Diabetes mellitus (DM) is the metabolic disorder most frequently associated with pregnancy. Approximately 1% of all pregnant women have pre-gestational DM (PGDM), and 12% or more, depending on the diagnostic strategy used, will have gestational diabetes mellitus (GDM). Of the women who have diabetes during pregnancy, it is estimated that approximately 87.5% have GDM, 7.5% have type 1 DM (DM1) and the remaining 5% have type 2 DM (DM2).
In Spain, the prevalence of DM1, and especially that of DM2, has increased in recent years. Recent data shows that the incidence of GDM is also increasing as a result of higher rates of obesity and more pregnancies in older women.
This article focuses on areas where additional or different care should be offered to women with diabetes and their newborns, based on the available evidence (full practice guideline available at: https://www.sediabetes.org/grupos_de_trabajo/diabetes-y-embarazo/; https://sego.es/Guias_de_Asistencia_Practica#perinatal).
Classification of pregnancy-related diabetesAll diabetes diagnosed before pregnancy is considered PGDM. Within this group, we can find DM1, DM2 and other specific types, such as monogenic diabetes. During pregnancy, it is important to rule out the presence of frank diabetes at the first antenatal visit, thus excluding the need for a diagnosis of GDM.
Pre-gestational diabetesPreconception controlOptimal glycaemic control in the periconceptional period and during pregnancy is associated with better maternal and fetal outcomes, including reduced risk of malformations and perinatal mortality. Therefore, all women of childbearing age with diabetes should receive regular preconception advice from the healthcare team that cares for them, whether in primary or specialised care (gynaecology/endocrinology). The risk for each patient wishing to become pregnant will be assessed individually and glycaemic control and treatment of associated complications and comorbidities will be optimised, suspending or replacing potentially teratogenic drugs with others that are safer for pregnancy.
- •
Glycaemic control target: HbA1c <6.5% (48mmol/l), if it can be achieved with low risk of hypoglycaemia. In women with DM1, the use of continuous glucose monitoring and/or continuous subcutaneous insulin infusion may be considered.
- •
Daily supplementation with folic acid (at least 400μg) and iodine (at least 200μg).
- •
Health education: reducing weight in case of obesity and abandoning the consumption of tobacco and other toxins.
- •
Contraception: recommended until the right conditions for pregnancy are met.
The same goals are recommended as in the preconception period:
- •
Basal blood glucose: 70–95mg/dl (3.9–5.3mmol/l).
- •
Postprandial blood glucose (1h): 110–140mg/dl (6.1–7.8mmol/l).
- •
Postprandial blood glucose (2h): 100–120mg/dl (5.5–6.7mmol/l).
- •
Continuous glucose monitoring: time in range (63–140mg/dl) >70%; time <63mg/dl: <4%; time >140mg/dl: <25%.
- •
Mean HbA1c ±2 SD (4.8–5.7% or 29–38.8mmol/l); <6.5% according to NICE; <6.5% in the first trimester, and <6.0% in the second and third, according to ADA.
- •
Absence of ketonuria and hypoglycaemia.
- •
Adapt the diet and recommend the practice of daily moderate physical exercise.
- •
Regarding treatment with oral hypoglycaemic agents, metformin may be justified in pregnant women with DM2 in conjunction with insulin, to avoid using large amounts of insulin.
- •
Regarding insulin treatment, a basal-bolus regimen or continuous insulin infuser can be used, preferably implemented in the preconception period. Be aware of changes in insulin sensitivity in relation to hormonal changes.
- •
Home self-monitoring: three preprandial capillary blood glucose tests and three daily postprandial blood glucose tests are recommended in cases of blood glucose >200mg/dl, with analysis of baseline ketonuria, to rule out ketosis/ketoacidosis.
- •
Use flash or real-time continuous glucose monitoring whenever possible.
- •
Provide glucagon to use in case of severe hypoglycaemia.
- •
Measurement of HbA1c every 4–8 weeks.
- •
Joint follow-up by obstetrician and diabetologist every 2–4 weeks Fig. 1.
Pregnancy can cause progression of diabetic retinopathy, especially if it is severe. It is advisable to examine the fundus at least prior to pregnancy and at 28 weeks. If no previous examination has been done recently, do one also in the first trimester Table 1, Table 2.
Diagnostic criteria for frank diabetes during pregnancy.
| Fasting plasma glucose | ≥126mg/dl* (7.0mmol/l) on more than two occasions (IADPSG, WHO, ADA) |
|---|---|
| Glucose 2h after 75g OGTT | ≥200mg/dl* (11.1mmol/l) (WHO, ADA) |
| Clinical symptomatology of diabetes and random glucose | ≥200mg/dl** (11.1mmol/l) (IADPSG, WHO, ADA) |
| HbA1c | ≥6.5%* (47.5mmol/mol) (IADPSG, ADA) |
OGTT: oral glucose tolerance test; ADA: American Diabetes Association; IADPSG: International Association of Diabetes and Pregnancy Study Groups; WHO: World Health Organization.
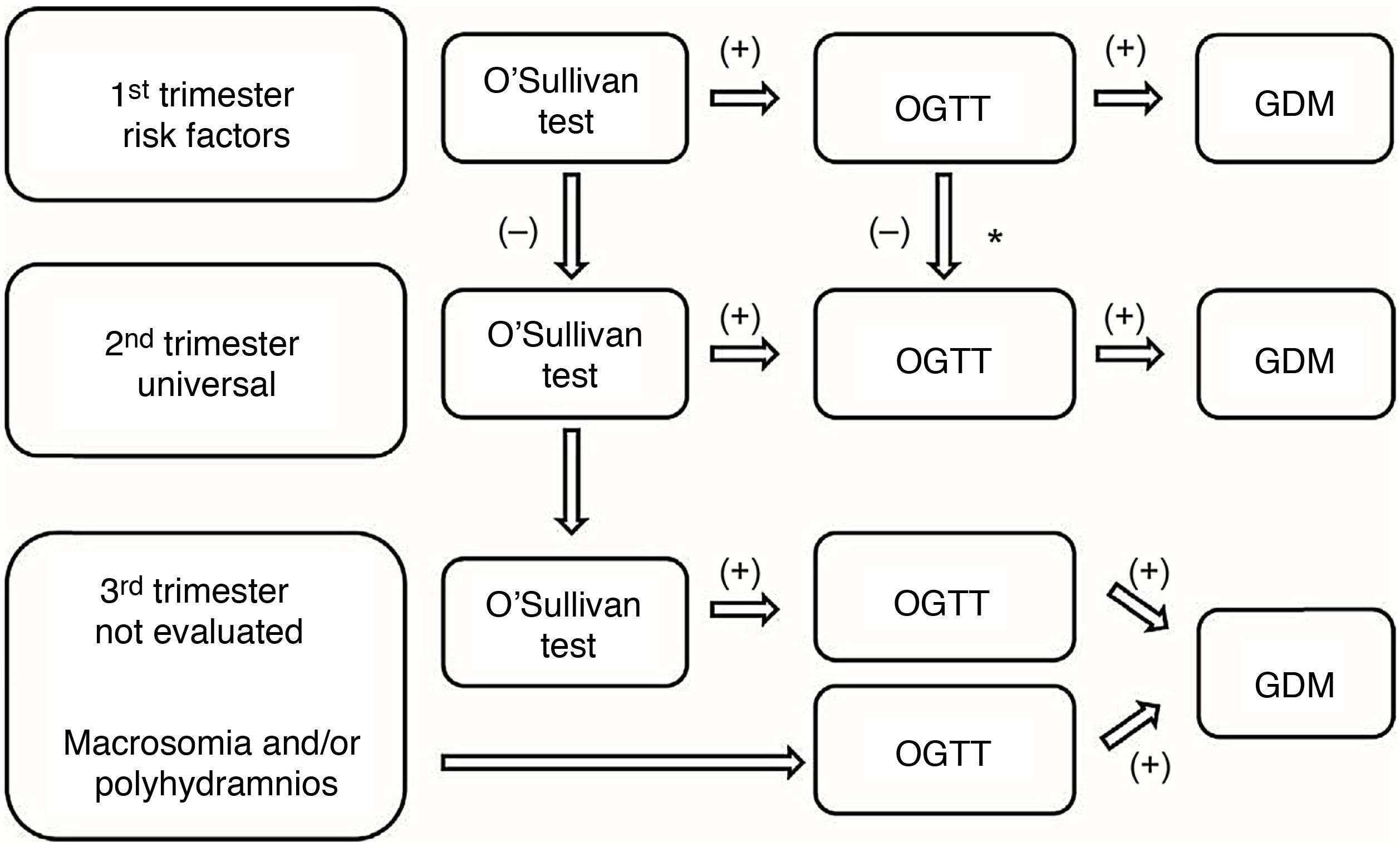
Indications for gestational diabetes screening.
| When? | In whom? | How? | Where? |
|---|---|---|---|
| 1st trimester (at 10–12 weeks) | High-risk pregnant women (recommendation B): Age ≥35 years Obesity (BMI≥30) Personal history of GDM, or poor obstetric history (macrosomia or polyhydramnios) Family history of DM in first-degree relatives Ethnic minorities with a high prevalence of DM (pregnant women from Latin America, from Southeast Asia, …) | O’Sullivan test (50g OGTT) | At the health centre |
| 2nd trimester (at 24–28 weeks) | UNIVERSAL (recommendation A) For all pregnant women not previously diagnosed | ||
| 3rd trimester | Complications associated with GDM (macrosomia or hydramnios) in pregnant women not previously diagnosed | 100g OGTT | Usually in hospital |
Determination of albuminuria and creatinine is recommended every trimester. Suspend treatment with ACE inhibitors and ARBs, replacing them with others with less risk to the fetus (alpha-methyldopa, labetalol and calcium antagonists).
Obstetric monitoring completion of pregnancy and mode of delivery
It should be aimed at preventing pre-eclampsia and early diagnosis of a possible appearance of structural malformations, fetal cardiomyopathy and macrosomia.
PGDM is a risk factor for pre-eclampsia, so screening in the first trimester is recommended. If screening is high risk, the ADA recommends starting with acetylsalicylic acid (ASA) at low doses of 60–150mg/day. Although there is controversy about the optimal dose of ASA, since doses of 150mg/day were used in the ASPRE study, there are several meta-analyses that have found a reduction in cases of pre-eclampsia with doses ≥100mg/day, so we should recommend a dose of 100–150mg/day from 12 weeks of gestation and up to 36+6 weeks. If screening is not available, preventive treatment with ASA is recommended in all pregnant women with diabetes.
Ultrasound control is recommended to monitor fetal growth, amniotic fluid volume and placental characteristics, which should be performed monthly from 28–30 weeks. It is recommended to perform an early echocardiography at week 14–16, especially in pregnant women with a higher risk of malformations (BMI≥30kg/m2, unplanned pregnancy, HbA1c >8%, polyhydramnios, ketoacidosis, severe diabetic nephropathy), as well as another fetal echocardiography at week 28–32 for the study of hypertrophic cardiomyopathy, mainly in pregnant women with poor metabolic control.
Induction of labour at term is generally accepted to reduce the risk of complications. With correct metabolic control and adequate monitoring of fetal well-being, the pregnancy should be allowed to progress until the spontaneous onset of labour, with induction of labour appropriate from week 38+6.When there are no guarantees of adequate follow-up, there is suboptimal glycaemic control or there are other maternal or fetal complications (maternal vasculopathy, worsening renal failure, active proliferative retinopathy, pre-eclampsia, IUGR), termination of the pregnancy from week 36+0 will be considered.
If it is necessary to terminate pregnancy before week 34+6, corticosteroids should be administered to accelerate fetal lung maturation, taking into account the corresponding adequacy of insulin treatment.
Atosiban and nifedipine are the drugs of choice for the treatment of threatened preterm labour. β-mimetics are not recommended due to their hyperglycaemic effect. The method of induction will depend on the cervical conditions. With a favourable cervix (Bishop index >6): amniotomy, cardiotocographic monitoring and oxytocin infusion; with an unfavourable cervix: prior cervical ripening with prostaglandins or with mechanical methods to reduce the risk of uterine hyperstimulation (fetal macrosomia or polyhydramnios).
The route of choice for delivery will be vaginal. The indications for caesarean section are the same as for pregnant women without diabetes, except when the estimated fetal weight exceeds 4,500g or there is a history of shoulder dystocia, in which case caesarean section is recommended in order to avoid obstetric trauma. Induction of labour should be avoided when fetal macrosomia is suspected, as this intervention has not been proven to improve maternal or fetal outcomes and may increase the rate of cesarean sections. There is no contraindication to attempting vaginal delivery in women with a history of previous cesarean section, although the rate of vaginal delivery appears to be lower than in women without diabetes.
Diabetic retinopathy is not a contraindication for vaginal delivery, although in the case of severe proliferative retinopathy it is recommended to shorten the expulsive period to avoid the development of retinal haemorrhages, and the use of locoregional anaesthesia during delivery is also recommended.
Intrapartum metabolic controlIts objective is to avoid maternal metabolic complications and contribute to reducing neonatal morbidity. Neonatal hypoglycaemia is mostly related to intra-pregnancy control, but intrapartum hyperglycaemia (>140–180mg/dl) also contributes.
With little evidence from clinical trials, the following is recommended:
- •
Control objective: capillary blood glucose between 70–110mg/dl (3.9–6.1mmol/l), trying to minimise maternal hypoglycaemia.
- •
Carbohydrate intake: 5% dextrose serum at a rate of 125ml/h (500 cc/4h) to minimise ketogenesis.
- •
Insulin intake: administer fast-acting insulin, preferably by intravenous infusion, due to the flexibility that this route provides.
- •
Monitoring: hourly control of capillary blood glucose to adjust the rhythm of glucose and/or insulin infusions.
There are observational data that support the use of a subcutaneous insulin pump and continuous glucose monitoring as an alternative, provided that institutional protocols are available in this regard.
Breastfeeding and puerperiumNewborn care differs from that established for pregnant women without diabetes in the need to prevent, detect and treat neonatal hypoglycaemia. After delivery, insulin treatment will be discontinued and glycaemic controls will be carried out to confirm the metabolic situation in the immediate postpartum period. Breastfeeding is recommended. The need to adjust insulin treatment and diet, the recommendations for other diabetes treatments in this period and the specific puerperal controls for each patient should be clarified.
Special considerations in women with diabetes and of childbearing ageThe indications and efficacy of the different contraceptive methods available are similar to those for the general population. Contraceptive methods that combine oestrogen and progestin have been shown to be safe in women with type 1 and 2 diabetes. However, in patients with vasculopathy, the potential risk of thrombotic phenomena must be taken into account and other options must be evaluated, such as methods that only use gestagens (pill, levonorgestrel IUD, subdermal implants) or copper IUD, since all of them are associated with a lower rate of thrombotic effects.
Gestational diabetesDiagnosisThere are two strategies for diagnosis:
- •
One-step strategy. With 75g oral glucose tolerance test (OGTT).
- •
Two-step strategy. 50g OGTT screening test and, if positive (≥140mg/dl), 100g OGTT diagnostic test.
The screening/diagnostic test will be carried out:
- •
In the first trimester if there are risk factors for GDM.
- •
In the second trimester (week 24–28 of gestation) in all pregnant women not previously diagnosed.
- •
In the third trimester in those not previously studied and/or who develop complications (polyhydramnios, macrosomia).
The GEDE [Grupo Español de Diabetes y Embarazo (Spanish Diabetes and Pregnancy Group)] continues to recommend a two-step diagnosis and the use of the diagnostic criteria of the National Diabetes Data Group (NDDG) and the 3rd Workshop-Conference on Gestational Diabetes Mellitus (two or more values equal to or greater than the following: basal blood glucose 105mg/dl, 190mg/dl at one hour, 165mg/dl at two hours and 145mg/dl at three hours), considering that there is insufficient evidence with randomised controlled trials that show benefits in terms of pregnancy outcomes with the diagnosis and treatment of GDM with the IADPSG criteria versus these previous criteria.
Control during pregnancy and childbirthMetabolic controlTreatment begins with a diet plan, physical activity, weight control and blood glucose control to achieve the objectives:
- •
Fasting blood glucose <95mg/dl (5.3mmol/l).
- •
One-hour postprandial blood glucose <140mg/dl (7.8mmol/l) or two-hour postprandial glucose <120mg/dl (6.7mmol/l).
Most GDM patients can control blood glucose levels with lifestyle modification.
Nutritional control and exerciseCaloric needs will be similar to the rest of pregnant women, not recommending diets with fewer than 1,700kcal and promoting weight gain in accordance with the recommendations of the Institute of Medicine (2009). A minimum intake of 175g of carbohydrates is recommended, limiting fast-absorbing carbohydrates, which represents 40-50% of total calories, along with a fibre intake of 28g per day. The diet should emphasise monounsaturated and polyunsaturated fats, limit saturated fats and avoid trans fats, recommending that they should contribute 30% to 40% of total calories. The minimum protein intake will be 71g per day, based on weight.
Aspartame, sucralose and stevia are considered safe non-caloric sweeteners, when consumed in moderation. The use of saccharin and cyclamate is not recommended. Daily physical exercise of 20–60min three to four days a week is recommended.
Self-monitoring of capillary blood glucoseIn general, the recommendation is to carry out four capillary blood glucose checks per day: preprandial and postprandial at breakfast and preprandial and postprandial at lunch or dinner (every other day). In general, at diagnosis it will be recommended daily, modifying this frequency according to the results of the glycaemic profile. Carry out one or two weekly 6-point profiles, including pre-meal and pre-dinner blood glucose levels and control of ketonuria.
If lifestyle modifications within one to two weeks do not achieve glycaemic control goals (two control levels above goals at the same time of day), or in the case of fetal overgrowth, pharmacological treatment may be necessary.
Pharmacological treatmentInsulin is the first-line agent. Basal insulin will be used in the case of high fasting blood glucose levels in two or more controls, with the starting dose being 0.1–0.2U/kg/day. For prandial insulin, 0.7–1.5IU (obesity)/10g of carbohydrates at breakfast and 0.5–1IU (obesity)/10g of carbohydrates at lunch and dinner could be an appropriate calculation. The insulin dose, both basal and prandial, will be adjusted according to the glycaemic control levels.
Metformin can be considered the pharmacological alternative in patients with difficult follow-up or who refuse to take insulin.
Obstetric control and termination of pregnancyObstetric control will be similar to that carried out in normal pregnant women, with some clarification. Follow-up in patients with GDM will include the recommendation to perform an additional ultrasound scan at week 28–30 to detect polyhydramnios and fetal macrosomia. Ultrasound at around week 36–38 can provide useful information for planning the termination of pregnancy.
Pregnant women with GDM associated with poor glycaemic control, macrosomia, obesity or the existence of other comorbidities are at risk of worse perinatal outcomes. The priority objective in this group will be to carry out stricter follow-up and control, until it is similar to pregnant women with PGDM in the most severe cases.
Termination of pregnancy in patients with well-controlled GDM will be similar to in the general population. However, in patients with risk factors, such as those who require insulin, the decision will be individualised, although prolonging gestation beyond 39–40 weeks is generally not recommended.
Intrapartum monitoringIn cases that require insulin, or in those with macrosomia, the same metabolic targets should be maintained as in PGDM, so capillary blood glucose should be monitored (target 70–110mg/dl, without ketonuria) Insulin treatment will preferably be by continuous intravenous infusion, supplying glucose at 5–10% and performing hourly monitoring of capillary blood glucose to adjust the rate of infusion.
Newborn care differs from that established for pregnant women without diabetes in the need to prevent, detect and treat neonatal hypoglycaemia. After delivery, insulin treatment will be discontinued and glycaemic controls will be carried out to confirm the metabolic situation in the immediate postpartum period.
Postpartum follow-upGDM identifies a group of women with a higher risk of developing diabetes mellitus, metabolic syndrome and cardiovascular disease throughout their lives. The evaluation of hydrocarbon metabolism will be carried out using a 75g OGTT, preferably between 6–12 weeks postpartum, although this period could be extended up to six months or after the end of lactation. A fasting glycaemia prior to discharge ≥100mg/dl allows us to identify those patients with a higher risk of persistent diabetes and who would benefit from earlier intervention.
There is no validated strategy for long-term follow-up after the first reassessment, although an annual metabolic review is recommended in cases of categories of increased risk of diabetes, and one every three years in case of normal tolerance to glucose, while other components of the metabolic syndrome should also be evaluated.
Breastfeeding should be recommended and encouraged due to its beneficial effects on the mother and the baby. Interventions aimed at optimising diet and lifestyle have been shown to be cost-effective in this group of patients, especially if they are started during pregnancy. The recommendations regarding contraception are similar to those for the general population.
Justification of diabetes and pregnancy clinics (DPCs)Multidisciplinary teams, made up primarily of obstetricians, endocrinologists/diabetologists and diabetes educators/dieticians, have been shown to improve glycaemic control and maternal-fetal outcomes in women with PGDM. Regarding the management of women with GDM, there is no uniform position in most of the guides on the role of DPCs, but there is uniformity in recommending the skills that are needed for their management, especially in nutritional treatment.
Adequate management and follow-up of pregnant women with PGDM and GDM requires specialised units that can handle two levels of care complexity, as well as adequate coordination to enable early referral of patients between them.
- •
Level A (outpatient primary and specialised care): will basically carry out the diagnosis of GDM, its follow-up if it is controlled with diet and exercise, and postpartum control and follow-up.
- •
Level B (referral hospital): will carry out, above all, the management of PGDM and its preconception planning, as well as the management of GDM that is difficult to control or requires drugs.