 15 años en un único centro. Cumplieron los criterios de inclusión establecidos 24 pacientes (31 nódulos; edad: 15,2 ± 2,2 años; 18 mujeres): PAAF con clasificación de Bethesda y ecografía con clasificación EU-TIRADS reevaluadas de forma retrospectiva. A 14 de ellos se les realizó una cirugía y se obtuvo el diagnóstico histológico definitivo, esto permitió llevar a cabo los cálculos de sensibilidad, especificidad y valores predictivo positivo y negativo de la clasificación EU-TIRADS y Bethesda.
ResultadosDel global de 31 nódulos, la distribución por categoría EU-TIRADS (T) fue: T1 (3,2%), T2: 2 (6,4%), T3: 7 (22,6%), T4: 16 (51,6%) y T5: 5 (16,1%). Todos los nódulos malignos estaban incluidos en la categoría EU-TIRADS 4 o 5. En contraste, 13 de los 25 nódulos benignos fueron integrados también en la categoría EU-TIRADS 4, y uno de ellos, en la 5. La distribución por categorías de la clasificación de Bethesda (B): B I: 6 (19,4%), BII: 14 (45,2%), BIII: 5 (16,1%), BIV: 2 (6,5%), BV: 0 y BVI: 4 (12,9%). El diagnóstico anatomopatológico de los 14 pacientes en los que se realizó la cirugía fue: seis carcinomas papilares y ocho con lesiones benignas; seis hiperplasias nodulares y dos adenomas foliculares. El porcentaje de malignidad fue del 42%. La sensibilidad de la clasificación EU-TIRADS para detectar nódulos malignos fue del 100%, la especificidad del 25%, el valor predictivo positivo (VPP) del 44% y el valor predictivo negativo (VPN) del 100%. La sensibilidad de la clasificación Bethesda para identificar nódulos malignos fue del 86%, la especificidad del 75%, VPP 67% y VPN 90%. El análisis del diámetro mayor de los nódulos no demostró diferencias estadísticamente significativas entre lesiones benignas y malignas.
ConclusionesLos criterios ecográficos en combinación con la historia clínica representan un método adecuado y reproducible para identificar nódulos malignos en la edad pediátrica. La ecografía es una prueba diagnóstica fiable para decidir qué nódulos serán candidatos a PAAF.
The finding of a thyroid nodule (TN) in a paediatric patient requires a thorough study, since it reflects differentiated thyroid carcinoma (DTC) at a higher rate in paediatric versus adult patients1.
The incidence of TNs in paediatric patients is around 1%–1.5%. Its frequency increases with age and is higher in those with a family or personal history of thyroid disease and those with a history of radiotherapy, as well as carriers of certain genetic mutations (RET, BRAF and DICER), among others2. Rates of malignancy in published paediatric series range from 18% to 25% and are as high as 40% in patients exposed to radiotherapy3.
DTC is considered a rare cancer in childhood, although it should be noted that its incidence in the paediatric population has been increasing in recent years by 1.1% per year4. Follicular cell-derived carcinomas are the most common and include DTC, poorly differentiated carcinoma and anaplastic carcinoma; however, the latter two are rarely seen in paediatrics. These three, taken together, account for more than 85%–90% of cases of thyroid carcinoma. DTC, for its part, includes papillary carcinoma (75%–80%) and follicular carcinoma (10%).
Ultrasound and cytology evaluation of samples obtained by fine needle aspiration biopsy (FNAB) are the cornerstones of the diagnostic and therapeutic management of TNs, along with clinical presentation and disease history, particularly in relation to other tumours and radiotherapy treatments.
The challenge in ultrasound is distinguishing nodules that raise suspicion of malignancy and will require cytological study from those that will only need clinical follow-up. Various scientific associations have attempted to group and stratify TN malignancy risk in a single classification to recommend puncture and cytological analysis. One of the best known is the Thyroid Imaging Reporting and Data System (TIRADS), in its American version (ACR-TIRADS) and its European version (EU-TIRADS). However, as yet, there is no single scale for the adult population5 and it has not been validated for the paediatric population, although some studies have been published6,7. The TIRADS scale5 evaluates some nodule characteristics such as structure (solid, cystic or mixed) and echogenicity, in addition to “classic” signs of malignancy (hypoechogenicity, presence of microcalcifications, taller-than-wide shape and regularity of margins), yielding a score that classifies the TN in one of five categories associated with the expected risk of malignancy. Those five categories are 1: normal, 2: benign, 3: low risk of malignancy, 4: intermediate risk of malignancy and 5: high risk of malignancy. In both versions, American and European, stratification of risk by ultrasound characteristics is scored and detailed, and therefore easily reproducible. The difference between the two lies in the diameter of the nodules subject to biopsy. The European version is slightly more aggressive only in category 3. EU-TIRADS recommends biopsy in category 3 when the nodule has a diameter of 20 mm or more (ACR-TIRADS suggests biopsy when the diameter of the nodule is greater than or equal to 25 mm), in category 4 when it exceeds 15 mm and in category 5 when it is greater than 10 mm. FNAB is not indicated for categories 1 and 2, except when performed for therapeutic purposes. In our clinical practice, given that we work with paediatric patients, who present DTC at higher rates than adults, we use the EU-TIRADS classification and analyse all thyroid nodules measuring 20 mm or more and meeting the category 3 criteria.
The Bethesda system for the cytological diagnosis of TNs is more extensive and its goal is to standardise diagnosis, assigning each category an expected percentage of malignancy, and providing suggestions for clinical and/or surgical management. It classifies the specimen collected in TN FNAB in one of six diagnostic categories; I: nondiagnostic, II: benign, III: atypia of undetermined significance, IV: follicular neoplasm, V: suspicious for malignancy and VI: malignant. It does not include specific recommendations for assessing paediatric nodules, nor does it include rates of expected malignancy corrected specifically for children6.
The objective of this study was to evaluate, for the first time in a sample of paediatric patients, the usefulness and accuracy of the ultrasound criteria defined by EU-TIRADS in 2017 for adult patients5 in order to: 1. Classify nodules in paediatric patients by risk of malignancy. 2. Assess said EU-TIRADS criteria as a guide for the indication of cytology evaluation by FNAB in children with TNs.
Patients and methodsThis was a retrospective study of 24 paediatric patients with TNs seen at a tertiary university paediatric hospital in the past 15 years (2005–2020). The inclusion criteria were having an ultrasound study and a cytological examination obtained by FNAB. Thyroid ultrasounds were re-evaluated retrospectively and independently by two paediatric radiologists and scored according to the EU-TIRADS classification, with no knowledge of the results of the cytological examination. The different scores from each radiologist did not change the TIRADS category of the nodules, and therefore had no impact on management. Data on the largest diameters of the nodules measured by ultrasound were collected so that their median and upper and lower limits could be calculated. As the Bethesda classification came into use at our hospital in 2012, FNABs performed before then were retrospectively reviewed and assigned a diagnostic category. In cases in which surgical removal of the TN had been indicated, the type of surgery performed and the definitive pathology result were also collected. The final sample consisted of 24 patients and 31 nodules. Nodules that did not undergo surgery (17/31) were not included in the statistical analysis as histological confirmation could not be obtained; at present, they remain in follow-up.
All the patients signed an assent form to participate in the study.
Statistical analyses were performed with the IBM SPSS statistics software programme, version 20. Categorical variables are expressed in terms of absolute and relative frequency. Quantitative variables are expressed in terms of mean and standard deviation or median and interquartile range, as appropriate. In addition, the diagnostic performance of the Bethesda and TIRADS classifications was evaluated compared to surgery to detect nodule malignancy by means of estimation of predictive values of sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). For these calculations, just 14 cases having undergone surgery with histological confirmation available were analysed, as histological confirmation was considered definitive. Altogether, 19 ultrasounds and 19 FNABs were examined (five patients had two ultrasounds and two FNABs). Nodules belonging to the following categories were considered “suspicious for malignancy or malignant”: EU-TIRADS categories 4 and 5, Bethesda categories III and IV (being considered indeterminate and therefore subject to repeat FNAB or surgery), and Bethesda categories V and VI.
ResultsIn the 24 patients analysed, the mean age was 15.3 ± 2.2 years. Among them, 18 were female (75%) and 19 (80%) were postpubertal. The reasons for indicating the TN study were: incidental finding in a physical examination (10 patients, 42%), goitre study (seven patients, 30%), history of radiotherapy (four patients, 16%), genetic syndrome with a predisposition to thyroid cancer (two patients, 8%) and ultrasound finding in follow-up for prior thyroid surgery (one patient, 4%). In 14 cases, TN removal was performed (in 10 cases by means of total thyroidectomy, and in four cases by means of hemithyroidectomy with isthmectomy).
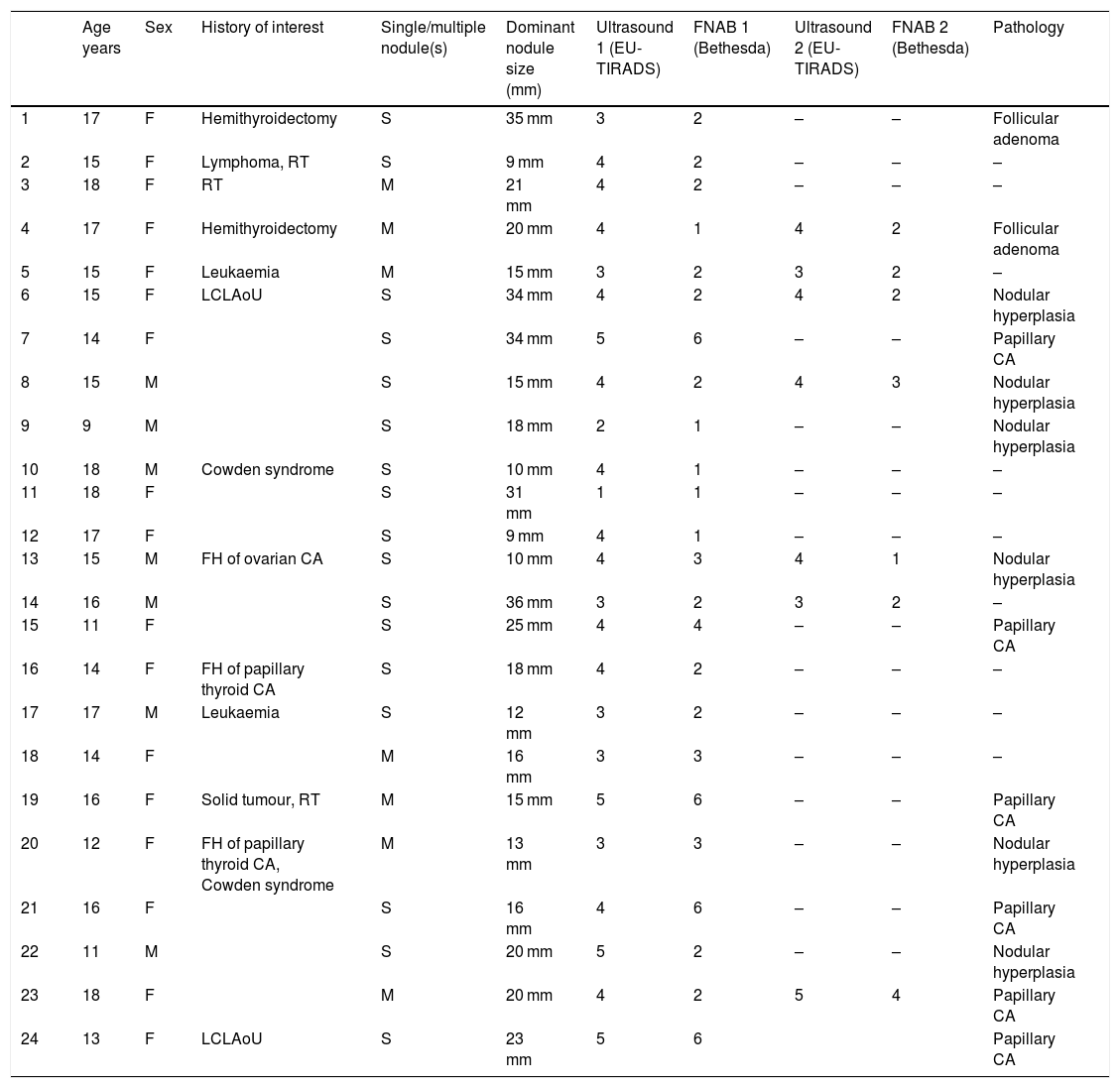
Table 1 describes the 24 patients (31 nodules) analysed in terms of clinical characteristics, EU-TIRADS score, Bethesda category and pathology diagnosis of the surgical specimen for 14 nodules: six cases of papillary carcinoma and eight cases of benign lesions (corresponding to six cases of nodular hyperplasia and two cases of follicular adenoma).
Description of the patients' clinical characteristics, EU-TIRADS score, Bethesda category and definitive histological diagnosis.
| Age years | Sex | History of interest | Single/multiple nodule(s) | Dominant nodule size (mm) | Ultrasound 1 (EU-TIRADS) | FNAB 1 (Bethesda) | Ultrasound 2 (EU-TIRADS) | FNAB 2 (Bethesda) | Pathology | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 17 | F | Hemithyroidectomy | S | 35 mm | 3 | 2 | – | – | Follicular adenoma |
| 2 | 15 | F | Lymphoma, RT | S | 9 mm | 4 | 2 | – | – | – |
| 3 | 18 | F | RT | M | 21 mm | 4 | 2 | – | – | – |
| 4 | 17 | F | Hemithyroidectomy | M | 20 mm | 4 | 1 | 4 | 2 | Follicular adenoma |
| 5 | 15 | F | Leukaemia | M | 15 mm | 3 | 2 | 3 | 2 | – |
| 6 | 15 | F | LCLAoU | S | 34 mm | 4 | 2 | 4 | 2 | Nodular hyperplasia |
| 7 | 14 | F | S | 34 mm | 5 | 6 | – | – | Papillary CA | |
| 8 | 15 | M | S | 15 mm | 4 | 2 | 4 | 3 | Nodular hyperplasia | |
| 9 | 9 | M | S | 18 mm | 2 | 1 | – | – | Nodular hyperplasia | |
| 10 | 18 | M | Cowden syndrome | S | 10 mm | 4 | 1 | – | – | – |
| 11 | 18 | F | S | 31 mm | 1 | 1 | – | – | – | |
| 12 | 17 | F | S | 9 mm | 4 | 1 | – | – | – | |
| 13 | 15 | M | FH of ovarian CA | S | 10 mm | 4 | 3 | 4 | 1 | Nodular hyperplasia |
| 14 | 16 | M | S | 36 mm | 3 | 2 | 3 | 2 | – | |
| 15 | 11 | F | S | 25 mm | 4 | 4 | – | – | Papillary CA | |
| 16 | 14 | F | FH of papillary thyroid CA | S | 18 mm | 4 | 2 | – | – | – |
| 17 | 17 | M | Leukaemia | S | 12 mm | 3 | 2 | – | – | – |
| 18 | 14 | F | M | 16 mm | 3 | 3 | – | – | – | |
| 19 | 16 | F | Solid tumour, RT | M | 15 mm | 5 | 6 | – | – | Papillary CA |
| 20 | 12 | F | FH of papillary thyroid CA, Cowden syndrome | M | 13 mm | 3 | 3 | – | – | Nodular hyperplasia |
| 21 | 16 | F | S | 16 mm | 4 | 6 | – | – | Papillary CA | |
| 22 | 11 | M | S | 20 mm | 5 | 2 | – | – | Nodular hyperplasia | |
| 23 | 18 | F | M | 20 mm | 4 | 2 | 5 | 4 | Papillary CA | |
| 24 | 13 | F | LCLAoU | S | 23 mm | 5 | 6 | Papillary CA |
RT: radiotherapy; FH: family history; CA: carcinoma; LCLAoU: lateral cervical lymphadenopathy on ultrasound; M: male; F: female; S: single nodule; M: multiple nodules.
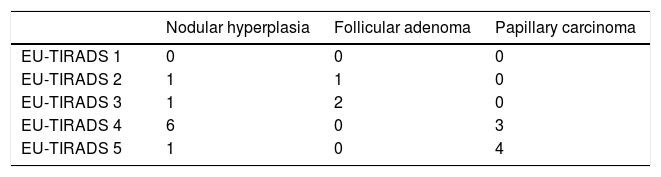
Out of the total of 31 nodules, the distribution by EU-TIRADS category was as follows: 1 (3.2%), 2: 2 (6.4%), 3: 7 (22.6%), 4: 16 (51.6%) and 5: 5 (16.1%). All malignant nodules were included in EU-TIRADS categories 4 and 5. On the other hand, 13 of the 25 benign nodules were also included in EU-TIRADS category 4, and one of them was included in category 5. The distribution by Bethesda classification category was the following: I: 6 (19.4%), II: 14 (45.2%), III: 5 (16.1%), IV: 2 (6.5%), V: 0 and VI: 4 (12.9%).
Tables 2a and 2b describe the distribution by EU-TIRADS category and by Bethesda category as well as the correlation thereof with the histological diagnosis for the 19 samples (corresponding to 14 nodules) with a definitive histological diagnosis. The rate of malignancy was 42% (six cases of papillary carcinoma were found in 14 nodules with a definitive histological result).
The EU-TIRADS classification had a sensitivity of 100%, a specificity of 25%, a PPV of 44% and an NPV of 100% for detecting malignant nodules. The results are shown in Table 3a. All cases of papillary carcinoma obtained a score of 4 or 5 on the EU-TIRADS classification. By contrast, nine ultrasounds of benign lesions also obtained an EU-TIRADS classification score of 4 or 5. According to the EU-TIRADS guidelines, two patients did not meet the criteria for FNAB, although it was performed anyway due to a history of Cowden syndrome (patient 10) or radiotherapy (patient 19); neither patient had carcinoma.
Results of EU-TIRADS classification versus definitive histological diagnosis.
| EU-TIRADS versus definitive diagnosis | Malignant | Benign |
|---|---|---|
| EU-TIRADS 4 and 5 | 7 | 9 |
| EU-TIRADS 1−3 | 0 | 3 |
Sensitivity (Se) = 100%, Specificity (Sp) = 25%, Positive Predictive Value (PPV) = 44% and Negative Predictive Value (NPV) = 100%.
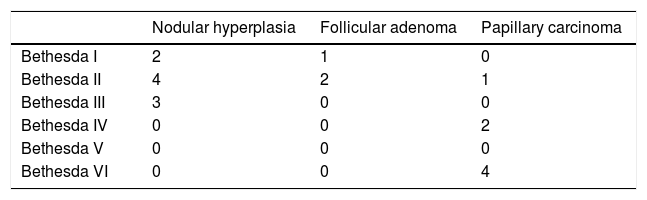
The Bethesda classification had a sensitivity of 86%, a specificity of 75%, a PPV of 67% and an NPV of 90% for the detection of malignant nodules. The results are shown in Table 3b. The rate of malignancy for the indeterminate categories of the Bethesda classification (III and IV) was 10.5% (2/19). One case of papillary carcinoma (patient 23) obtained a Bethesda classification score of II in the first FNAB but IV in the second FNAB, and therefore was also a candidate for surgery.
Results of Bethesda classification versus definitive histological diagnosis.
| Bethesda versus definitive diagnosis | Malignant | Benign |
|---|---|---|
| Bethesda III-VI | 6 (2 III-IV; 4 VI) | 3 |
| Bethesda I-II | 1 | 9 |
Sensitivity (Se) = 86%, Specificity (Sp) = 75%, Positive Predictive Value (PPV) = 67% and Negative Predictive Value (NPV) = 90%.
Regarding the largest diameter of the nodules, measured by ultrasound, the upper and lower limits were 9 mm and 36 mm, respectively, and the median was 18 mm. The upper and lower limits of the six papillary carcinomas were 15 mm and 34 mm and the median was 21.5 mm. The upper and lower limits of the eight benign lesions were 10 mm and 35 mm and the median was 20 mm. Analysis of the largest diameter of the nodules showed no statistically significant differences between benign and malignant lesions.
DiscussionThe initial evaluation of a TN that is clinically obvious or incidentally discovered includes neck ultrasound and evaluation of clinical risk factors. Based on the results, the use of other diagnostic instruments, such as FNAB, will be indicated for purposes of cytological study, and occasionally molecular study of samples with indeterminate cytology by means of next-generation sequencing molecular panels including most of the genetic variants involved in thyroid cancer.
The challenge in ultrasound is identifying nodules that raise suspicion of malignancy and will require cytology evaluation and distinguishing them from nodules that will require clinical follow-up alone. Until the latest paediatric guidelines were published7, DTC had been managed in children by extrapolating from the guidelines for adults, such as the American Thyroid Association (ATA) guidelines8. However, DTC differs in terms of clinical presentation and behaviour in children versus adults, as children present larger volume nodules, earlier capsular invasion, greater extension and involvement of lymph nodes, and pulmonary metastases. Paediatric patients show better treatment response and better mid- and long-term survival rates, meaning that the long-term sequelae of their treatments take on greater importance9,10.
From our series of paediatric patients with TNs, despite its limited sample with just 31 nodules, it can be deduced that the use of the EU-TIRADS ultrasound criteria identified malignant nodules with high sensitivity, but was less effective in distinguishing benign nodules. In our sample, these criteria would have allowed us to diagnose the six patients with papillary carcinoma, since four had a score of 5 and the other two had a score of 4 on the EU-TIRADS classification. All of them would have been candidates for FNAB, and none of them would have been left undiagnosed.
Our results were similar to those reported by Lim-Dunham et al.7, in which just one malignant nodule obtained a TIRADS score below 4. By contrast, a study by Richman et al.11 in an extensive series of paediatric patients estimated that 22% of malignant nodules (17 out of 77) had not been diagnosed. Both studies7,11 used the American version of the TIRADS (ACR-TIRADS). With respect to benign nodules, 3/8 (37.5%) obtained an EU-TIRADS score less than or equal to 3; the rest obtained an EU-TIRADS score of 4–5, which would have led to an unnecessary FNAB with a benign histological result. These results were similar to those of Lim-Dunham et al.7 (35%) and Martínez-Rios et al.12 (82%). Concerning nodule size, our study provided no data for a change in the management of paediatric nodules.
Unlike studies by Richman et al.11 and Dinauer13, in which large nodules (40 mm) and low TIRADS categories (ACR-TIRADS scale) yielded false negative cytology results, none of our benign or malignant nodules showed a false negative result; just one case of papillary carcinoma obtained Bethesda classification scores of II in the first FNAB and IV in the second FNAB, rendering the patient a candidate for surgery. However, in line with the other studies11,14, we believe that in the stratification of the level of risk of paediatric nodules, all nodules should be subject to close follow-up, including smaller ones (10 mm), and that this follow-up should be tailored to each patient.
We considered nodules with clinical follow-up but no surgery to be non-evaluable as pathology results were not available. This might account for the differences seen between our study and others15,16 in which nodules were considered benign if no significant changes were seen in mid-term follow-up (two years). We realise that this decision may have introduced bias in our study, since criteria for surgery may include other warning signs; however, we feel that we pursued the most academic option as it allowed us to compare the diagnostic tests evaluated to a definitive histological examination.
Our study showed that the Bethesda classification is highly reliable in categories V-VI, and that in its indeterminate categories (III-IV), surgery or repeat FNAB should be considered, since expected malignancy rates are higher in the paediatric population than in the adult population9. In our sample, the six cases of papillary carcinoma would have been candidates for surgery, since four obtained a Bethesda score of VI and two obtained Bethesda score of IV. The rate of cytological examinations with indeterminate results in our series was 22.6% (for all 31 nodules) and similar to those reported in other paediatric and adult series17,18. The problem lies in determining which of these nodules are benign and can be monitored over time, and which are malignant and require surgery. Supplementary use of molecular panels along with cytology is, for now, the only tool that seems to help in this challenging situation19. At present, there is a dearth of studies, especially in paediatric patients20, that definitively calibrate the actual usefulness thereof with regard to clinical practice, costs and patient quality of life. There is also a lack of recommendations on long-term follow-up and need for cytological/molecular re-evaluation in these patients19.
A Bethesda I classification, which is by definition non-diagnostic, corresponded to 20% of our sample. This rate was similar to those of other published series21. Bethesda I classification rates vary widely across published paediatric series (from 5.5% to 20%)21. One reason for this high rate could be the absence of in situ assessment, or rapid on-site evaluation (ROSE). In our series, ROSE was not performed in older cases, which might explain the high rate of non-diagnostic cases in our sample.
In conclusion, our study found that the ultrasound criteria of the EU-TIRADS scale represent a suitable, reproducible method for estimating levels of suspicion for malignancy in paediatric thyroid nodules. In our study, it proved a good tool for identifying malignant nodules, but a less effective tool for identifying benign ones. The limitations of our study include its retrospective nature (although the other studies cited were also retrospective) and the limited number of nodules evaluated in the sample. All things considered, we believe that TN assessment in paediatric patients should involve more specific paediatric instruments for these patients' diagnosis and treatment.
FundingThis study has not received any type of funding.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Yeste Fernández D, Vega Amenabar E, Coma Muñoz A, Arciniegas Vallejo L, Clemente León M, Planes-Conangla M, et al. Criterios ecográficos (EU-TIRADS) para identificar el riesgo de malignidad de los nódulos tiroideos en adolescentes. Correlación con los hallazgos cito-histológicos. Endocrinol Diabetes Nutr. 2021;68:728–734.