To assess the prevalence of peripheral artery disease and the validity of clinical signs for its diagnosis in patients with type 2 diabetes.
MethodsSetting: Health center (Mariñamansa, Orense).
PeriodJanuary 2011–January 2013.
Inclusion criteriaPatients with type 2 diabetes, informed consent.
MeasurementsAge, sex, diabetes duration, body mass index, Charlson index, blood pressure, ankle-brachial index (ABI), cholesterol levels, smoking. Cardiovascular risk (UKPDS). Edinburgh Claudication Questionnaire.
Sample sizen=323 (±5.5% accuracy, 95% confidence).
Statistical analysis: multivariate logistic regression analysis. Sensitivity, specificity, predictive values, and agreement were estimated.
Informed consent and ethics committee approval were obtained (2010/278).
ResultsMean patient age was 71.56±12.73 years, and mean diabetes duration 12.38±9.96 years.
Symptoms of intermittent claudication were reported by 26.4% of patients, ABI was normal (0.9–1.1) in 37.2% of patients, less than 0.9 in 26.5%, and higher than 1.10 in 36.2% of patients.
The kappa index of agreement of peripheral artery disease according to the Edinburgh Claudication Questionnaire and the ankle-brachial index was 0.33.
The questionnaire showed a sensitivity of 50.7% for predicting the diagnosis of peripheral artery disease (ABI<0.9) with a specificity of 82.6%, with positive and negative predictive values of 48.6% and 83.8% respectively.
ConclusionsOne-fourth of patients with type 2 diabetes had peripheral artery disease.
There was a low level of agreement between the evaluation of symptoms of intermittent claudication and the results of the ankle-brachial index.
Presence or absence of symptoms of claudication did not allow for confirming or ruling out peripheral artery disease.
Determinar en pacientes diabéticos tipo 2 la prevalencia de arteriopatía periférica y la validez de las manifestaciones clínicas para su diagnóstico.
MétodosÁmbito: Centro de Salud (Mariñamansa, Orense).
PeriodoEnero de 2011 - enero de 2013.
Criterios inclusiónPacientes diabéticos tipo 2, con consentimiento informado.
MedicionesEdad, sexo, tiempo de evolución de diabetes, índice de masa corporal, índice de Charlson, presión arterial, índice tobillo-brazo, niveles de colesterol, hábito tabáquico. Riesgo cardiovascular (UKPDS). Cuestionario de Edimburgo.
Tamaño muestral: n=323 (±5,5% precisión; 95% seguridad).
Análisis estadísticoAnálisis multivariado de regresión logística. Estudio de sensibilidad, especificidad valores predictivos y concordancia.
Aprobado por el Comité Ético de Investigación (2010/278).
ResultadosLa edad media fue de 71,56±12,73 años, la media de evolución de la diabetes tipo 2 fue de 12,38±9,96 años.
El 26,4% referían síntomas de claudicación intermitente. El 37,2% presentaban un índice tobillo-brazo normal (ITB 0,9-1,1), un 26,5%<0,9 y un 36,2%>1,10.
La concordancia de la arteriopatía periférica según el cuestionario de Edimburgo y el ITB fue reducida (índice Kappa=0,33).
El cuestionario de Edimburgo mostró una sensibilidad del 50,7% para predecir el diagnóstico de arteriopatía periférica (ITB< 0,9) una especificidad del 82,6%, un valor predictivo positivo y negativo de 48,6 y 83,8% respectivamente.
ConclusionesUna cuarta parte de los pacientes diabéticos tipo 2 presenta arteriopatía periférica.
Existe una baja concordancia entre la evaluación de síntomas de claudicación intermitente y los resultados del ITB.
La presencia de los síntomas de claudicación o su ausencia no permiten descartar ni confirmar la enfermedad arterial periférica.
Diabetes is associated with a significant increase in cardiovascular diseases such as coronary disease, cerebrovascular disease, peripheral vascular disease and heart failure.
Cardiovascular risk is from two to four-fold higher among type 2 diabetics than in non-diabetics. This risk in turn is higher in females than in males. Diabetes doubles the risk of coronary disease in males and increases it four-fold in females.1–3
Of the three main clinical manifestations of arteriosclerosis, the early detection of peripheral artery disease (PAD)—which is often silent—is of great importance, since its presence is an indicator of systemic arteriosclerotic disease and therefore points to a high risk of coronary or cerebrovascular events.4–6 The prevalence of PAD in the general population is estimated to be 12%, though this figure varies greatly according to the population considered. The ESTIME study carried out in Spain among individuals over 50 years of age found the prevalence of PAD to be 8.5% (10.2% in males and 6.3% in females).7 In the Hermex study,8 the prevalence of PAD was 3.7% (5.0% in males and 2.6% in females). The cumulative prevalences from the age of 50, 60 and 70 years were 6.2%, 9.1% and 13.1%, respectively. The disease was found to be asymptomatic in 13% of the cases. The prevalence of PAD among diabetic patients is much higher: 14.4% according to the study published by Agarwal et al.9 and 39.28% according to Ali et al.10 The ankle-branchial index (ABI) for the screening of PAD in the general population is not advised.11
The clinical manifestation of PAD—intermittent claudication—is characterized by the absence of symptoms at rest and the appearance of ischemic pain on walking. The pain generally affects the region of the calf muscles, and less often the entire extremity. The pain typically appears after walking a predictable distance and disappears after 2–3min of rest. Validated questionnaires are available for the diagnosis of intermittent claudication. The Edinburgh questionnaire12 is one of the most widely used tools and has a sensitivity of 91% and a specificity of 99%. The ABI in turn is the most useful noninvasive test for diagnosing PAD. In routine clinical practice PAD screening is carried out based on the clinical assessment, while the ABI is used to confirm the diagnosis.
The objective of this study was to evaluate the prevalence of PAD and the validity of the Edinburgh questionnaire in determining the presence of PAD in type 2 diabetics.
Material and methodsA prevalence observational study was made in Mariñamansa Primary Care Center (Ourense, Spain).
This center has a recruitment population of 28,470 inhabitants, of which 2810 (1450 males and 1360 females) meet the criteria of type 2 diabetes (representing a prevalence of 11.2%).
Randomized sampling stratified according to age and gender was performed after categorizing the patients with type 2 diabetes according to the international classification of primary care (ICPC-2), based on the electronic case history, by gender and age groups: under 40 years, 41–64 years, 65–80 years, and 81 years or older.
We randomly selected 350 patients, which allowed us to estimate the parameters of interest with a confidence level of 95% (α=0.05) and a precision of ±5.5%. We finally studied 323 patients, due to the loss of 27 subjects (7.7%).
The data collection period was between January 2011 and December 2012.
The confidentiality of the data obtained in the study was guaranteed in compliance with the Spanish Organic Act 15/1999, of December 13, referring to personal data protection. All the participants gave their written informed consent to participation in the study, which was evaluated and approved by the Clinical Research Ethics Committee of Galicia (Spain) (Registry code 2010/278).
The following variables were recorded for each patient included in the study: age, gender, duration of diabetes, weight and height at the time of the study, the body mass index (BMI), basal glycemia, glycosylated hemoglobin (HbA1c), total cholesterol, HDL-cholesterol, ldl-cholesterol, triglycerides, plasma creatinine, the estimated glomerular filtration rate (GFR) based on the Modification of Diet in Renal Disease (MDRD) abbreviated formula, and using the Cockcroft–Gault equation. Proteinuria was determined from the albumin/creatinine ratio in a first morning urine sample.
Comorbidity: arterial hypertension, retinopathy, smoking and comorbidity were calculated from the Charlson score.
Cardiovascular risk was estimated from the UKPDS score.
The number of visits during the previous year to the physician, nurse and other primary care professionals was recorded. We also documented the number of visits to other specialists (ophthalmologist, endocrinologist, cardiologist, neurologist). The number of emergency service consultations (both within and outside the hospital) was documented, as well as the number of hospital admissions.
Clinical evaluation of PAD was carried out based on the Edinburgh questionnaire11 and the ABI. Patients with mobility handicaps were excluded from the questionnaire: bedridden subjects, people in wheelchairs, amputees or patients with any other problem precluding the assessment of pain or discomfort on walking. The Edinburgh questionnaire was completed in the course of an interview by the investigator.
Statistical analysisQuantitative variables were reported as the mean±standard deviation (SD). Qualitative variables were presented as absolute and relative frequencies (%), with estimation of the corresponding 95% confidence interval (95%CI).
The comparison of means was made with the Student's t-test or Mann–Whitney U-test, as applicable, after normal data distribution was assessed with the Kolmogorov–Smirnov test. Multiple comparisons of means were carried out by analysis of variance (ANOVA) or the Kruskal–Wallis test. Associations between qualitative variables were evaluated by means of the chi-squared test.
The concordance between different qualitative variables was estimated based on the Kappa index. Sensitivity, specificity, predictive values and likelihood ratios were calculated to determine the validity and reliability of the Edinburgh questionnaire, using the ABI as the reference test.
Multivariate logistic regression analysis was used to identify those variables associated with the events of interest.
Data analysis was performed using the Statistical Package for the Social Sciences (SPSS), version 19.
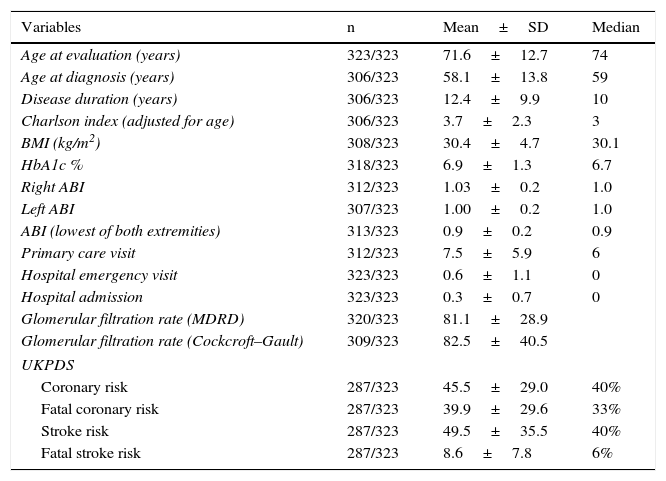
ResultsTable 1 describes the characteristics of the study sample. The mean patient age was 71.56±12.73 years; 50.5% were males.
General characteristics of the study sample.
| Variables | n | Mean±SD | Median |
|---|---|---|---|
| Age at evaluation (years) | 323/323 | 71.6±12.7 | 74 |
| Age at diagnosis (years) | 306/323 | 58.1±13.8 | 59 |
| Disease duration (years) | 306/323 | 12.4±9.9 | 10 |
| Charlson index (adjusted for age) | 306/323 | 3.7±2.3 | 3 |
| BMI (kg/m2) | 308/323 | 30.4±4.7 | 30.1 |
| HbA1c % | 318/323 | 6.9±1.3 | 6.7 |
| Right ABI | 312/323 | 1.03±0.2 | 1.0 |
| Left ABI | 307/323 | 1.00±0.2 | 1.0 |
| ABI (lowest of both extremities) | 313/323 | 0.9±0.2 | 0.9 |
| Primary care visit | 312/323 | 7.5±5.9 | 6 |
| Hospital emergency visit | 323/323 | 0.6±1.1 | 0 |
| Hospital admission | 323/323 | 0.3±0.7 | 0 |
| Glomerular filtration rate (MDRD) | 320/323 | 81.1±28.9 | |
| Glomerular filtration rate (Cockcroft–Gault) | 309/323 | 82.5±40.5 | |
| UKPDS | |||
| Coronary risk | 287/323 | 45.5±29.0 | 40% |
| Fatal coronary risk | 287/323 | 39.9±29.6 | 33% |
| Stroke risk | 287/323 | 49.5±35.5 | 40% |
| Fatal stroke risk | 287/323 | 8.6±7.8 | 6% |
| n | % | 95%CI | |
|---|---|---|---|
| Gender | |||
| Males | 163/323 | 50.5% | 44.8–56.0 |
| Females | 160/323 | 49.5% | 43.9–55.1 |
| AHT | 247/323 | 76.5% | 71.7–81.2 |
| Obesity (≥30) | 157/308 | 51.0% | 45.2–56.7 |
| Retinopathy | 46/270 | 17% | 12.3–21.7 |
| Glomerular filtration rate <60ml/min (MDRD) | 68/320 | 21.3% | 16.6–25.9 |
| Glomerular filtration rate <60ml/min (Cockcroft–Gault) | 104/309 | 33.7% | 33.6–28.2 |
| Smokers | 29/322 | 9% | 5.7–12.2 |
| Edinburgh questionnaire | 288/323 | ||
| No intermittent claudication | 212/288 | 73.6 | 68.3–78.8 |
| Defined intermittent claudication | 41/288 | 14.2 | 10.2–18.4 |
| Atypical intermittent claudication | 35/288 | 12.2 | 8.2–16.1 |
| Ankle-brachial index | 309/323 | ||
| ABI<0.9 | 82/309 | 26.5 | 21.4–31.6 |
| ABI 0.9–1.10 | 115/309 | 37.2 | 31.6–42.7 |
| ABI >1.10 | 112/309 | 36.2 | 30.7–41.7 |
SD: standard deviation; GF: glomerular filtration; AHT: arterial hypertension; BMI: body mass index; ABI: ankle-branchial index.
A total of 26.4% of the diabetic patients reported intermittent claudication according to the Edinburgh questionnaire (defined 14.2% and atypical 12.2%). On assessing PAD with the ABI, 37.2% of the patients had a normal ABI (0.9–1.10), 26.5% presented an ABI<0.9, and 36.2% presented an ABI>1.10 (Table 1).
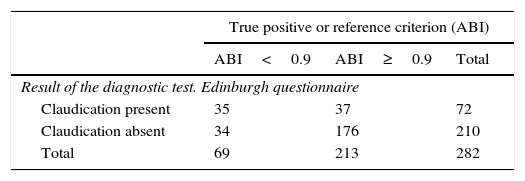
The analysis of concordance between the two diagnostic tests revealed a discordance between the Edinburgh questionnaire and the ABI in diagnosing PAD (Kappa index=0.33; p=0.000) (Table 2).
Validity of the Edinburgh questionnaire in predicting peripheral artery disease according to the ankle-branchial index (ABI<0.9).
| True positive or reference criterion (ABI) | |||
|---|---|---|---|
| ABI<0.9 | ABI≥0.9 | Total | |
| Result of the diagnostic test. Edinburgh questionnaire | |||
| Claudication present | 35 | 37 | 72 |
| Claudication absent | 34 | 176 | 210 |
| Total | 69 | 213 | 282 |
| 95%CI | |||
|---|---|---|---|
| % | Lower limit | Upper limit | |
| Disease prevalence | 24.5 | 19.6 | 29.9 |
| Patients correctly diagnosed | 74.8 | 69.3 | 79.7 |
| Sensitivity | 50.7 | 38.5 | 62.8 |
| Specificity | 82.6 | 76.7 | 87.3 |
| Positive predictive value | 48.6 | 36.8 | 60.6 |
| Negative predictive value | 83.8 | 77.9 | 88.4 |
| Positive likelihood ratio | 2.92 | 2.01 | 4.24 |
| Negative likelihood ratio | 0.60 | 0.47 | 0.76 |
CI: confidence interval; ABI: ankle-branchial index.
The Edinburgh questionnaire showed a sensitivity of 50.7% in predicting the diagnosis (ABI<0.9) and a specificity of 82.6%. The positive and negative predictive values were 48.6% and 83.8%, respectively (Table 2).
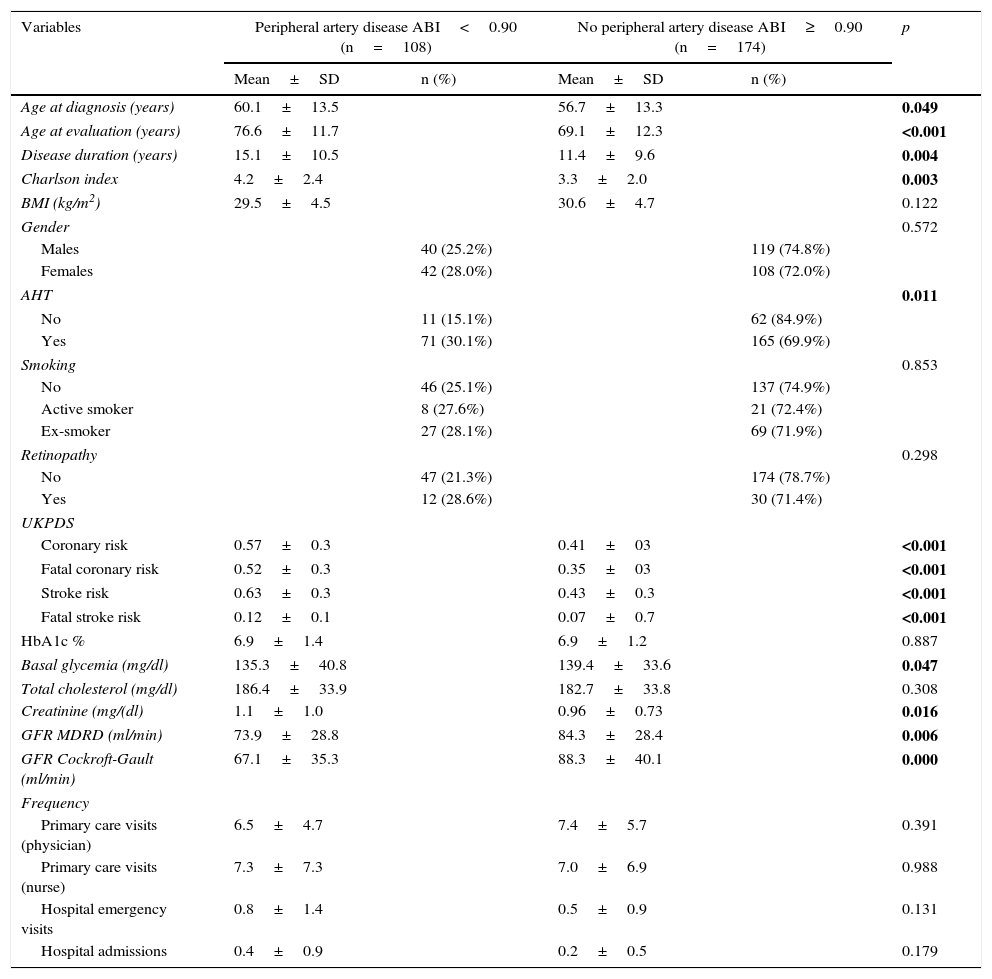
In the bivariate analysis, the variables associated with the presence of PAD (ABI<0.9) were found to be patient age, age at the time of diagnosis, disease duration, the Charlson morbidity score, a history of arterial hypertension, the risk of cardiovascular events calculated from the UKPDS score, the estimated glomerular filtration rate, and glycemia (Table 3).
Variables associated with the presence of peripheral artery disease (ABI<0.90).
| Variables | Peripheral artery disease ABI<0.90 (n=108) | No peripheral artery disease ABI≥0.90 (n=174) | p | ||
|---|---|---|---|---|---|
| Mean±SD | n (%) | Mean±SD | n (%) | ||
| Age at diagnosis (years) | 60.1±13.5 | 56.7±13.3 | 0.049 | ||
| Age at evaluation (years) | 76.6±11.7 | 69.1±12.3 | <0.001 | ||
| Disease duration (years) | 15.1±10.5 | 11.4±9.6 | 0.004 | ||
| Charlson index | 4.2±2.4 | 3.3±2.0 | 0.003 | ||
| BMI (kg/m2) | 29.5±4.5 | 30.6±4.7 | 0.122 | ||
| Gender | 0.572 | ||||
| Males | 40 (25.2%) | 119 (74.8%) | |||
| Females | 42 (28.0%) | 108 (72.0%) | |||
| AHT | 0.011 | ||||
| No | 11 (15.1%) | 62 (84.9%) | |||
| Yes | 71 (30.1%) | 165 (69.9%) | |||
| Smoking | 0.853 | ||||
| No | 46 (25.1%) | 137 (74.9%) | |||
| Active smoker | 8 (27.6%) | 21 (72.4%) | |||
| Ex-smoker | 27 (28.1%) | 69 (71.9%) | |||
| Retinopathy | 0.298 | ||||
| No | 47 (21.3%) | 174 (78.7%) | |||
| Yes | 12 (28.6%) | 30 (71.4%) | |||
| UKPDS | |||||
| Coronary risk | 0.57±0.3 | 0.41±03 | <0.001 | ||
| Fatal coronary risk | 0.52±0.3 | 0.35±03 | <0.001 | ||
| Stroke risk | 0.63±0.3 | 0.43±0.3 | <0.001 | ||
| Fatal stroke risk | 0.12±0.1 | 0.07±0.7 | <0.001 | ||
| HbA1c % | 6.9±1.4 | 6.9±1.2 | 0.887 | ||
| Basal glycemia (mg/dl) | 135.3±40.8 | 139.4±33.6 | 0.047 | ||
| Total cholesterol (mg/dl) | 186.4±33.9 | 182.7±33.8 | 0.308 | ||
| Creatinine (mg/(dl) | 1.1±1.0 | 0.96±0.73 | 0.016 | ||
| GFR MDRD (ml/min) | 73.9±28.8 | 84.3±28.4 | 0.006 | ||
| GFR Cockroft-Gault (ml/min) | 67.1±35.3 | 88.3±40.1 | 0.000 | ||
| Frequency | |||||
| Primary care visits (physician) | 6.5±4.7 | 7.4±5.7 | 0.391 | ||
| Primary care visits (nurse) | 7.3±7.3 | 7.0±6.9 | 0.988 | ||
| Hospital emergency visits | 0.8±1.4 | 0.5±0.9 | 0.131 | ||
| Hospital admissions | 0.4±0.9 | 0.2±0.5 | 0.179 | ||
The values in boldface indicate statistically significant differences.
SD: standard deviation; GF: glomerular filtration; AHT: arterial hypertension; BMI: body mass index; ABI: ankle-branchial index.
As regards the frequency of visits to the medical services, the patients with PAD attended the hospital more often, with both more admissions and more visits to the nurse each year, compared with the patients without PAD, though the difference failed to reach statistical significance (Table 3).
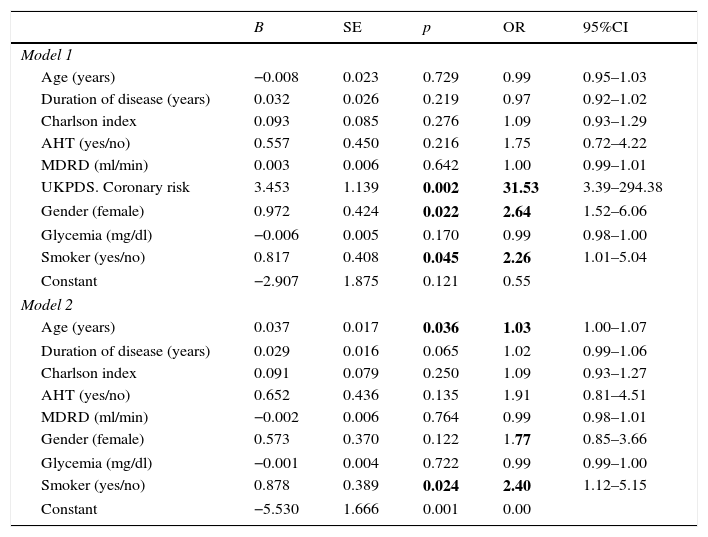
On entering the variables identified in the univariate analysis as being associated with PAD or which proved clinically relevant (gender) into the multivariate logistic regression model, we found the variables with an independent effect in predicting PAD to be the UKPDS cardiovascular risk score (odds ratio [OR]=31.6), smoking (OR=2.3), and female gender (OR=2.6) (Table 4, Model 1).
Logistic regression model for predicting peripheral artery disease adjusted for different covariables.
| B | SE | p | OR | 95%CI | |
|---|---|---|---|---|---|
| Model 1 | |||||
| Age (years) | −0.008 | 0.023 | 0.729 | 0.99 | 0.95–1.03 |
| Duration of disease (years) | 0.032 | 0.026 | 0.219 | 0.97 | 0.92–1.02 |
| Charlson index | 0.093 | 0.085 | 0.276 | 1.09 | 0.93–1.29 |
| AHT (yes/no) | 0.557 | 0.450 | 0.216 | 1.75 | 0.72–4.22 |
| MDRD (ml/min) | 0.003 | 0.006 | 0.642 | 1.00 | 0.99–1.01 |
| UKPDS. Coronary risk | 3.453 | 1.139 | 0.002 | 31.53 | 3.39–294.38 |
| Gender (female) | 0.972 | 0.424 | 0.022 | 2.64 | 1.52–6.06 |
| Glycemia (mg/dl) | −0.006 | 0.005 | 0.170 | 0.99 | 0.98–1.00 |
| Smoker (yes/no) | 0.817 | 0.408 | 0.045 | 2.26 | 1.01–5.04 |
| Constant | −2.907 | 1.875 | 0.121 | 0.55 | |
| Model 2 | |||||
| Age (years) | 0.037 | 0.017 | 0.036 | 1.03 | 1.00–1.07 |
| Duration of disease (years) | 0.029 | 0.016 | 0.065 | 1.02 | 0.99–1.06 |
| Charlson index | 0.091 | 0.079 | 0.250 | 1.09 | 0.93–1.27 |
| AHT (yes/no) | 0.652 | 0.436 | 0.135 | 1.91 | 0.81–4.51 |
| MDRD (ml/min) | −0.002 | 0.006 | 0.764 | 0.99 | 0.98–1.01 |
| Gender (female) | 0.573 | 0.370 | 0.122 | 1.77 | 0.85–3.66 |
| Glycemia (mg/dl) | −0.001 | 0.004 | 0.722 | 0.99 | 0.99–1.00 |
| Smoker (yes/no) | 0.878 | 0.389 | 0.024 | 2.40 | 1.12–5.15 |
| Constant | −5.530 | 1.666 | 0.001 | 0.00 | |
The values in boldface indicate statistically significant differences.
B: regression coefficient; SE: standard error; AHT: arterial hypertension; CI: confidence interval; OR: odds ratio.
On eliminating the UKDS score from the regression model, the variables with an independent effect in predicting PAD were found to be patient age (OR=1.03) and smoking (OR=2.4), with diabetes duration at the limit of statistical significance (OR=1.02) (Table 4, Model 2).
DiscussionDiabetic patients have a high risk of suffering from PAD. The clinical presentation of the latter is highly variable, ranging from the absence of symptoms to the presence of ulcers, gangrene or resting ischemic pain (so-called critical ischemia). In most cases PAD manifests as intermittent claudication (pain, discomfort or fatigue in the region of the calf muscles or other muscles of the lower extremity that increase on walking and disappear with rest). Due to the inconsistency of the clinical findings of PAD, and since a noninvasive and relatively simple test is available (the ABI), the latter is regarded as the method of choice for diagnosing PAD. An ABI score of under 0.9 is not only diagnostic of PAD but constitutes an independent risk marker for mortality and cardiovascular morbidity.4,13,14
The prevalence of PAD is higher among diabetic patients than in the general population.13–15 The NHANES studies recorded a higher risk of PAD in diabetic individuals (OR=2.71, 95%CI: 1.03–7.12), this risk level only being exceeded by smokers (OR=4.46, 95%CI: 2.25–8.84).16
The true prevalence of PAD in diabetics is difficult to establish, not only because many patients are asymptomatic, but also because the different studies use different diagnostic methods and include different diabetic patient profiles. Asymptomatic PAD is more frequent in diabetic patients in relation to associated comorbidity, altered pain perception or physical inactivity. Although the role of the ABI in the detection of PAD has now been well established, the first studies were made based on the absence of pulses or the presence of intermittent claudication.
Our study identified a prevalence of PAD (defined as an ABI<0.90) of 26.5% (95%CI: 21.4–31.6). The findings of this study are consistent with the prevalence of PAD documented in the literature, with values between 14.4% and 60.6%.9,10,17–20 The variability of the different findings is related to the characteristics of each individual study. Some of the reasons for such variability in the prevalence of the disease are the use of different sampling methods, differences in age ranges and in the duration of diabetes, and different risk factor distributions. In this study, the Edinburgh questionnaire was completed with the help of a professional, since the results have been found to be more reliable than when it is self-administered by the patient.21
Escobar et al.17 recorded a very high prevalence of PAD (70.9%) in a population of 1462 diabetics. However, the fact that the patients were over 70 years of age is sufficient in itself to explain such a high rate.
Puras-Mallagray et al.20 in turn studied 477 type 1 (n=119) and type 2 diabetics (n=358) seen in endocrinology clinics. The mean age was 59±16.1 years, and 53.2% were males. The prevalence of PAD in the clinics was 37.3% (mild-moderate in 34.6% of the cases and severe in 2.6%). The prevalence of PAD was seen to increase with age, in males, in the presence of metabolic syndrome, and with a longer duration of diabetic disease.
Agarwal et al.9 analyzed a sample of 146 type 2 diabetics with a mean age of 59.4±7.2 years (54.1% males), and with a mean duration of diabetes of 8.8±3.8 years. The prevalence of PAD, using an ABI<0.9 as the diagnostic criterion, was 14.4%, the figure being slightly higher in females (14.9%) than in males (13.9%). The risk factors significantly associated with PAD were found to be older age, a longer duration of diabetes disease, arterial hypertension, smoking and higher HbA1c levels.
Ali et al.10 studied 387 type 2 diabetics with a mean age of 52.22±9.6 years (33.1% males) and with a mean duration of diabetes of 8.8±3.8 years. The prevalence of PAD was found to be 39.28%, with a higher prevalence in females (46.71%) than in males (24.22%). Arterial hypertension was seen to be associated with the risk of PAD.
In this study, women had a significantly higher prevalence of PAD than men (28.0% versus 25.2%), which agrees with the observations of other authors.22,23 In our series, and in coincidence with the literature, active smoking and age were correlated to the presence of PAD.
As is well-known, the Edinburgh questionnaire represents one of the ways to explore PAD from the clinical perspective.
Based on the Edinburgh questionnaire, our study identified a prevalence of PAD of 26.4% (95%CI: 21.3–31.6).
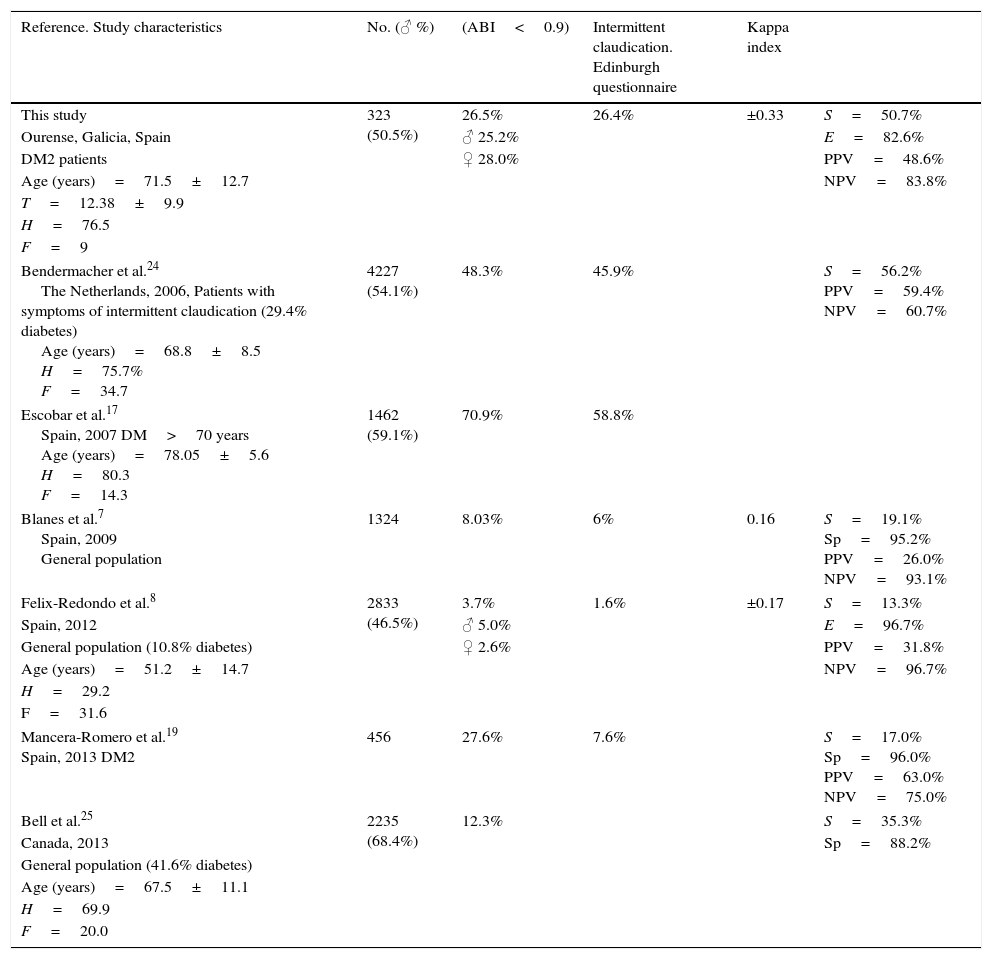
Different studies that have used the Edinburgh questionnaire for the diagnosis of PAD have reported values of between 1.6 and 58.8%7,8,17,19,24,25 (Table 5).
Edinburgh questionnaire predictive capacity regarding peripheral artery disease (ABI-Edinburgh questionnaire) in different studies.
| Reference. Study characteristics | No. (♂ %) | (ABI<0.9) | Intermittent claudication. Edinburgh questionnaire | Kappa index | |
|---|---|---|---|---|---|
| This study | 323 (50.5%) | 26.5% | 26.4% | ±0.33 | S=50.7% |
| Ourense, Galicia, Spain | ♂ 25.2% | E=82.6% | |||
| DM2 patients | ♀ 28.0% | PPV=48.6% | |||
| Age (years)=71.5±12.7 | NPV=83.8% | ||||
| T=12.38±9.9 | |||||
| H=76.5 | |||||
| F=9 | |||||
| Bendermacher et al.24 The Netherlands, 2006, Patients with symptoms of intermittent claudication (29.4% diabetes) Age (years)=68.8±8.5 H=75.7% F=34.7 | 4227 (54.1%) | 48.3% | 45.9% | S=56.2% PPV=59.4% NPV=60.7% | |
| Escobar et al.17 Spain, 2007 DM>70 years Age (years)=78.05±5.6 H=80.3 F=14.3 | 1462 (59.1%) | 70.9% | 58.8% | ||
| Blanes et al.7 Spain, 2009 General population | 1324 | 8.03% | 6% | 0.16 | S=19.1% Sp=95.2% PPV=26.0% NPV=93.1% |
| Felix-Redondo et al.8 | 2833 (46.5%) | 3.7% | 1.6% | ±0.17 | S=13.3% |
| Spain, 2012 | ♂ 5.0% | E=96.7% | |||
| General population (10.8% diabetes) | ♀ 2.6% | PPV=31.8% | |||
| Age (years)=51.2±14.7 | NPV=96.7% | ||||
| H=29.2 | |||||
| F=31.6 | |||||
| Mancera-Romero et al.19 Spain, 2013 DM2 | 456 | 27.6% | 7.6% | S=17.0% Sp=96.0% PPV=63.0% NPV=75.0% | |
| Bell et al.25 | 2235 (68.4%) | 12.3% | S=35.3% | ||
| Canada, 2013 | Sp=88.2% | ||||
| General population (41.6% diabetes) | |||||
| Age (years)=67.5±11.1 | |||||
| H=69.9 | |||||
| F=20.0 | |||||
DM: diabetes mellitus; Sp: specificity; F: smokers (%); H: history of arterial hypertension (%): ABI: ankle-branchial index; S: sensitivity; T: duration of disease (years); NPV: negative predictive value; PPV: positive predictive value.
There is a clear discrepancy between the findings of the clinical measurements based on the Edinburgh questionnaire and the ABI results. In our study, concordance measured with the Kappa index between the Edinburgh questionnaire and the ABI was 0.33. Different studies have reported Kappa indexes of about 0.16–0.17, though in the general population, not in diabetics.7,8,17,19,24,25
Using the ABI as the gold standard, this study found the sensitivity of the Edinburgh questionnaire in predicting PAD to be 50.7%, with a specificity of 82.6%.
The positive likelihood ratio was 2.92, this indicating the number of times a patient with a positive Edinburgh questionnaire result is more likely to have PAD than a patient without a positive Edinburgh questionnaire result.
The negative likelihood ratio was 0.60. These low likelihood ratios indicate that a positive Edinburgh questionnaire result does not allow us to confirm the presence of PAD, while a negative questionnaire result is unable to rule out PAD.
These results indicate that the Edinburgh questionnaire has a low predictive capacity regarding PAD, specificity being high but sensitivity low. The above findings are similar to those of other previous studies,7,8,17,19,24,25 possibly because the Edinburgh questionnaire is only a measure of symptomatic disease, and the symptoms are not only related to the degree of atherosclerosis but also to many other factors, including the level of patient physical exercise.
The sensitivity of the Edinburgh questionnaire varies greatly from 13.3% in the Hermex study8 conducted in the Spanish general population (with a 10.8% prevalence of diabetics) to 56.2% according to the study of Bendermacher et al.24 in 4227 patients visiting the physician due to symptoms suggestive of intermittent claudication.
The specificity performance of the questionnaire is more uniform in the different studies, with values in all cases that exceed the 82.6% recorded in our study, and reaching 96.7% in the Hermex trial.
In conclusion, there is a high prevalence of peripheral artery disease that increases with age, smoking, and the duration of diabetes disease. The concordance between the ABI and the Edinburgh questionnaire in diagnosing PAD is weak.
Consequently, the ABI must be determined in order to assess the presence of PAD.
Strong points
- -
Questionnaires and procedures validated by trained professionals were used.
- -
The study sample consisted of consecutive diabetic patients in which the prevalence of PAD was determined and a global assessment made of cardiovascular risk using the UKPDS score, together with comorbidity, quality of life, metabolic control and renal function.
- -
Missing values were minimized, with the patient being contacted to complete the required information.
Weak points
- -
Although this represented a consecutive sample of patients seen in a healthcare center, it was not necessarily representative of the general population. However, the results are consistent with the data found in the literature, thus warranting the internal validity of the study.
- -
As this is a prevalence study, it is not possible to determine the causality of the events; a patient follow-up study would be needed for this purpose.
The authors declare that they have no conflicts of interest.
Please cite this article as: Pita-Fernández S, Modroño-Freire MJ, Pértega-Díaz S, Herrera-Díaz L, Seoane-Pillado T, Paz-Solís A, et al. Validez del cuestionario de Edimburgo para el diagnóstico de arteriopatía periférica en pacientes diabéticos tipo 2. Endocrinol Diabetes Nutr. 2017;64:471–479.