Hyponatremia is the most prevalent electrolyte disorder in the outpatient and inpatient settings. Despite this frequency, hyponatremia, including severe hyponatremia, is frequently underestimated and inadequately treated, thus highlighting the need to produce consensus documents and clinical practice guidelines geared towards improving the diagnostic and therapeutic approach to it in a structured fashion.
Material and methodsMembers of the Acqua Group of the Spanish Society of Endocrinology and Nutrition (SEEN) met using a networking methodology over a period of 20 months (between October 2019 and August 2021) with the aim of discussing and developing an updated guideline for the management of hyponatraemia. A literature search of the available scientific evidence for each section presented in this document was performed.
ResultsA document with 8 sections was produced, which sets out to provide updated guidance on the most clinically relevant questions in the management of hyponatraemia. The management of severe hyponatraemia is based on the i.v. administration of a 3% hypertonic solution. For the management of chronic euvolemic hyponatraemia, algorithms for the initiation of treatment with the two pharmacological therapeutic options currently available in Spain are presented: urea and tolvaptan.
ConclusionsThis document sets out to simplify the approach to and the treatment of hyponatraemia, making it easier to learn and thus improve the clinical approach to hyponatremia.
La hiponatremia es el trastorno electrolítico más prevalente tanto en medio ambulatorio como hospitalario. A pesar de esta frecuencia, la hiponatremia, incluso la grave, es frecuentemente infravalorada e inadecuadamente tratada, lo que pone de manifiesto la necesidad de elaborar documentos de consenso y guías de práctica clínica orientadas a mejorar su abordaje diagnóstico y terapéutico de forma estructurada.
Material y métodosMiembros del Grupo Acqua de la Sociedad Española de Endocrinología y Nutrición (SEEN) se reunieron mediante una metodología de trabajo en red durante un periodo de 20 meses (entre Octubre de 2019 y Agosto de 2021), con la finalidad de discutir y elaborar una guía actualizada para el manejo de la hiponatremia. Se realizó una búsqueda bibliográfica de la evidencia científica disponible para cada apartado expuesto en el presente documento.
ResultadosSe ha elaborado un documento que a través de 8 secciones pretende resolver la mayoría de las preguntas en el manejo clínico de la hiponatremia. El manejo de la hiponatremia grave se basa en la administración de solución hipertónica al 3% iv. Para el manejo de la hiponatremia crónica euvolémica se exponen algoritmos para el inicio del tratamiento con las dos opciones terapéuticas farmacológicas disponibles actualmente en nuestro medio: urea y tolvaptán.
ConclusionesEste documento pretende simplificar el abordaje y tratamiento de la hiponatremia, permitiendo facilitar su aprendizaje y mejorar así el abordaje clínico de la misma.
Hyponatraemia, defined as a decrease in serum sodium concentration (Na) below 135mmol/l, is the most prevalent electrolyte disorder in both outpatient and hospital settings,1 affecting 19% of hospitalised patients2 and 7% of those treated on an outpatient basis.3
Acute hyponatraemia, which by definition develops in less than 48h, is serious in itself, capable of inducing deep cerebral oedema, cerebral herniation and, therefore, high mortality. On the other hand, while chronic hyponatraemia, which takes more than 48h to develop or has an unknown onset time, can also be serious, it is usually mild to moderate, given its more latent development. However, it is not an indolent condition, as it is associated with gait instability and risk of falls and fractures,4,5 as well as increased hospital stay, and both in-hospital6,7 and outpatient mortality in the immediate period after discharge.2 Given its multiple aetiologies, hyponatraemia is often the first sign of pathologies with important prognostic repercussions. In addition, its presence notably increases the costs of healthcare,8 not only due to its direct effects, but also due to the important association with a lower functional capacity and quality of life, especially in elderly patients.
After correction of severe hyponatraemia, whether acute or chronic, or its overcorrection, complete neurological recovery may take weeks, and some patients may present permanent neurological sequelae.9 Therefore, early treatment of severe hyponatraemia, with an increase in Na of at least 4−6mmol/l in the first 4−6h, is essential and has been shown to improve the survival prognosis.10 Likewise, correction of chronic hyponatraemia, after reaching sustained levels of eunatraemia (>135mmol/l), has been associated with a significant improvement in gait stability11 and very probably in mortality, as suggested by Corona et al.12 in their latest meta-analysis. However, if the increase in Na is very rapid and exceeds the established limits, it can cause the appearance of osmotic demyelination syndrome (ODS), especially in patients with chronic hyponatraemia and associated risk factors, such as alcoholism, malnutrition, prolonged use of diuretics or hypokalaemia.13,14
Despite the aforementioned impact and the currently available therapeutic options, hyponatraemia, even severe hyponatraemia, is frequently underestimated and inadequately treated, which has highlighted the need to develop consensus statements and clinical practice guidelines aimed at improving the diagnostic and therapeutic approach to this condition in a structured way.
These documents include a consensus guide from a Spanish multidisciplinary group15 made up of two specialists in nephrology, one in hospital pharmacy, two in internal medicine and two in endocrinology, which was drawn up in 2013 with the aim of providing a specific guide and a detailed description of the treatment of hyponatraemia induced by the syndrome of inappropriate antidiuretic hormone secretion (SIADH).
With the intention of giving continuity to the aforementioned guide, the water metabolism working group (Acqua Group) of the Área de Conocimiento de Neuroendocrinología [Neuroendocrinology Knowledge Area] of the Sociedad Española de Endocrinología y Nutrición [Spanish Society of Endocrinology and Nutrition] (SEEN) has prepared this update document.
Material and methodsMembers of the Acqua Group met through a networking methodology over a period of 20 months (between October 2019 and August 2021), in order to discuss and develop an updated guide for the management of hyponatraemia. A bibliographic search of the available scientific evidence was carried out for each section presented in this document.
Given the lack of evidence that characterises many aspects of the treatment of hyponatraemia, the algorithms developed below are largely based on widely accepted recommendations in routine clinical practice, expert opinion and previous consensus guidelines, as well as on the clinical experience of the authors. As is always the case in clinical practice situations, the instructions in the document must be applied in a flexible manner and adapted to each patient and socio-health context.
Pathogenesis of hyponatraemiaPhysiologically, to maintain water balance and avoid states of overhydration, dehydration and decreased tissue perfusion, the plasmatic release of antidiuretic hormone (ADH), also known as arginine vasopressin (AVP) or argipressin, is regulated from the neurohypophysis mainly through two ways: via the osmoreceptors of the anterior hypothalamus and via the systemic baroreceptors. ADH, once released into the bloodstream, has a half-life of approximately 20min.16 Through its action on V2 receptors in the collecting duct of the nephron, it will promote increased reabsorption of water through aquaporin-2 channels in the collecting duct, with a consequent reduction in free water excretion, and increased intravascular water/sodium volume and ratio.
Via osmoreceptors, changes in plasma osmolality (pOsm) regulate the release of ADH. It has been observed that the blood release of ADH is markedly inhibited with effective pOsm less than 280mOsm/kg, while its maximum concentrations are observed with effective pOsm ≥290mOsm/l.17,18 Via baroreceptors (at the level of the left atrium, carotids, aortic arch, pulmonary capillaries or afferent arteriole of the nephron), changes in the effective circulating volume (ECV) – or intravascular volume – can be detected, which will determine the concentration of ADH in the blood. Small decreases in ECV, such as those observed with reductions of 5% or more in blood pressure,19 can be detected by the baroreceptors and cause a direct stimulus via the sympathetic system,19 and an indirect stimulus via the renin-angiotensin-aldosterone system to the hypothalamic nuclei for the synthesis of and subsequent release of ADH.20 Conversely, an increase in ECV will inhibit ADH release via these pathways. The sensitivity of the osmoreceptors for causing changes in ADH release is greater than that of the baroreceptors, although the potency of the stimulus is greater in the baroreceptors. However, both stimuli are acute and rapidly inhibited when the precipitating conditions are corrected.19
Hyponatraemia can be produced by dependent (non-osmotic secretion) or non-dependent mechanisms of ADH release. The first group is the most common, and includes clinical situations in which there is a marked release of ADH via baroreceptors or by other non-osmotic stimuli. In the second group, conditions such as primary polydipsia and iatrogenic fluid overload can be found, in which there is a decrease in Na and pOsm as a consequence of excess intravascular water. This occurs due to an inability of the kidneys to excrete excess free water, generally associated with an absence of sufficient solute to generate an adequate osmotic gradient in the renal medulla.21
In addition to the classic ADH stimuli, such as increased plasma osmolality and hypovolaemia, we find non-osmotic stimuli other than those of the baroreceptor pathway, such as cortisol deficiency, hypoglycaemia,16 septic-inflammatory states,22,23 nausea or vomiting,18 or pain.24 On the other hand, the mechanisms of action of some drugs (Table 1), through their interaction with some central neurotransmitters (cholinergic, serotonergic, adrenaline, dopaminergic) and/or with angiotensin-II, can stimulate the release of hypothalamic ADH. In addition, inappropriately high levels of ADH can be produced ectopically by some tumours and cause hyponatraemia (typically SIADH), a situation commonly seen in small cell lung carcinoma.25
List of drugs that can cause or contribute to hyponatraemia.
| Diuretics |
| Acetazolamide, loop, thiazides, amiloride |
| Drugs that interfere with aldosterone secretion/action |
| Heparin, amiloride, trimethoprim, pentamidine, NSAIDs, cyclosporine, tacrolimus, spironolactone, eplerenone, ACE inhibitors, ARBs |
| Psychotropics |
| Tricyclic antidepressants, serotonin reuptake inhibitors |
| Antipsychotics: phenothiazines, butyrophenones |
| Antiepileptics: carbamazepine, oxcarbazepine, valproate |
| Chemotherapy |
| Vinca alkaloids: vincristine, vinblastine |
| Alkylating agents: cyclophosphamide, melphalan, ifosfamide |
| Cisplatin, carboplatin |
| Analgesics |
| Nonsteroidal anti-inflammatory drugs, opioids |
| Other |
| Methotrexate, interferon alpha and gamma, proton pump inhibitors |
NSAIDs: nonsteroidal anti-inflammatory drugs; ARBs: angiotensin receptor blockers; ACE inhibitors: angiotensin-converting enzyme inhibitors.
SIADH is the most common aetiology of hyponatraemia,27 both in hospital28 and outpatient29 settings. In this condition, there is an excessive concentration of urine for the existing low pOsm, in the absence of a decrease in ECV, leading to a low excretion of free water and the consequent hyponatraemia. Its diagnosis is by exclusion, having to rule out the existence of adrenal insufficiency, the use of diuretics and other physiological and non-osmotic stimuli of ADH. Although hypothyroidism is one of the conditions to rule out before diagnosing SIADH, it is important to point out that it is a rare disorder and that for it to cause hyponatraemia it must be severe, and the hyponatraemia associated with this condition is usually mild.30 The diagnostic characteristics of SIADH are summarised in Table 2.
Diagnostic criteria for SIADH.
| Euvolaemic state |
| Effective plasma osmolality <275mOsmol/kg |
| Urinary osmolality >100mOsmol/kg with preserved renal function |
| Urinary sodium >30mmol/l with adequate sodium and water intake |
| Rule out: |
| Use of diuretics, hypocortisolism, hypothyroidism, and other physiological causes (nausea/vomiting, pain) |
SIADH: syndrome of inappropriate antidiuretic hormone secretion.
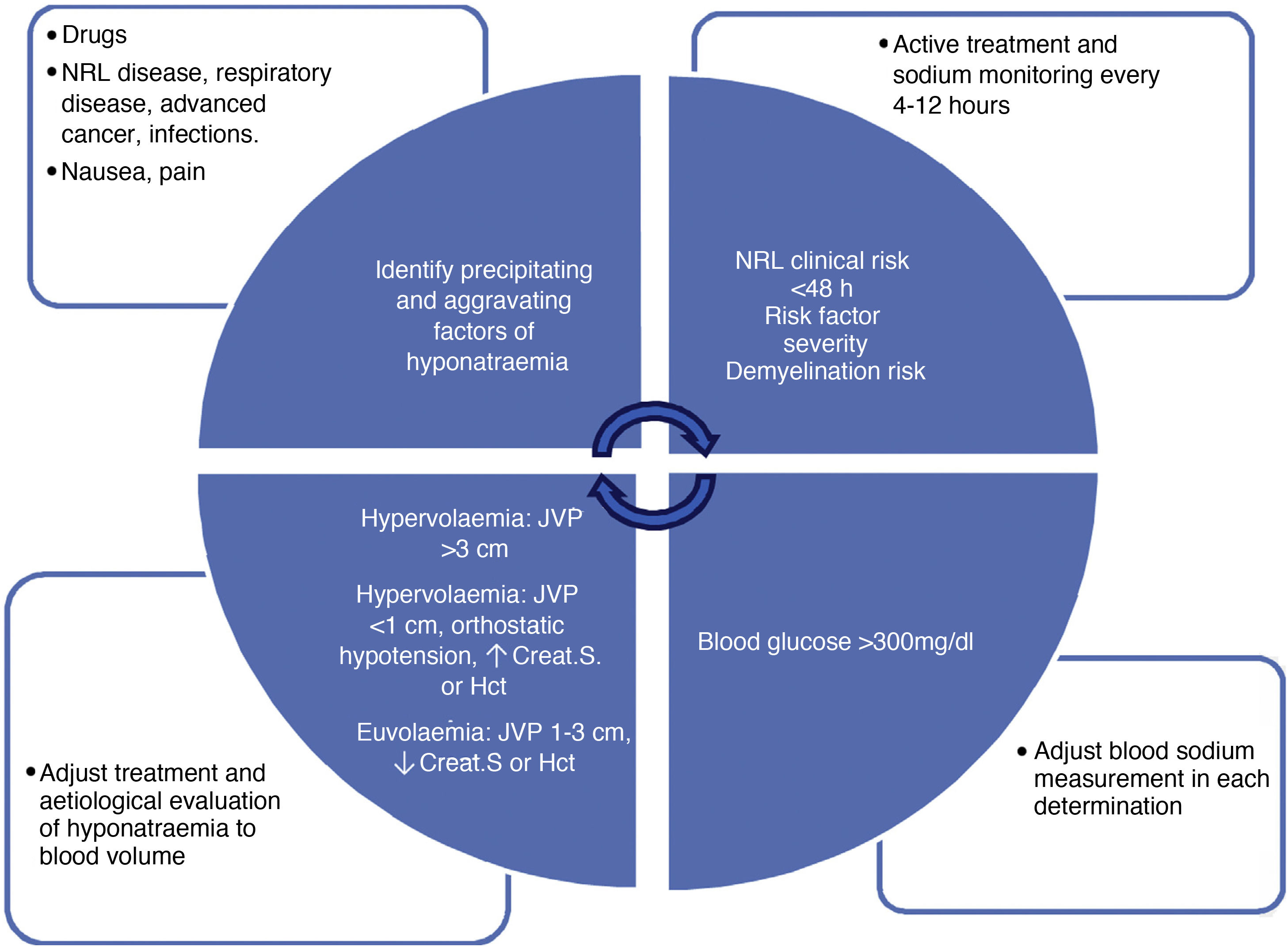
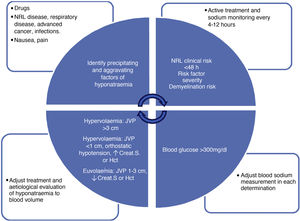
The diagnosis of hyponatraemia must be made early and accurately, since its treatment and, therefore, the success of its resolution will depend on it. The diagnosis can be summarised schematically in four steps of continuous evaluation that must be repeated in each assessment of the patient (Fig. 1):
- 1.
Confirm the actual level of Na.
- 2.
Stage the severity of hyponatraemia.
- 3.
Classify the volume.
- 4.
Identify predisposing and aggravating factors.
Diagnostic algorithm for hyponatraemia: it describes the factors to be evaluated serially in all patients with hyponatraemia. The upper left box assesses factors for the onset and progression of hyponatraemia. The upper right box evaluates severity factors: clinical neurological risk (obtundation, seizures, impaired consciousness), progression in <48h (also allows faster correction of hyponatraemia), severity risk factors (hypoxemia, disseminated cancer, advanced heart failure or liver disease) and demyelination (liver disease, alcoholism, malnutrition, hypokalaemia, natraemia <105mEq/l). The lower right box specifies the conditions in which we must correct the measurement of natraemia (hypoproteinaemia, hypertriglyceridaemia, hyperglycaemia). Finally, the lower left box indicates the blood volume classification of patients with hyponatraemia, which should be done serially in all patients. Crea.S: serum creatinine; Hct: haematocrit; SSRIs: selective serotonin reuptake inhibitors; NRL: neurological; JVP: jugular vein pulse.
In the presence of a state of hypotonicity (low effective osmolality), the analytical value of Na must be corrected based on blood glucose levels31,32 (when it is >140mg/dl), which is the most relevant and common factor in clinical practice. To correct Na according to blood glucose, the measured Na value must be increased by 1.6mmol/l for every 100mg/dl of blood glucose above 100mg/dl up to values of 400mg/dl (corrected Na=measured Na+0.016 * blood glucose [mg/dl]–100). With higher values, the increase should be 4mmol/l of Na for every 100mg/dl of blood glucose.31 Na corrections with total protein and triglycerides are not clearly validated. In addition, the change observed in natraemia figures with the evaluated formulas is not clinically relevant, so we do not recommend its routine application.
Classify the severity of hyponatraemiaVarious factors, such as young age or female sex33,34, can increase the clinical severity of hyponatraemia, but the speed of onset is one of the most determining factors. A decrease in natraemia >10mmol/l in 48h should be considered as severe hyponatraemia.
Traditionally, the classification of the severity of hyponatraemia has been based on Na levels. In this way, hyponatraemia is considered mild when Na is between 130.1 and 134.9mmol/l, moderate when it is between 120.1 and 130mmol/l, and severe when it is ≤120mmol/l. However, recent reviews suggest changing the Na threshold level to <125mmol/l to define severe hyponatraemia,35–37 fundamentally if it is accompanied by neurological symptoms, given that the clinical repercussion is greater from this figure onwards.11 In addition, higher overcorrection rates have been observed when Na is <125mmol/l37, and there is therefore a need for stricter monitoring of Na to avoid the appearance of associated complications.38 However, beyond these Na figures, one should be cautious about attributing moderate to severe neurological manifestations to hyponatraemia.
It is precisely the neurological manifestations, related to the degree of cerebral oedema produced35, which determine the classification of the severity of hyponatraemia. It should be taken into account that, although the symptoms/signs associated with greater severity in hyponatraemia are homogeneously recognised in the medical literature, there are discrepancies between different authors as to classifying the condition as mild or moderate (Table 3).
Classification of hyponatraemia according to symptoms.
| Mild symptoms | Moderate symptoms | Severe symptoms | |
|---|---|---|---|
| Peri et al.48 | Difficulty concentrating, headache, nausea, mood swings | Vomiting, unsteady gait/falls, confusion, drowsiness | Stupor, respiratory distress, convulsions, coma |
| Broch et al.35 | Headache, anorexia, asthenia, attention deficit, memory or gait disturbances, bradypsychia | Nausea, vomiting, disorientation, delirium, confusion, drowsiness | Stupor, seizures, coma, brain herniation, non-cardiogenic pulmonary oedema |
| Clinical practice guidelines: | Nausea, confusion, headache | Vomiting, respiratory distress, convulsions, drowsiness, stupor, coma | |
| USA:Verbalis et al.42 | |||
| Europe: Spasovski et al.43 | |||
| Clinical practice guidelines: | Headache, attention deficit, memory disturbance, gait disturbance, bradypsychia | Nausea, vomiting, disorientation, drowsiness, confusion | Stupor, coma, convulsions, respiratory distress |
| Spain: Runkle et al.15 |
When hyponatraemia appears acutely, it is associated with a higher risk of developing cerebral oedema,39 and often presents with moderate to severe neurological symptoms/signs. This situation is usually observed mainly in the postoperative period of neurosurgical interventions or when acute water intoxication occurs, such as in potomania or primary polydipsia, or due to iatrogenesis. In these cases, it may be advisable to determine natraemia by arterial blood gas analysis, since it will allow changes in Na to be detected more quickly given the speed of onset of hyponatraemia, and thus for treatment to be better adjusted.40 On the other hand, chronic hyponatraemia has a lower risk of cerebral oedema,41 and a milder and more subtle clinical presentation, although it is not without risk, so depending on the clinical situation, it could also manifest as severe.
Regardless of the criteria used to define the severity of hyponatraemia, the differences in patients' immediate progress make it necessary to consider a different therapeutic approach, being more aggressive in correcting hyponatraemia when it appears quickly or is severe, either clinically or biochemically, or is accompanied by factors predisposing to permanent neurological injury or death, such as hypoxia, women of childbearing age, children or the presence of intracranial tumour.
Classify the blood volumeFrom a pathophysiologic perspective, hyponatraemia can be classified based on clinical blood volume. Thus, we distinguish between hypovolaemic, hypervolaemic and euvolaemic hyponatraemia.44 An adequate initial classification of blood volume is crucial for the correct treatment of hyponatraemia.36,45–47 In both hypovolaemic and hypervolaemic hyponatraemia, ECV is decreased,48 so hyponatraemia is the consequence of a non-osmotic release of ADH via baroreceptors. In contrast, in euvolaemic hyponatraemia, ECV is normal or relatively elevated, and there is no baroreceptor or osmotic stimulus for ADH release. In this latter group, situations are found in which the plasma concentration of ADH may be elevated (e.g. SIADH) or decreased/inhibited (e.g. primary polydipsia). One way to estimate whether or not hyponatraemia depends on ADH is by measuring urinary osmolality (uOsm), which has a very high direct correlation with ADH activity in the collecting duct, and with its plasma levels. Thus, in situations of low ECV, or ADH-dependent hyponatraemia, the uOsm will usually be greater than the pOsm. On the contrary, in the presence of normal/elevated ECV and if the hyponatraemia does not depend on ADH, the expected mOsm is <100mOsm/kg.49 In cases of uOsm >100mOsm/kg but less than or equal to the pOsm, a double component (fluid overload and inadequate ADH stimulation) should be suspected.
Differentiating between mild hypovolaemia and euvolaemia can be a challenge,44,46,50–52 and it is necessary to integrate the analysis of several clinical-analytical parameters to carry out an adequate blood volume classification. However, it is important to point out that the evaluation of volume status must be continuous. The same patient may have euvolaemic hyponatraemia and later hypovolaemic hyponatraemia, or vice versa, depending on the appearance and/or resolution of precipitating or aggravating factors.
Table 4 details the clinical and biochemical parameters available in routine clinical practice, which will help differentiate between a hypovolaemic and euvolaemic state. Based on our experience, we recommend having at least three positive parameters suggestive of hypovolaemia for greater certainty of a correct classification of blood volume, since the individual specificity of the parameters to differentiate between hypovolaemia and euvolaemia may be low depending on different clinical situations.
Diagnostic aid parameters to classify hyponatraemia as hypovolaemic or euvolaemic.
| Clinical | Hypovolaemia | Euvolaemia | Limitationsa |
|---|---|---|---|
| Orthostatic symptoms/signs | Present | Absent | Requires training/experience Difficulty differentiating from vertiginous syndrome in the anamnesis. |
| Internal jugular vein pulse height measurement | Below the sternal angle (or angle of Louis) in chest tilt ≤30° | 1−3cm above the angle of Louis in chest tilt between 30°and 45° | High interobserver variability, requires training, dependent on experience |
| Heart rate | ≥90bpm | <90bpm | Low sensitivity since it requires >15% loss of blood volume |
| False positives due to anxiety, fever, thyrotoxicosis, arrhythmias | |||
| False negatives due to bradycardic drugs or marked hypothyroidism | |||
| Blood pressure | ≤90/60mmHg | >90/60mmHg | Low sensitivity since it requires >30% loss of blood volume |
| High pressures can lead to an incorrect assumption of euvolaemia | |||
| Eye palpation | Hardness, no rebound | Turgid, with rebound | Interobserver variability, requires training, depends on experience, there are no clearly studied parameters |
| Greater value in children | |||
| Distal venous return of the upper extremities while sitting | Distal venous filling occurs when the angle of the elbow is below the xiphoid process. | Distal venous filling occurs when the angle of the elbow is between the xiphoid process and the clavicle. | Interobserver variability, requires training, depends on experience, there are no clearly studied parameters |
| Mucous membrane moisture | Dry | Moist | Not very specific |
| Urine sodium | <30mmol/l | >30mmol/l | Only useful for the differential diagnosis of hypovolaemia: extrarenal vs. renal losses; but not for euvolaemia Patients with urinary losses (diuretics, tubulopathies, cerebral salt wasting syndrome, isolated hypoaldosteronism or primary adrenal insufficiency) usually present high values |
| False positives in the case of urinary dilution, so it should be assessed in the presence of a urine osmolality equal to or greater than plasma osmolality | |||
| Sodium excretion fraction | <1% | >1% | Only useful for differential diagnosis of hypovolaemia: extrarenal vs. renal losses. Patients with urinary losses (diuretics, tubulopathies, cerebral salt wasting syndrome, isolated hypoaldosteronism or primary adrenal insufficiency) usually present high values |
| False positives in the case of urinary dilution, so it should be assessed in the presence of a urine osmolality equal to or greater than plasma osmolality | |||
| Observation of change in serum creatinine and/or serum urea | Creatinine or urea increase during hyponatraemia, compared with previous levels in eunatraemia | Creatinine and/or urea remain the same or decrease during hyponatraemia, compared to previous levels in eunatraemia | Requires a prior eunatraemia analysis |
| Changes in muscular constitution can alter the interpretation | |||
| Hypervolaemic hyponatraemia has the same kinetics as hypovolaemic hyponatraemia. |
bpm: beats per minute.
In case of doubt, a response test to saline fluid therapy with NaCl 0.9% can be performed.46,53 Based on this, the administration of 25ml/kg of NaCl 0.9% in 24h would lead to an increase of more than 4mmol/l of natraemia in patients with hypovolaemic hyponatraemia.54 However, it must be taken into account that, in addition to not observing such a response, some patients with euvolaemic hyponatraemia could show a worsening of Na, especially if the urinary osmolality (uOsm) is >500mOsm/kg55, since in this case the excretion of free water will be less than the intake included in NaCl 0.9%, so caution is advised with this test.
Identify predisposing and aggravating factorsHyponatraemia can be exacerbated or triggered by various clinical situations. Therefore, each factor must be monitored and addressed where appropriate. Below, we describe the most common aetiological and aggravating factors of hyponatraemia in clinical practice.
- a.
Aetiological factors: mainly drugs (Table 1), respiratory, renal, hepatic, cardiovascular and/or neurological pathology, infections/sepsis, pain, nausea/vomiting and surgery.
- b.
Aggravating factors: often, although not exclusively, the prognostic impact of hyponatraemia is linked to the underlying pathologies of the patient (cancer, liver disease, respiratory diseases, etc.) by multiple mechanisms. Therefore, the treatment of hyponatraemia should be included in the global approach to the patient and its severity factors. Among these, hypoxaemia56 is the factor that most worsens the prognosis of patients with hyponatraemia, mainly when it is severe.
Severe hyponatraemia is a medical emergency, and as such, should always be treated as soon as possible. The assessment of the patient's blood volume status should not condition the therapeutic attitude in the case of severe hyponatraemia, since the priority objective in these patients is a reduction of cerebral oedema rather than an aetiological diagnosis. Rapid and active correction of severe hyponatraemia improves survival compared to slow correction, and also reduces the risk of neurological sequelae,57 while a watchful waiting approach is associated with an increase in morbidity and mortality.2
The clinical manifestations in patients with hyponatraemia result from the interaction between the severity of the electrolyte disturbance, its speed of onset and the predisposing factors of severity. This makes it difficult to properly classify the severity of the disease. We recommend considering and treating as serious all cases of hyponatraemia that entail a situation of high risk of cerebral oedema and/or herniation if active measures are not taken, which are: acute hyponatraemia (<48h), Na ≤120mmol/l, severe clinical neurological manifestations (regardless of plasma Na levels and onset time), and Na <125mmol/l in paediatric patients or women of childbearing age.
The treatment of severe hyponatraemia is based on three pillars: increase blood sodium level to reach the correction target as soon as possible, stop the increase once the target has been reached, and decrease blood sodium level if the target has been exceeded. The treatment of choice for severe hyponatraemia is the administration of 3% hypertonic saline solution (HSS 3%), regardless of the type and aetiology of the hyponatraemia. There are commercial preparations of HSS 3% ready to be administered by peripheral venous route, although alternatively, it can also be obtained by mixing saline solutions in different proportions (Table 5). Co-administration of other specific measures to correct hyponatraemia in the first 24h after initiation of HSS 3% should be avoided to reduce the risk of overcorrection. Exceptions to this rule are the use of furosemide in patients with heart failure, hydrocortisone in patients with adrenal insufficiency, and potassium chloride in patients with hypokalaemia.
Ways to prepare a hypertonic saline solution 3% (NaCl 3%).
| 500ml of NaCl 3% | A) 28ml NaCl 20%+472ml NaCl 2% |
| B) 55ml NaCl 20%+445ml NaCl 0.9% | |
| C)a 40ml NaCl 20%+250ml distilled H2O |
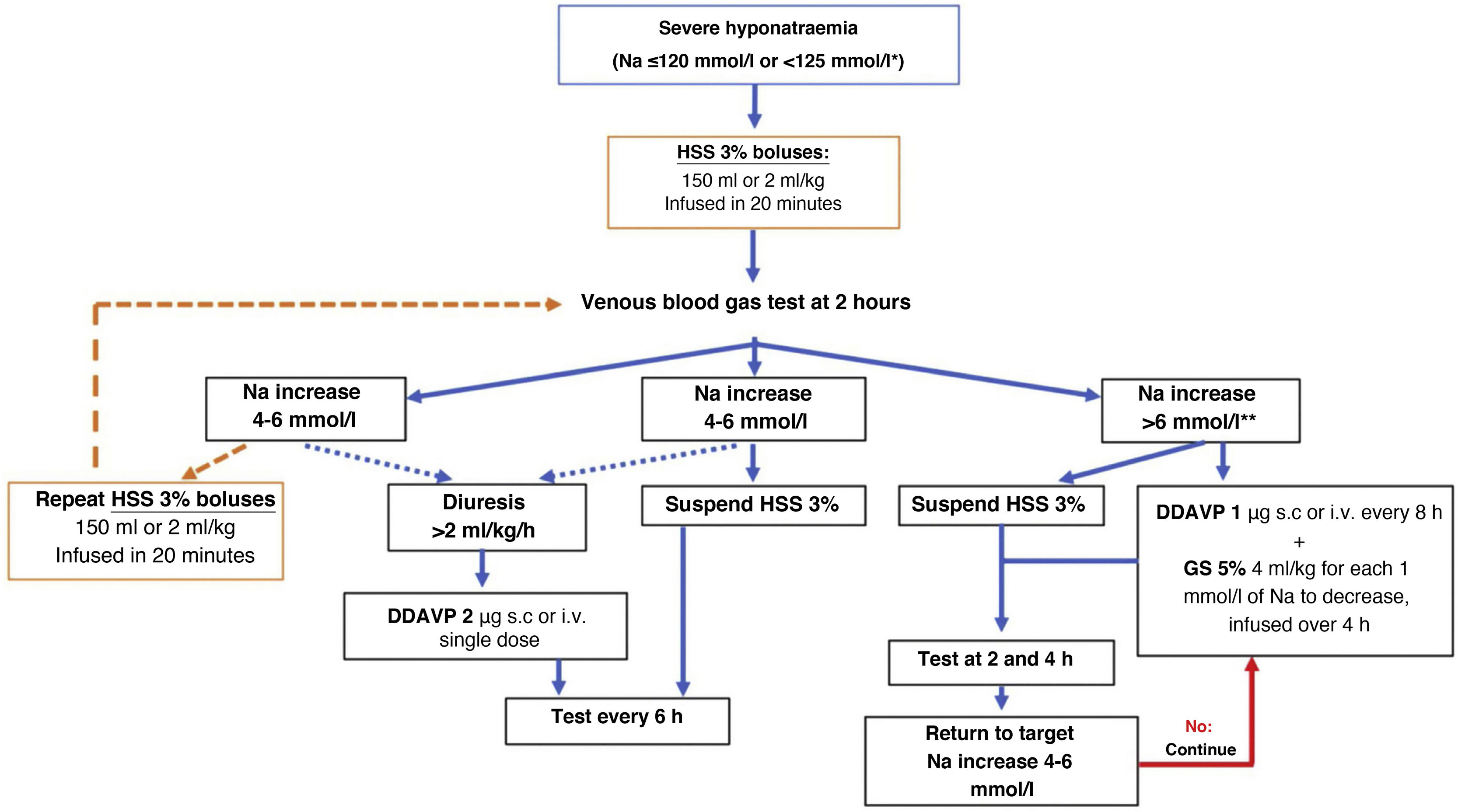
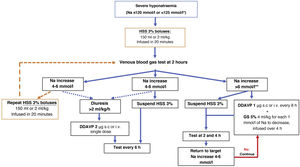
In routine practice, continuous infusion of HSS 3% has been frequently used with serial analytical tests to adjust its rate. Recently, a prospective study in 50 patients with severe hyponatraemia due to SIADH documented that the use of up to two 100ml boluses of HSS 3%, compared with continuous infusion of HSS 3% at 20ml/h, achieved a more rapid rise in Na at six hours of treatment, associated with an improvement in the level of consciousness.58 Likewise, the results of the SALSA study59 show the same efficacy rate but with less need for corrective measures with the use of HSS 3% boluses at doses of 2−4ml/kg in 20−40min compared to the continuous infusion strategy at doses of 0.5−1ml/kg/h, in patients with Na <125mmol/l. For this reason, we recommend the use of HSS 3% in boluses of 100−150ml administered over 10−20min, repeating them as many times as necessary until the desired clinical and biochemical response is achieved (Fig. 2). However, in patients with hypokalaemia, the use of continuous infusion would be more convenient, since it allows for the addition of 20mmol of potassium chloride to each 500ml of HSS 3%.
Treatment algorithm for severe hyponatraemia.
*Na <125mmol/l in the presence of neurological symptoms or in special situations: paediatric age, women of childbearing age, oxygen saturation ≤90% and sepsis.
**In cases of severe acute-onset hyponatraemia, it is not usually necessary to apply corrective measures, so this limit can be raised.
DDAVP: desmopressin; h: hour; Na: serum sodium; HSS 3%: hypertonic saline solution 3%.
At the beginning of treatment with HSS 3%, Na must be closely controlled, requesting frequent analytical tests (usually every 2h) until reaching the individualised correction objective based on the level of risk of ODS of each patient (Table 6). These can be spaced out more later once the clinical picture has stabilised. It is also very important to monitor urine output, since an increase in free water excretion, detected as a significant increase in urine output (>2ml/kg/h or >100ml/h43 and/or a decrease in uOsm relative to the test done prior to treatment, will mean a greater increase in Na, and can serve as an indicator of a potential overcorrection (see section on specific management).
Objectives and limits of correction in chronic hyponatraemia, depending on the presence of risk factors for ODS.
| Target before the first 6 h | Target at 24 h | Limit at 24 h | Limit at 48 h | |
|---|---|---|---|---|
| Without ODS factors | 4−6mmol/l | 4−8mmol/l | <10mmol/l | <18mmol/l |
| With ODS factors | 4−6mmol/l | 4−6mmol/l | <8mmol/l | <16mmol/l |
| Malnutritiona | ||||
| Hypokalaemiaa | ||||
| Liver diseasea | ||||
| Alcohol usea | ||||
| Low Na (≤120mmol/l) |
ODS: osmotic demyelination syndrome.
Mild to moderate hyponatraemia is often chronic, facilitating better osmotic adaptation by the central nervous system. Despite this, a hypoosmolar internal medium has a clinically significant impact on patients, so Na+/water homeostasis must be restored.
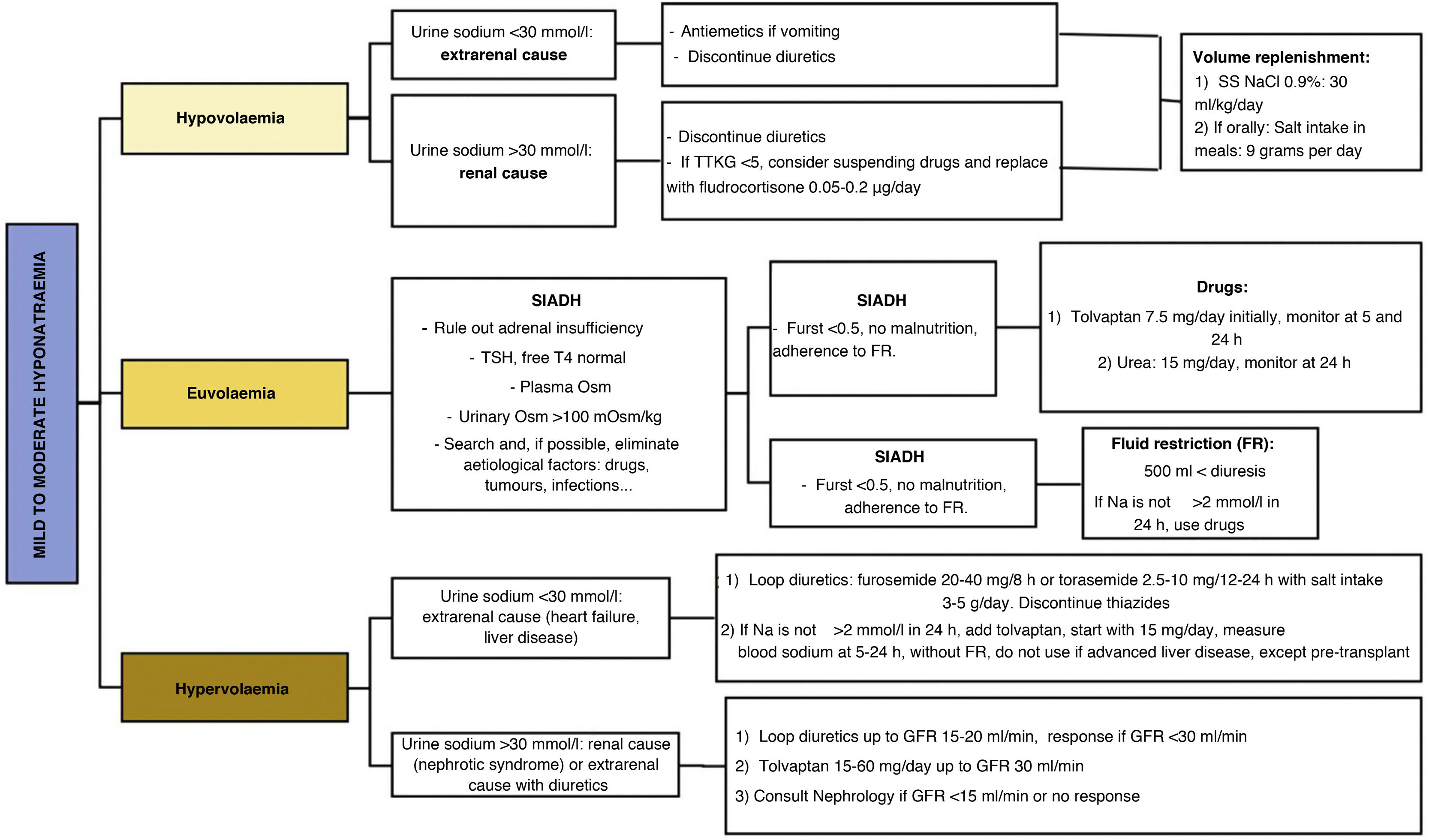
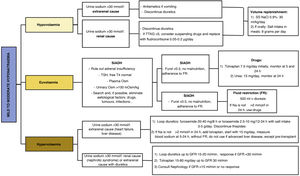
Evaluation of the patient's blood volume is the determining point for the aetiological assessment of hyponatraemia and, therefore, for the adoption of appropriate therapeutic measures (Fig. 3). Currently, we have different pharmacological treatments for the different types of hyponatraemia (Table 7). However, there is a notable lack of quality scientific evidence validating their use.
Pharmacological options for the treatment of hyponatraemia.
| Vaptans | Urea | SGLT2i | Loop diuretics | Isotonic saline solution | |
|---|---|---|---|---|---|
| Indication | SIADH | SIADH | SIADH | SIADH | Hypovolaemic hyponatraemia |
| Hypovolaemic hyponatraemia due to CHF | Hypervolaemic hyponatraemia due to CHF | ||||
| Daily dosage (a) | Tolvaptan: 7.5−60mg | 15−60mg | 10−25mg | Furosemide: 20−40mg/12h | NaCl 0.9 %: 23−30ml/kg |
| Torasemide: 2.5−10mg/24h | |||||
| Glomerular filtrate for use | Up to 15ml/min | Up to 30ml/min | Up to 60ml/min | Unlimited | Unlimited |
| Factors for ineffective response | Water intoxication | uOsm <350mOsm | |||
| Nephrogenic antidiuresis | |||||
| Expected Na increase | 4mmol/l in 24 h | 4mmol/l in 2−7days60 | ? | Variable, usually 2mmol/l in first 48h | ? |
| Overcorrection risk | <2%61 | ? | ? | ? | ? |
| Precautions/Contraindications | Avoid in pregnancy | Renal insufficiency | Hypovolaemia | Hypovolaemia | Hypervolaemia |
| Precaution CYP3A4 | Liver disease | ||||
| Dehydration risk |
CHF: congestive heart failure; SGLT2i: sodium-glucose cotransporter-2; min: minute; SIAHD: syndrome of inappropriate antidiuretic hormone secretion.
Hypovolaemic hyponatraemia results from the compensatory response of ADH to loss of solutes and water, either through the digestive system (vomiting, diarrhoea, etc.), renal route (overdose of diuretics, tubulopathy, hypoaldosteronism, primary adrenal insufficiency)62 or haemorrhage. Aetiological treatment (e.g. antiemetics or discontinuation of diuretics) together with adequate volume replacement, either intravenously or orally, is essential.
- a)
Intravenous route: we recommend using physiological saline (NaCl 0.9%), at a dose of 23−30ml/kg-day54, with which a high rate of correction has been seen within the desired objectives, together with a low risk of overcorrection. However, in the event of haemodynamic instability/shock, massive volume replacement will prevail according to the specific guidelines, monitoring the increase in Na and correcting it when necessary.
- b)
Oral route: feasible in clinically and haemodynamically stable patients, with the ability to ingest and absorb liquids. A minimum intake of sodium similar to that provided by one litre of NaCl 0.9% (equivalent to a supply of 9g of sodium chloride orally) must be guaranteed, divided into several daily intakes, together with meals for greater palatability, and with adequate fluid intake.
It is important to note that treatment of hypovolaemic hyponatraemia is associated with the risk of overcorrection, particularly if other aggravating factors of hyponatraemia are effectively treated (e.g. pain therapy and nausea in a patient with acute gastroenteritis and hypovolaemic hyponatraemia) that resolve the aetiology of the clinical profile. For this reason, it is important to monitor natraemia in the first 6−8h after starting correction in moderate hyponatraemia, and in the first 24h in mild hyponatraemia, and apply, if necessary, the necessary measures to avoid overcorrection (see section on overcorrection treatment).
Mild to moderate hypervolaemic hyponatraemiaThe causes of hypervolaemic hyponatraemia are basically congestive heart failure63, advanced liver disease64 and nephrotic syndrome. In all of them, despite increased extracellular fluid volume, ECV is decreased due to low cardiac output (e.g. heart failure), redistribution of blood circulation (e.g. liver disease), or loss of oncotic pressure (e.g. nephrotic syndrome). It is important to clinically and continuously assess blood volume in these patients, since, as in other types of hyponatraemia, they may present with hyponatraemia of another origin (e.g. hypovolaemic due to overdose of diuretics or gastrointestinal bleeding), in which case the treatment must be adapted.
The treatment of hypervolaemic hyponatraemia is based on the staggered application of the following measures:
- a.
Fluid restriction: although it is the first therapy in the pathology reference guides, it often cannot be used in patients with advanced liver or heart disease due to the malnutrition that they usually present. However, it is important to indicate that the patient should only drink fluids when thirsty and monitor fluid intake and diuresis.
- b.
Loop diuretics: they inhibit the sodium-potassium-chlorine co-transporter in the ascending limb of the loop of Henle, which reduces the osmolality of the renal medulla, and thus the osmotic gradient that urine faces in the collecting duct. This effect reduces ADH-mediated water reabsorption. Therefore, loop diuretics are effective for the treatment of hypervolaemic and euvolaemic hyponatraemia. They are the first line of treatment for hypervolaemic hyponatraemia, at doses of 20−40mg of furosemide every 8−12h. They are particularly effective when uOsm is >350mOsm/kg65 and when there is a salt intake that guarantees their action (4−5g/day of sodium chloride).
- c.
Tolvaptan: is a treatment indicated for hypervolaemic hyponatraemia. The EVEREST study demonstrated efficacy in hypervolaemic hyponatraemia due to heart failure, with an increase in natraemia of 5−6mmol/l in seven days.66 The dose necessary to obtain efficacy is greater than in SIADH; it begins with a dose of 15mg per day and does not require intermediate control at six hours due to less risk of overcorrection. It should not be co-administered with furosemide in this situation. Tolvaptan can be associated with hypertransaminasemia, so it is contraindicated in patients with advanced liver disease, except in pre-transplant situations,67 in whom, although there is no unanimous consensus, the use of tolvaptan in refractory hyponatraemia is accepted.
- d.
Albumin: it only has a role in the treatment of hypervolaemic hyponatraemia due to advanced liver disease within the multidisciplinary treatment of these patients,66 directed by hepatologists. Intravenous albumin infusion may play an adjuvant role in the treatment of hypervolaemic hyponatraemia by temporarily restoring oncotic pressure and increasing ECV, which decreases the stimulus on ADH. For it to be effective, it is important to constantly maintain plasma albumin levels close to the normal range, so it should be administered every 6−8h, given its short half-life. It is also usually administered in association with paracentesis, at a dose of >40g/day.
- e.
Urea: there is no clear mechanism of action of urea in the treatment of hyponatraemia. Its administration is believed to exert an osmotic effect on the proximal tubule that increases renal excretion of free water. Clinical cases of mild to moderate hypervolaemic hyponatraemia due to heart failure successfully treated with urea60 at doses of 15−60g/day have been described. However, these are not randomised studies, so we recommend selecting this treatment option on an individual basis.
- f.
Sodium-glucose cotransporter-2 (SGLT2) Inhibitors: although the osmotic diuresis produced by these drugs could help correct hypervolaemic hyponatraemia due to heart failure, there are no current data on efficacy and dosage in this clinical situation, so its use as a treatment for this type of hyponatraemia should be kept as a last resort, when the treatments indicated by the algorithms have not produced the desired response or are contraindicated, and in no case as a measure for the treatment of severe hyponatraemia.
Considering that the most common cause of mild to moderate euvolaemic hyponatraemia is SIADH, we must first consider the need for a diagnosis of exclusion of other causes of euvolaemic hyponatraemia before starting treatment for SIADH. In the case of other causes, we must manage the triggering aetiological treatment (hypocortisolism, hypothyroidism, physiological elevation of ADH, use of drugs, paraneoplastic, respiratory diseases, etc.) when feasible. If hyponatraemia persists, or if the diagnosis of SIADH is established, there are different therapeutic management options:
a) Fluid restriction: it is based on the fact that the renal excretion of free water exceeds the oral and parenteral intake of fluids. Although fluid restriction (FR) is considered to be the first treatment for euvolaemic hyponatraemia in the European and American guidelines for the treatment of hyponatraemia,42,43 its efficacy is not high. In the Hyponatremia Registry,55 FR achieved an acceptable correction of natraemia in only 44% of patients, with similar correction rates between those who received FR and those who received no treatment. Recently, in the only randomised clinical trial to date evaluating FR versus no treatment in patients with SIADH, it was observed that nearly one-third of SIADH patients treated with FR failed to achieve Na >130mmol/l after three days of treatment.68 However, education on fluid intake – "drink only when you are thirsty and when you eat"69 – seems to be effective as a treatment. In fact, it is likely that this measure alone may be sufficient to achieve eunatraemia in patients with chronic mild hyponatraemia due to SIADH and high fluid intake, as shown in a retrospective series of 34 patients with SIADH with a mean age of 72 years and a pre-measured Na of 130mmol/l, in which the use of this measurement alone managed to normalise natraemia in 76.5% of cases.70
It should be taken into account that FR slowly corrects hyponatraemia when there is a response, at a rate of 1−2mEq/l every 24−48h and for a maximum of 3−4 days, with a median increase of 3mmol/l on the fourth day and 4mmol/l after one month of treatment.68 Likewise, those factors considered as predictors for the non-efficacy of FR42,54 (uOsm <500mOsm/kg, diuresis <1,500ml/day and a Furst ratio >1) can be seen in almost 60% of patients with SIADH,71 limiting its applicability. Therefore, if the goal of treatment is to achieve an increase in Na greater than 4−5mmol/l, and it is necessary to maintain it over time, or if there are factors that can lead to a rapid deterioration of natraemia (e.g. invasive procedures or initiation of treatments that require a large supply of parenteral fluids), FR should not be the first choice of treatment.72 In addition, several factors, which are detailed below, must be taken into consideration before starting treatment with FR:
- □
Renal function must be preserved. Although with a glomerular filtration rate >30ml/min free water excretion capacity can be maintained in healthy people, the ability to maintain a complete water balance could already be reduced from filtration rates of <60ml/min73 in some individuals, mainly in older adults.
- □
There must be capacity to excrete free water. For which the Furst ratio74 is used (ratio of urinary Na+urinary K/plasma Na), whose interpretation is as follows:
- i.
Furst ratio >1: minimal or no excretion of urinary free water, FR is not recommended.
- ii
Furst ratio 0.5−1: low excretion of urinary free water. If FR is applied, it must be <0.5l/day, something that is clinically unfeasible and that threatens the nutritional status. Therefore, we do not recommend the use of FR.
- iii.
Furst ratio <0.5: sufficient excretion of urinary free water. Moderate FR can be introduced with an intake of up to 1l/day. Evaluate use if there is clinical viability.
- i.
- □
There should be no clinical situation that prevents restricting fluid intake. FR is contraindicated in the following situations:
- i.
Impossibility of patient collaboration.
- ii.
Malnutrition, since it would make it difficult to recover the nutritional status.
- iii.
Need for the provision of essential parenteral fluid therapy that equals or exceeds 1l/day.
- iv.
Adverse weather conditions: high temperatures, low humidity.
- i.
b) Loop diuretics: due to the mechanism of action referred to above (see hypervolaemic hyponatraemia section), loop diuretics are effective for the treatment of euvolaemic hyponatraemia, particularly when the uOsm is >350mOsm/kg.65 To date, there are no randomised clinical trials documenting the efficacy, safety and outcome of patients with euvolaemic hyponatraemia treated with loop diuretics specifically for this indication.
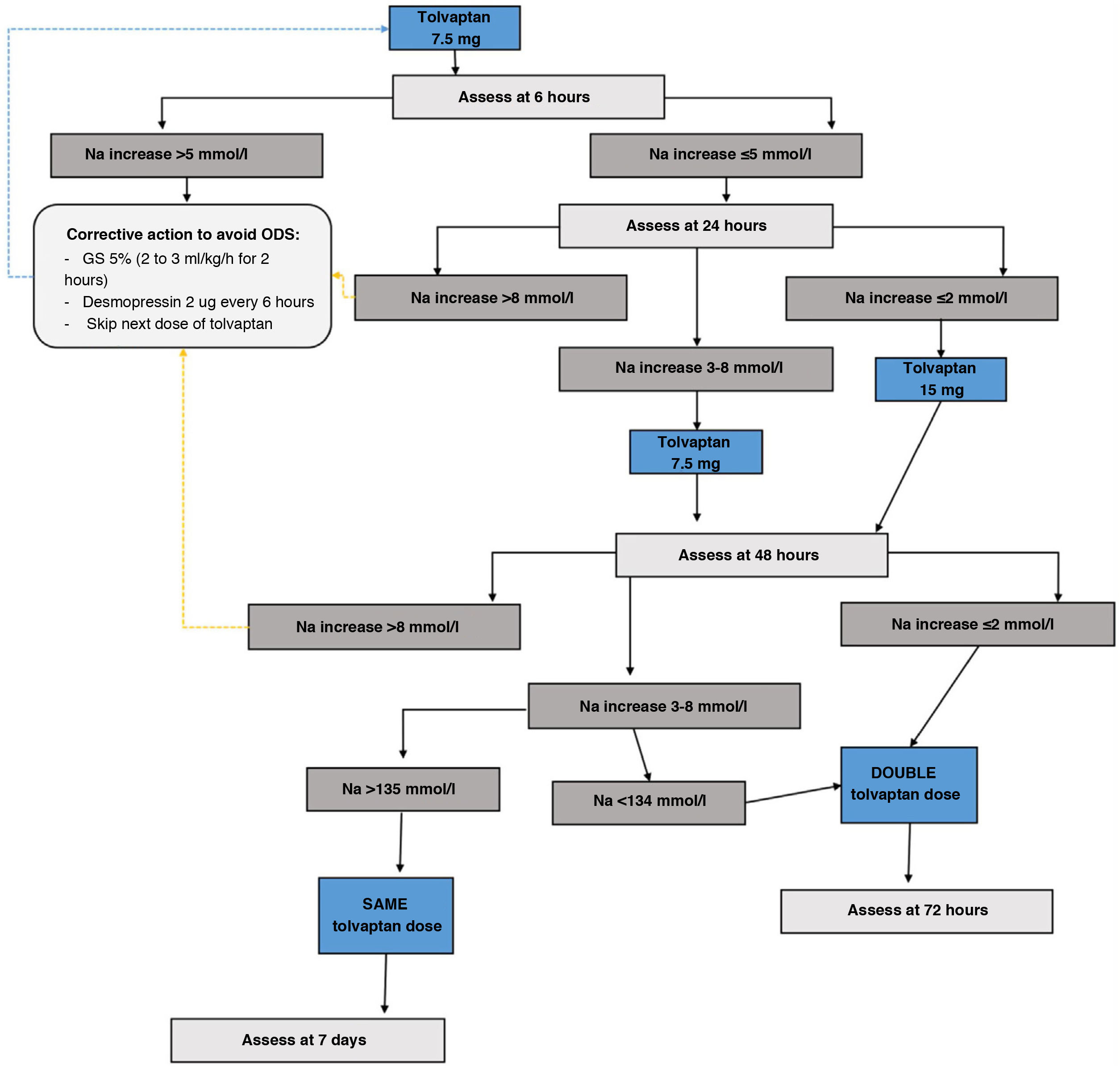
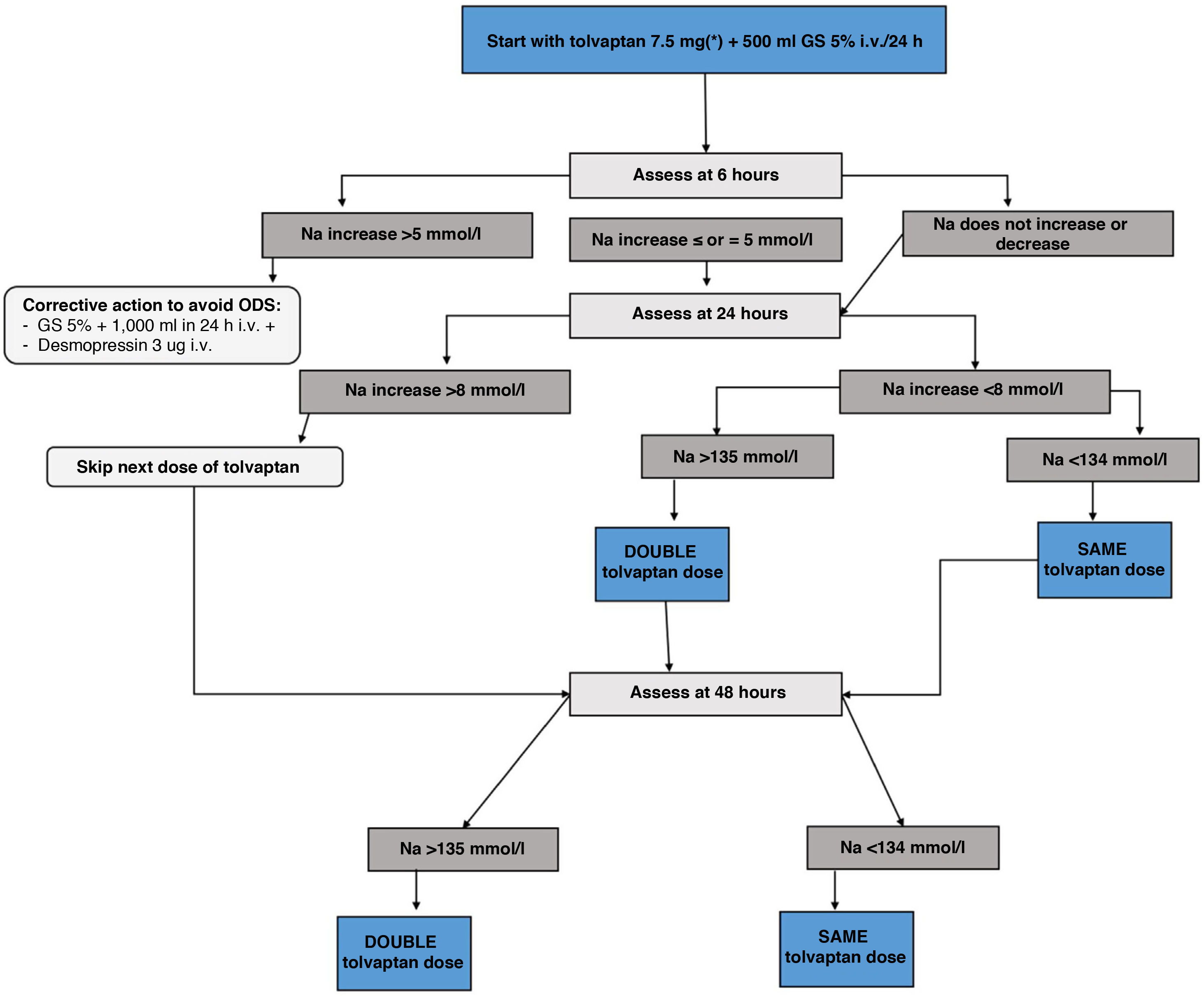
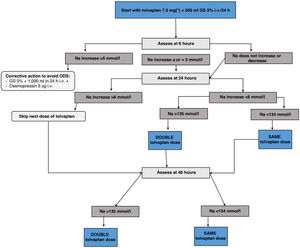
c) Tolvaptan: V2 receptor inhibitors increase free water excretion and natraemia.75 Currently, they are the drugs with the most evidence for the treatment of mild to moderate euvolaemic hyponatraemia, based on the results of clinical trials and real-life studies of up to four years of treatment.64 Likewise, tolvaptan has shown efficacy in the treatment of transient SIADH after neurosurgical surgery.76 However, its use must be directed by personnel trained in the field of hyponatraemia and with adequate hospital conditions that allow for action at home in the event of overcorrection. Below we provide an adapted version of the tolvaptan treatment initiation protocol of the Department of Endocrinology and Nutrition of the Hospital Clínico San Carlos, based on a two-day monitoring regimen, which has shown a success rate in achieving eunatraemia in 48h of 66.7%, with an overcorrection rate of 0%.77
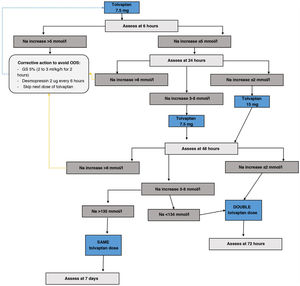
Tolvaptan initiation protocol- i.
Day 1 of treatment. Basal pre-treatment analytical tests are performed. Start dose of 7.5mg first thing in the morning, 20min before breakfast, instructing the patient to drink freely according to thirst. Monitor analytical parameters in blood and urine at 6, 24 and 48h (Fig. 4)
- ii.
If Na at 6h increases <5mmol/l no action is required and the second analytical tests are performed at 24h. If the correction was ≥5mmol/l, the “braking” protocol is established: administer 2μg of desmopressin subcutaneously if the increase was only 5mmol/l, and also an intravenous infusion of dextrose/glucose 5% at a rate of 2ml/kg/h for two hours in case of a 6mmol/l increase in Na, or 3ml/kg/h for three hours in case of an increase in Na >8mmol/l.
- iii.
Day 2 of treatment. Follow-up analytical tests are performed at 24h. If the patient has achieved a correction of Na ≤8mmol/l, the tolvaptan dose will be increased to 15mg under the same administration conditions as the previous day. Those patients who exceed the indicated threshold will continue with the dose of 7.5mg. If the patient were to require the “braking” protocol, the dose of tolvaptan to be administered would be 7.5mg. Likewise, if the patient is underweight (<45kg), it is recommended to maintain this dose on the second day.
- iv.
Day 3 of treatment. Analytical tests are repeated at 48h. If the patient achieves the eunatraemia goal, continue with the previous dose. If the goal is not reached, the dose will be doubled compared to the previous day.
- v.
Day 4 or 7 of treatment. Analytical tests are performed to monitor the treatment and adjust it for follow-up.
- i.
Follow-up tests and appointment one week after the start of treatment, always indicating to drink freely according to thirst.
- ii.
If eunatraemia is achieved, maintain a minimum dose of tolvaptan depending on the presence of increased thirst or diuresis reported by the patient.
- i.
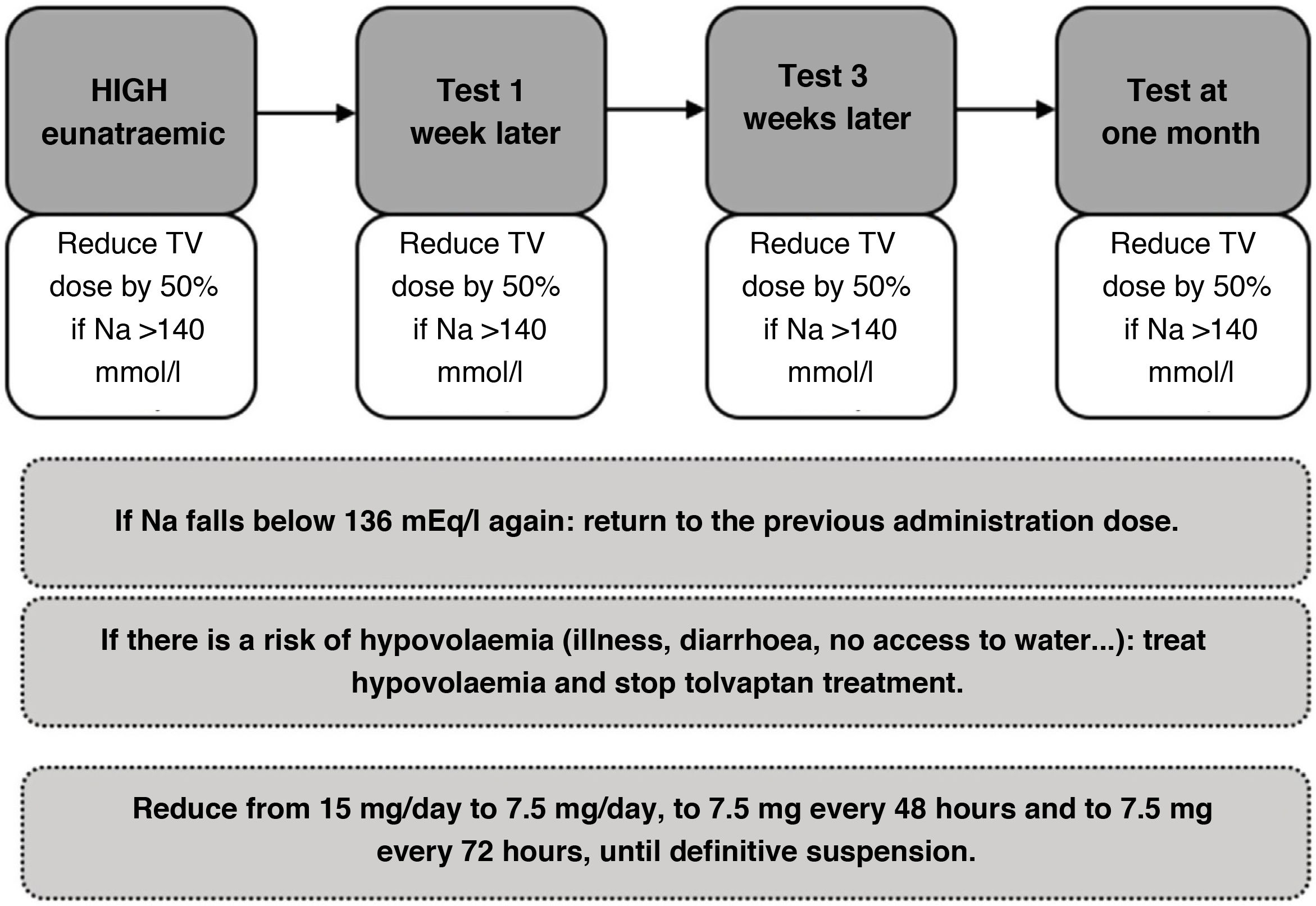
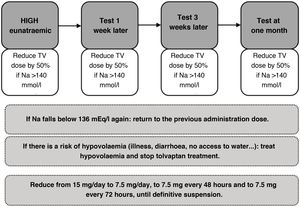
Initially, it is recommended to carry out a close follow-up (for example, one week after discharge, then every month, and, in the long term, every three months) of the patient treated on an outpatient basis with tolvaptan, in which the patient should be evaluated through regular tests and physical examination, considering dose reductions of 50% according to tolerance until complete withdrawal if possible (Fig. 5).
Figure 5.Adjustment protocol for outpatient tolvaptan treatment in patients with syndrome of inappropriate ADH secretion (SIADH).65
NaS: serum sodium; GS: glucose serum; TV: tolvaptan.
(0.34MB). - ii.
Special precaution is essential in the event of intercurrent hypovolaemia situations (diarrhoea, illness, lack of access to water, etc.) that require treatment to be suspended and a vigilant and proactive attitude to treat hypovolaemia. The patient and their relatives should be informed of the proactive attitude in these situations and of the risk of hypernatraemia in the event of fluid loss or oral intolerance.
d) Urea: urea supplementation produces an increase in uraemia that causes osmotic diuresis. Despite its use as a traditional alternative in the management of hyponatraemia due to SIADH, we have little evidence to support its efficacy and safety, usually being retrospective and without a placebo group.78 Available case series analyses suggest a lower frequency of overcorrection, and no cases of osmotic demyelination associated with urea treatment have been reported.79,80
There are no comparative studies between urea and tolvaptan, except for a study with important limitations of 12 patients that combined the use of urea and vaptans (only two patients were treated with tolvaptan, the rest with satavaptan), apparently showing similar efficacy in both groups.81
Due to the limitation from the lack of evidence with solid methodology, its use would not be the first choice in the treatment of hyponatraemia if vaptans are available, except in the nephrogenic syndrome of inappropriate antidiuresis, in which an activating mutation of the V2 receptors would render treatment with vaptans of little use, but the response to urea would be maintained.82 Special mention should be given to the approach to primary polydipsia, where treatment with urea has been shown to be an effective therapeutic option in refractory cases.83
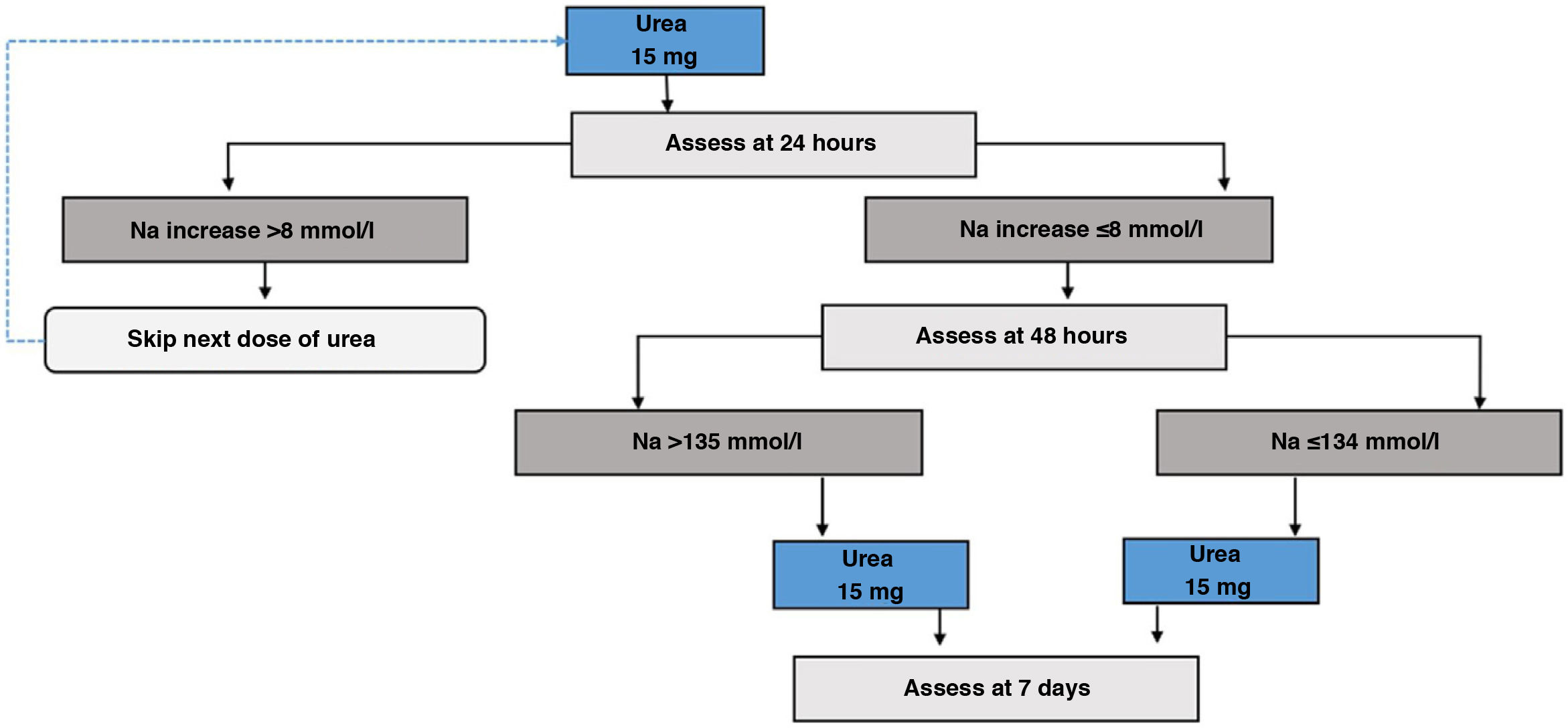
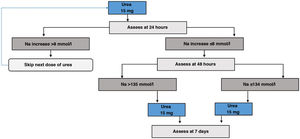
For treatment with urea, starting treatment with a dose of 15g/day84 should be considered, and subsequently evaluate whether to increase or reduce the dose based on Na tests. To our knowledge, there is no validated protocol for its initiation or for subsequent follow-up. Based on our experience, as well as the usual start of treatment on an outpatient basis and the more delayed effect of urea on the increase in natraemia compared to tolvaptan, we recommend monitoring at 24 and 48h after the start of treatment, and depending on the increase in natraemia, consider adjusting the dose of urea.
Urea initiation protocol- i.
Day 1 of treatment. Basal pre-treatment analytical tests are performed. Start 15g dose first thing in the morning (Fig. 6).
- ii.
Day 2 of treatment. Follow-up analytical tests are performed at 24h. If correction limits are exceeded, the dose should be omitted, otherwise continue with 15g dose.
- iii.
Day 3 of treatment. Analytical tests are repeated at 48h. If the patient achieves the eunatraemia goal, continue with the previous dose. Otherwise, it will be increased to 30g/day.
- iv.
Day 7 of treatment. Analytical tests are performed to monitor the treatment and adjust it for follow-up.
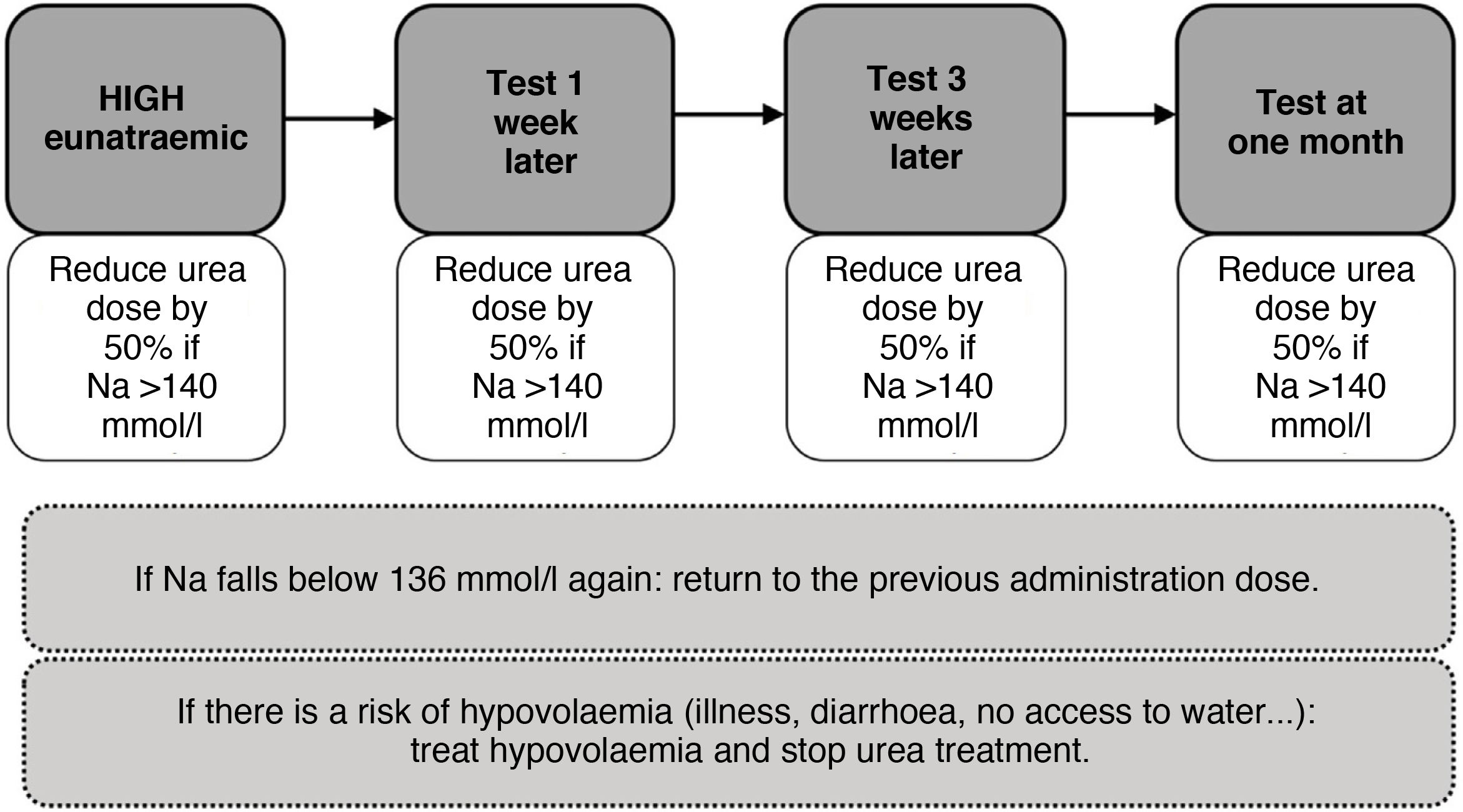
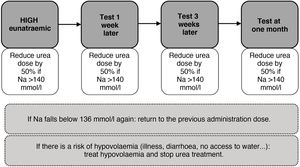
As with tolvaptan, initially close follow-up should be carried out and an attempt should be made to gradually reduce the dose until it is withdrawn. Likewise, in states of hypovolaemia, its withdrawal or dose reduction would be necessary (Fig. 7).
e) SGLT2 inhibitors: a recent clinical trial has shown that empaglifozin at a dose of 25mg/day was more effective in the treatment of hyponatraemia due to SIADH compared to placebo, with a proportion of patients with diabetes of 14% in both groups,85 with a median increase of 10mmol/l in Na in the empagliflozin group versus 7mmol/L in the control group after four days of treatment. However, although the future of this drug seems to be promising in this field, there is no more evidence to be able to currently recommend it as a specific treatment for SIADH, so its use for this indication should be restricted to individualised clinical situations in which other measures are not effective or available.
f) Other accessory measures in the management of euvolaemic hyponatraemia:
- □
Na intake: although hyponatraemia is a disorder due to a relative excess of water, to restore eunatraemia we must ensure that there is an adequate intake of salt. In general, we recommend adding 1−2g of common salt to each meal or administering an infusion made up of 20ml of NaCl 20% diluted in 500ml of NaCl 0.9% in 24h.
- □
Potassium intake: potassium is the main intracellular osmotic agent. Given its exchange with sodium, both at the tissue level and in the renal tubule, the intake of potassium, especially in hypokalaemia, facilitates the increase in natraemia. If intravenous supply is needed, it is recommended to provide the requirements in the form of potassium chloride. We do not recommend the addition of potassium bicarbonate, except in situations of hypokalaemia associated with metabolic acidosis (e.g. type 2 renal tubular acidosis or the use of acetazolamide), since bicarbonate can be excreted in the urine together with sodium, which can induce hypovolaemia, making it difficult to recover from hyponatraemia and hypokalaemia due to its exchange in the distal nephron with sodium, as well as generating an alkalotic state that will also make it difficult to correct potassium levels.
In patients receiving artificial nutrition, the prevalence of hyponatraemia is higher than that described in the general hospital population, with a frequency of 23% in patients receiving enteral nutrition86 and 30% in patients receiving parenteral nutrition.87 In turn, SIADH has been identified as the most common aetiology, accounting for 46% of hyponatraemia in patients receiving parenteral nutrition88 and 67% in patients receiving enteral nutrition.89 Regarding its consequences, hyponatraemia is associated with high morbidity and mortality in these patients, especially persistent hyponatraemia,90 so it is advisable to start treatment early. As in patients without artificial nutrition, the treatment of SIADH must be individualised, taking into account the particularities of each type of nutritional support.
In the case of patients receiving parenteral nutrition, the selection of treatment will not be influenced by their renal capacity to excrete free water. According to the subanalysis of a multicentre study on the aetiology of hyponatraemia in parenteral nutrition, in 80% of patients with SIADH who were prescribed an increase in sodium intake together with fluid restriction, the mean volume intake received was 2.5l.90 This finding highlights the difficulty of restricting fluid intake to less than 1l in these patients, so this measure is not recommended. In turn, 70% presented an uOsm >350mOsmol/kg, which indicates a probable aquaretic response to furosemide,65 so an initial administration of furosemide would be a suitable option. The use of furosemide must be accompanied by a minimum intake of about 136mEq of sodium/day, to guarantee its therapeutic action, as well as a partial restriction of unnecessary fluids, that is, concentrating the intravenous medication and, if possible, the parenteral nutrition formula. In the event of little/no response to the previous regimen (elevated Na ≤2mmol/l/day), tolvaptan could be administered in patients with a functioning first section of the digestive tract. The starting dose of tolvaptan will be 7.5mg and will be accompanied by an infusion of glucose solution, as shown in the attached diagram (Fig. 8).91 Treatment will continue until the aetiology of SIADH is resolved.
For patients receiving enteral nutrition, we recommend reducing total fluid intake to <1l/day, if feasible. Failing that, and in patients with a Furst ratio >0.5, it is recommended to add furosemide if the uOsm is >350 mOsmol/kg, accompanied by a minimum sodium intake of 136mEq/day to guarantee its therapeutic action. If eunatraemia is not achieved despite this, it is recommended to start tolvaptan or urea through the nasogastric tube or gastrostomy tube. In the case of tolvaptan, it would be started following the same protocol as for patients with parenteral artificial nutrition (Fig. 8). On the contrary, if urea is used, it is recommended to start with 15g/day, carrying out a sodium blood test every 24h. If the increase in Na is <8 mEq/day and hyponatraemia persists, it is recommended to increase the dose to 30g/day. If hyponatraemia persists after 48h, it is recommended to increase the dose to 45g/day. As in patients receiving parenteral nutrition, treatment with tolvaptan or urea will be continued until the cause of SIADH has ceased to exist.
In patients with hyponatraemia receiving artificial nutrition, whether it is enteral or parenteral nutrition, it is necessary to closely monitor Na during hospitalisation (e.g. every 72h), adapting treatment to the multiple clinical situations that may occur. At the outpatient level, on the contrary, it would be prudent to do a sodium test one week after discharge, monthly during the first three months, and subsequently every three months if there is clinical stability. In patients with chronic SIADH, it is recommended to avoid the use of furosemide as maintenance treatment, since it requires a higher sodium intake to maintain its therapeutic effect, in addition to the risk of hypokalaemia with which it may be associated.91
Treatment of overcorrection of hyponatraemiaOvercorrection is defined as an increase in sodium levels above the limits initially established as safe in each individual case (Table 6). In general, it is not usually necessary to adopt readjustment measures in patients with acute hyponatraemia. However, in patients with chronic hyponatraemia, it is usually necessary to counteract the rapid change in natraemia due to the potential risk of an osmotic imbalance that could condition the appearance of ODS. To do this, the guidelines recommend reducing serum sodium through the use of enteral water, dextrose solution 5% (2−3ml/kg/h) and/or desmopressin (2−4μg i.v. or s.c.).43
In our experience, we recommend adopting measures to prevent overcorrection with the isolated administration of desmopressin in the event of an increase of Na greater than 5mmol/l in the first six hours. In the event that the increase of Na exceeds the objective set for the first 24h (Table 6), a therapeutic measure to re-lower blood sodium levels, consisting of the provision of desmopressin and/or dextrose serum, should be established. Desmopressin can be administered in doses of 1−3μg subcutaneously or intravenously, and at intervals of 8−12h, and dextrose serum will be 5% at a dose of 3−4ml/kg of weight/h for four hours, performing a subsequent sodium blood test. The infusion can be repeated until the level below the therapeutic limit of the first 24h is recovered, or the therapeutic limit of 48h is entered.42 It should be noted that targeted therapies to correct hyponatraemia (saline fluid, furosemide, tolvaptan, urea, HSS 3%) should be stopped when natraemia re-lowering therapy is started.