Coffee consumption has demonstrated an effect on the regulation of appetite, causing less hunger and/or greater satiety; however, its effects are not well known in woman with overweight or obesity. Therefore, this study aimed to evaluate the effect of coffee consumption on hunger, satiety, sensory specific desire (SSD), and dietary intake in women with overweight or obesity.
MethodologyA randomized crossover clinical trial was realized in 3 sessions: in the first session a clinical history, anthropometric measurements and body composition analysis were performed; in sessions 2 and 3 the participants randomly consumed 240mL of coffee with 6mg/caffeine/kg of weight or 240mL of water along with a standardized breakfast. At fasting and every 30min after breakfast for the next 3h, appetite sensations and SSD were recorded using visual analog scales. Blood samples were taken at fasting, 30 and 180min after breakfast. Dietary intake was recorded in the rest of the intervention days.
ResultsIn the coffee intervention there was an increased desire for sweet foods, higher fructose intake during the rest of the day, and higher triglyceride levels than with the water intervention. No differences were detected in ghrelin or cholecystokinin.
ConclusionsCoffee consumption may lead to higher triglycerides and higher intake of simple sugars, mainly fructose, through changes in the SSD.
Clinical Trial Registration: https://clinicaltrials.gov/NCT05774119.
El consumo de café ha demostrado un efecto sobre la regulación del apetito, provocando menos hambre y/o mayor saciedad; sin embargo, sus efectos no son bien conocidos en mujeres con sobrepeso u obesidad. Por ello, este estudio tuvo como objetivo evaluar el efecto del consumo de café sobre el hambre, la saciedad, el deseo sensorial específico (DSE) y la ingesta alimentaria en mujeres con sobrepeso u obesidad.
MetodologíaSe realizó un ensayo clínico cruzado aleatorizado de 3 sesiones. En la primera sesión se realizó una historia clínica, mediciones antropométricas y análisis de composición corporal; en las sesiones 2 y 3 las participantes consumieron aleatoriamente 240mL de café con 6mg/cafeína/kg de peso o 240mL de agua junto con un desayuno estandarizado. En ayunas y cada 30 minutos después del desayuno durante las 3 horas siguientes, se registraron las sensaciones de apetito y el DSE mediante escalas análogas visuales. Se tomaron muestras de sangre en ayunas, 30 y 180 minutos después del desayuno. Se registró la ingesta dietética del resto del día después de las sesiones 2 y 3.
ResultadosEn la intervención con café se observó un mayor deseo de alimentos dulces, una mayor ingesta de fructosa durante el resto del día y niveles de triglicéridos más elevados que con la intervención con agua. No se detectaron diferencias en la grelina ni en la colecistoquinina.
ConclusionesEl consumo de café puede conducir a un aumento de los triglicéridos y a una mayor ingesta de azúcares simples, principalmente fructosa, a través de cambios en el DSE.
Registro de ensayo clínico: https://clinicaltrials.gov/NCT05774119.
Obesity is a multifactorial disease, and two of the principal related factors are eating behavior and sedentarism, resulting in energy imbalance in the body. A key element impacting eating behavior is appetite, which influences energy intake and is stimulated by the availability and pleasure of the food. Within this system, the succession between hunger and satiety is one of the key features of energy homeostasis. The sensation of hunger is an impulse to eat, and it is accompanied for the peristalsis of the stomach that stimulates to the start of a meal. Hunger is gradually suppressed by consuming food, after which, the sensation of satiety appears during a certain period, until some stimulus initiates the desire for food again.1
Hunger and satiety signals are altered in people with overweight or obesity, which leads to unrestrained eating and inappropriate eating behaviors that promote weight gain.2 These signals involve orexigenic and anorexigenic gastrointestinal peptides such as ghrelin and cholecystokinin (CCK), respectively. Ghrelin is the most studied hunger hormone of the gastrointestinal track. It is produced in the gastric fundus and duodenum by P/D1 type cells, it peaks before nutrient intake and its concentrations fall rapidly in the postprandial state. CCK is secreted by I cells of the duodenum, jejunum, and enteric and central neurons in response to lipid and protein ingestion. Their signal in the brain is through the vagus nerve where it triggers signaling pathways that cause satiety.2,3
Physiological appetite signals, responsible for regulating metabolism, can be affected by consuming delicious foods, especially those rich in fats and sugars. These foods activate the brain's reward system, creating a hedonic effect that can lead to addictive behaviors and overwhelm normal physiological regulation.4,5 It is possible to observe these behaviors through food craving to particular sensory attributes; this is known as specific sensory desire (SSD). This concept is the motivation for specific tastes or textures, such as sweet, salty, sour, bitter, fatty, and spicy foods, impacting future food choices.5
Considering this, the wide availability of palatable sweetened beverages has been associated with the increased prevalence of obesity worldwide.5 Therefore, finding palatable alternatives that help boost the body's physiological signals to maintain energy homeostasis is crucial.
In this regard, coffee is a very popular beverage. Its consumption has been associated with a lower risk of type 2 diabetes, a 10% lower risk of mortality, and a lower risk of cardiovascular disease,6 and in recent years, its effects on the modulation of hunger, satiety, and energy intake have been studied. Gavrieli et al. found that instant coffee consumption during breakfast (6mg caffeine/kg body weight) in overweight or obese individuals significantly reduced energy intake during the rest of the day.7 It has also been reported that consuming instant coffee with 3mg caffeine/kg body weight decreases food desire.8 In addition, one study reported that CCK concentration was higher when subjects drank coffee than water,9 although further research has yet to be conducted.
Based on this background, the possibility that coffee may influence the regulation of hunger and satiety is raised. However, it is important to note that most of these studies have been conducted in mostly male populations or mixed cohorts without sex-differentiated analysis. Since appetite in women may be influenced by hormonal variations throughout the menstrual cycle,10 studies need to be conducted considering these variations. Therefore, this study aims to evaluate the impact of coffee consumption on hunger, satiety, DSE, and dietary intake in overweight or obese women.
MethodsSubjects and recruitmentThis was a randomized crossover clinical trial carried out from January 2022 to May 2023. Participants were recruited through the posting of flyers at several locations around university campus, along with recruitment posts on various social media. The sample size was calculated considering a statistical power of 80% and α=0.05. Inclusion criteria included age between 20 and 40 years, overweight (25–29.9kg/m2) or obesity (30–39.9kg/m2) according to body mass index (BMI) classification, regular menstrual cycle (23–32 days), moderate coffee consumption (up to 4 cups per day) and having the habit of breakfast. Exclusion criteria were suffering from chronic pathologies, smoking, vegetarianism, pregnant or lactating women, food allergies, current nutritional treatments to lose weight or weight loss ≥5% of their weight in the last 6 months, and use of medications that alter the appetite. The protocol was approved by the Ethics Committee, Research Ethics Committee, and Biosafety Committee (number: CI-03421) of the University. The welfare, integrity, dignity, and rights of the participants were respected in accordance with the Declaration of Helsinki.11 Written informed consent was obtained from all subjects. The study was registered in clinicaltrials.org (NCT05774119).
ProcedureThe subjects were cited three times in a fasting state. In pre-intervention session, anthropometric and body composition measurements were obtained. A medical history, a frequency of coffee consumption, the Three Factor Eating Questionnaire (TEFQ) and the International Physical Activity Questionnaire (IPAQ) were also applied. Subjects were randomized to receive the sequence AB or BA. In the phase 1 the subjects were summoned at the beginning of their menstrual cycle, and the phase 2 was carried out after a 7-day washout period between phases. In both phases, each subject consumed coffee or water along with a standard breakfast and for the next 3h, they did not consume any food and did not have access to digital content or conversations related to food. During the intervention, they were not to consume any extra food. Peripheral blood samples were taken at fasting (30min before breakfast), and at 30 and 180min after breakfast. Subjects completed the visual analog scales (VAS) to assess appetite in fasting (30min before breakfast), at the end of the breakfast (time 0) and at 30, 60, 90, 120, 150, and 180min after breakfast. Details are shown in Fig. 1A and B.
Study procedure. (A) Participants were randomly assigned to receive the intervention in either the AB or BA sequence. Participants in the AB sequence received breakfast with simple water and those in the BA sequence received breakfast with coffee during phase 1. This was followed by a 7-day washout period. In phase 2, those in the AB sequence received breakfast with coffee and those in the BA sequence received breakfast with water. In both phases, appetite was measured with ghrelin and cholecystokinin parameters and with VAS, at fasting (VAS −30′), immediately after breakfast (VAS 0′) and every 30min until 180min (VAS 30′ to VAS 180′). (B) Detailed procedure of the study. TEFQ: Three Factor Eating Questionnaire; IPAQ: International Physical Activity Questionnaire; VAS: visual analog scales.
Height was determined using a stadiometer with a precision of 0.1cm and a measuring range up to 205cm (SECA® stadiometer, SECA GMBH & Co., Hamburg, Germany; model 213). Body composition was analyzed by electrical bioimpedance (Inbody 370, Biospace Co., Seoul, Korea, 250kg capacity, 0.1kg precision). Waist circumference was measured in the narrowest diameter between the last rib and the iliac crest using a Lufkin Rosscraft® tape (Lufkin Rosscraft® metal tape measure, Houston, Texas, USA; model W606, range 0–200cm, accurate to 0.1cm). Blood pressure was measured with an Omron Automatic arm digital blood pressure monitor (HEM-7130 Omron Healthcare Co., Ltd., Kyoto, Japan) after 15min of rest, for which subjects were instructed to sit with their backs touching the back of the chair, to rest their arms on a horizontal surface, and to keep their legs without crossing.
InterventionA ground Mexican arabica coffee (1g of coffee had 1.5mg of caffeine) was used to prepare the coffee beverage in a coffee maker (Oster®, model number BVSTDCS12B013, Mexico). Each participant received coffee with 6mg caffeine/kg body weight prepared in 240mL of plain water at 65°C.
We chose 240mL of water at room temperature as the control beverage since it is not feasible to formulate a placebo beverage due to the organoleptic characteristics of coffee. From a methodological point of view, if it is not possible to have a placebo, it is recommended to use a control without effect in the variables of interest.12
Breakfast designBreakfast consisted of 45g of egg, vegetables (30g of carrot and 45g of zucchini), 2 slices of whole wheat bread, 10 pieces of whole almonds and 140g of pear. The energy composition of the breakfast was 400kcal, with a macronutrient distribution of 55% carbohydrates, 30% lipids and 15% protein. The breakfast was prepared in the Laboratorio de Servicios de Alimentos of the University. Participants had to eat breakfast in 30min on average.
QuestionnairesInternational Physical Activity QuestionnairePhysical activity was assessed by examining different dimensions of physical activity, such as time spent walking, moderate and vigorous intensity activities, and sedentary activities. Weekly physical activity was measured by recording metabolic rate units (METs) per min per week. After calculating the physical activity index, whose value corresponds to the product of intensity (in METs) by frequency, by duration of activity, subjects are classified into 3 categories, low<600MET-min/week, moderate≥600 to <1500MET-min/week and high≥1500 physical activity.13
The Three Factor Eating QuestionnaireThe Three Factor Eating Questionnaire (TFEQ) assesses three dimensions of eating behavior: (a) dietary restraint (factor 1), consisting of 21 items designed to measure the cognitive dominance of food intake, (b) disinhibition (factor 2), containing 16 items that assess the tendency to overeat and the loss of control overeating, and (c) susceptibility to hunger (factor 3), with 14 items assessing subjective feelings of hunger and desire for food.14 It has been reported that a score higher than 12 on factor 1 may influence the results of interventions and may have different responses in the sensation of hunger.15
Dietary evaluationThe assessment of diet intake previous to each intervention was evaluated with a 3-day food record. Dietary intake for the remainder of the day after each intervention was also assessed. A certified nutritionist trained each participant in the correct filling out of the consumption record where they were instructed to provide the correct amount and description of the food. For the training, food replicas (Nasco, Atkinson, Wisconsin, USA) were used for a better understanding of the portion sizes. The Nutritionist Pro™ Diet Analysis software (Axxya Systems, Stafford, TX, USA) was used for the diet composition analysis. In that software the available carbohydrates reported include those that can be digested and absorbed for the gastrointestinal system. Total sugars represent all sugars found naturally in food and added sugars are those that were added during the food processing.
Appetite parameters assessmentAppetite was assessed through the VAS, which evaluate hunger, fullness, satiety, desire to eat and prospective consumption. The VAS are composed of 100mm long lines, where at one end the term “None” or “Not at all” is placed and at the other end the term “Yes, a lot” or “As much as I have never felt”. The subject marks a vertical line between these two extremes and quantification is done by measuring the distance from the left end of the line to the mark he/she recorded.16 Also, the use of VAS assesses the SSD for sweet, salty, fatty, and savory which are designed and measured in the same way as the other appetite sensations.16
Biochemical determinationsPeripheral blood samples were collected by venipuncture after 12h of fasting and at 30 and 180min postprandially, and immediately centrifuged at 3500rpm for 15min at 4°C to obtain serum. The serum was stored at −80°C for later use. The concentration of glucose, triglycerides, total cholesterol, and high-density lipoprotein cholesterol (HDL-c) was determined with a dry chemistry analyzer Vitros 350 Chemistry (Ortho-Clinical Diagnostics, Johnson & Johnson Services Inc., Rochester, NY, USA). Low-density lipoprotein cholesterol (LDL-c) was calculated with the Friedewald formula, except when triglyceride levels were greater than 400mg/dL.5 Very-low-density lipoprotein cholesterol (VLDL-c) was calculated as total cholesterol minus the sum of LDL-c+HDL-c.17
Appetite parameters hormonesGhrelin and CCK have opposite activities. On the one hand ghrelin is the only hormone related to hunger; on the other hand, CCK secretion causes satiety. In addition, it has been observed that coffee consumption increases CCK concentrations.9 Serum concentrations were measured by ELISA assay (Enzyme-Linked ImmunoSorbent Assay) according to the manufacturer's recommendations. The serum ghrelin levels were determined using RayBio®, model Human GHRL/Ghrelin ELISA Kit catalog number ELH-GHRL (RayBiotech, Norcross, GA, USA). The serum CCK levels were determined using RayBio®, Human CCK Enzyme Immunoassay Kit catalog number EIA-CCK-1 (RayBiotech, Norcross, GA, USA). Both assay manuals reported an intra-assay coefficient of variation of <10% and an inter-assay of <15%. The samples were measured in duplicate and for further calculations the mean of the two measurements was used.
Statistical analysisThe descriptive analysis is showed as mean±standard deviation (SD), estimated mean and standard error, or frequency and percentage as appropriate. Due to its power to detect non-normality distribution, the Shapiro–Wilk test was used to check the distribution of quantitative variables. Variables non-normal distributed were log-transformed and in the case of the linear mixed model it is only necessary that the residuals, and not the dependent variable, be normally distributed. The randomization of participants to the sequence AB or BA was computer-generated with a random number table obtained in the SPSS® v.20 software (Armonk, NY, USA). The sample size was calculated using the formula for comparing two means with data previously reported by Douglas BR et al., who reported a significant difference (p<0.01) in CCK concentrations when drinking 165ml of regular coffee versus drinking 165ml of NaCl water.9 Using the values reported by the authors, an “n” of 7 subjects per intervention was obtained, with an effect size of 1.53 according to Cohen's d. The calculation is shown in the equation below. In addition, as reported by Horner et al., the sample size to detect a 20mm difference in visual analog scales to assess appetite between meals (post-breakfast–pre-breakfast) in a paired design with an α=0.05 and a power of 80%, is 10 subjects.18
Comparisons of dietary intake the 24h after intervention, hunger, satiety, sensory specific desire, and biochemical parameters (ghrelin, CCK, glucose, lipid profile) between the intervention with coffee or the intervention with simple water was performed with the linear mixed model, considering the intervention, the time and the intervention*time as fixed effects and participants as random effect variable. The mixed linear model allows to consider the effect of carry of a crossover design. And because each subjects is measured several times, this type of analysis permits to control the effect of previous measurements in the dependent variables of interest. For example, the permits to know the effect of the beverages interventions on appetite and biochemical variables. These analyses were realized in RStudio v. 4.2.2.19 A p<0.05 was considered statistically significant. The graphs and figures were made with GraphPad software v. 9.3.1 (Boston, MA, USA) and with BioRender.com, respectively.
ResultsParticipants and baseline characteristicsAt the beginning of the study 103 people were interested, but after analyzing whether they met the eligibility criteria, only 12 women were included after they signed the informed consent. During the interventions, one participant left the study because of pregnancy. The details are shown in Fig. 2.
The mean age of participants was 28.8±7.9 and a BMI of 30.01±3.5kg/m2, other body composition variables are shown in Table 1. Women of this study realized 849.7±765.7 METs per week, which is considered a moderate physical activity. Besides, the mean duration of the menstrual cycle was 30 days. Females consumed an average of 1 cup of coffee per day and obtained a score of 5.8±3.8 in factor 1 (dietary restraint factor) of the TFEQ.
Anthropometric and body composition.
| Variable | Womenn=11 |
|---|---|
| Age (year) | 28.8±7.9 |
| BMI (kg/m2) | 30.1±3.5 |
| Waist (cm) | 88.1±7.5 |
| Hips (cm) | 108.0±8.1 |
| Body fat (%) | 41.1±4.5 |
| Fat-free mass (kg) | 47.5±3.4 |
| Total body water (kg) | 34.7±2.5 |
| Mineral mass (kg) | 3.4±0.3 |
Values are expressed as mean±standard deviation.
Energy, macronutrients, and caffeine intake after the day of coffee intervention was compared to the dietary intake after day of simple water intervention. It was found that when women consumed coffee, during the rest of the day the intake of fructose were almost eight grams more than the day when the intervention was simple water. Other dietary variables were not different between treatments (Table 2). The means and SD of pre- and post-intervention diet values are shown in Table S1.
Dietary intake after the intervention.
| Variable | Estimated mean | Standard error | t-Value | p(>|t|) |
|---|---|---|---|---|
| Energy (kcal) | 132.400 | 211.3 | 0.626 | 0.538 |
| Carbohydrates (%) | 1.000 | 5.007 | 0.200 | 0.846 |
| Protein (%) | −1.755 | 3.244 | −0.541 | 0.595 |
| Lipids (%) | 0.755 | 3.922 | 0.192 | 0.851 |
| SFA (%) | 0.100 | 2.351 | 0.043 | 0.967 |
| MUFA (%) | −1.873 | 4.297 | −0.436 | 0.672 |
| PUFA (%) | 0.882 | 2.886 | 0.306 | 0.763 |
| Cholesterol (mg) | −31.060 | 39.220 | −0.732 | 0.447 |
| Available carbohydrates (g) | −31.060 | 39.220 | −0.792 | 0.447 |
| Total fiber (g) | 6.336 | 3.863 | 1.640 | 0.132 |
| Soluble fiber (g) | 0.036 | 0.202 | 0.180 | 0.861 |
| Insoluble fiber (g) | −0.045 | 0.489 | −0.093 | 0.928 |
| Sugars (g) | 8.491 | 14.446 | 0.588 | 0.570 |
| Added sugars (g) | −0.691 | 8.084 | −0.085 | 0.933 |
| Glucose (g) | 4.273 | 2.597 | 1.645 | 0.131 |
| Galactose (g) | −0.036 | 0.046 | −0.784 | 0.442 |
| Fructose (g) | 7.955 | 2.478 | 3.211 | 0.009 |
| Sucrose (g) | 2.945 | 2.258 | 1.305 | 0.221 |
| Caffeine (mg) | 11.055 | 5.768 | 1.917 | 0.084 |
Values as presented as estimated mean and standard error. The estimated mean is the predict mean of the linear mixed model that considered fixed and random effects with its corresponding standard error. The value of the estimated mean is related to coffee intake and simple water was the reference. Comparison between interventions were analyzed with the linear mixed model. A p<0.05 was considered statistically significant, denoted in bold numbers. SFA: saturated fatty acids; MUFA: monounsaturated fatty acids; PUFA: polyunsaturated fatty acids.
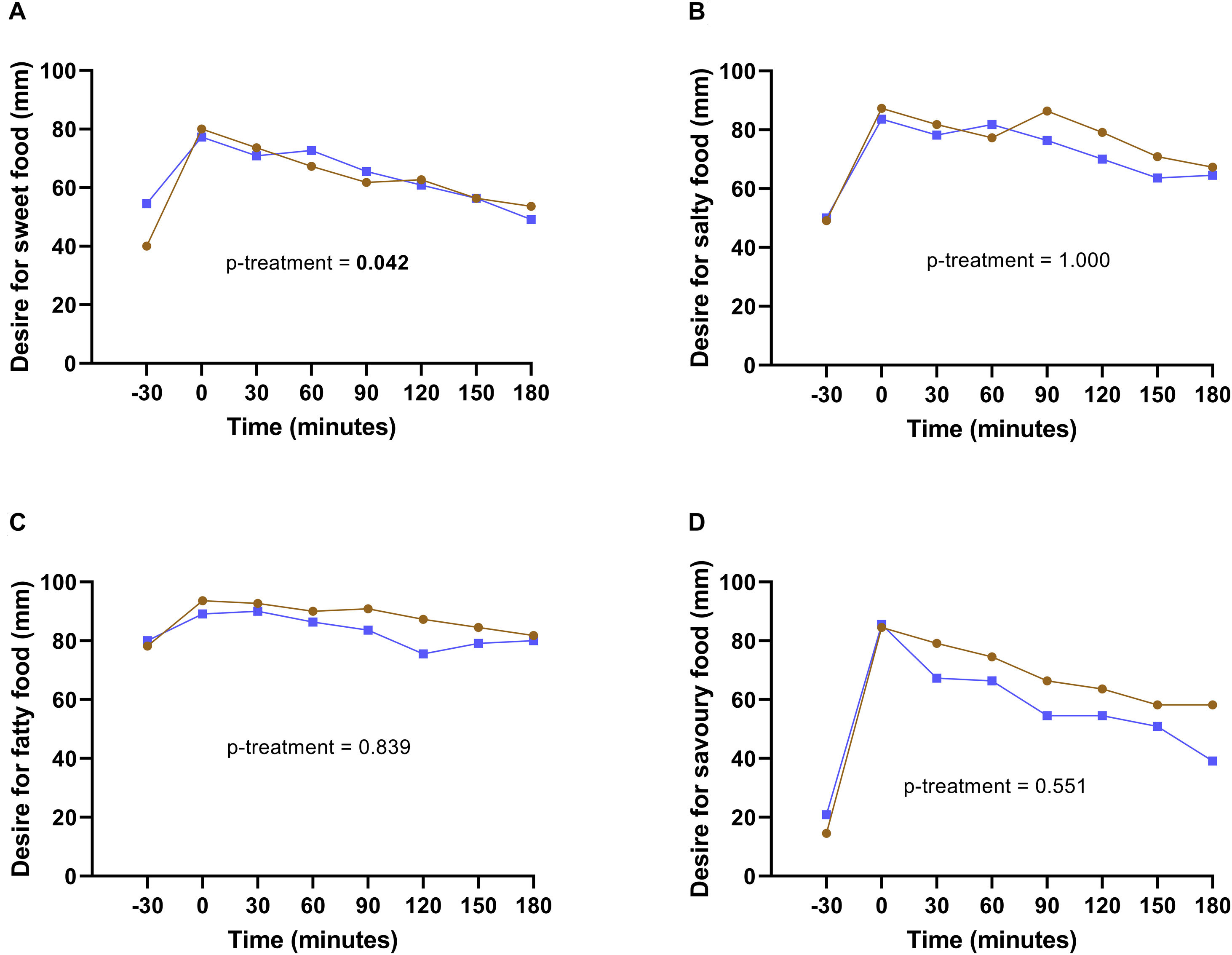
The subjective appetite sensations of hunger, fullness, satiety, desire to eat, and prospective food consumption was similar between coffee and water intake (data not shown). However, when comparing SSD between interventions, and considering only the differences between interventions and not time, a greater desire for something sweet was observed when participants drank coffee than plain water (16.1±7.9mm more in coffee than in plain water, p=0.042). Nevertheless, when considering the interaction time*treatment, a trend close to significance was observed at 180minutes with coffee consumption (p=0.061) (Fig. 3A). Another trend for the intervention*time interaction was detected in the desire for something fatty at 120min (p=0.073), and in the desire for something savory at 180min (p=0.070) (Fig. 3C and D).
Sensory specific desire in coffee and water interventions. Desire for something sweet (A), salty (B), fatty (C), and savory (D) in coffee (brown line) and simple water (blue line) interventions. Comparison between interventions was analyzed with the linear mixed model. A p<0.05 was considered statistically significant.
The appetite hormones ghrelin and CCK were compared between the two beverages, but no differences were found. Regarding glucose levels and total, HDL, and LDL cholesterol, no changes were detected either; but when women drank coffee an increase in triglycerides and VLDL levels compared to simple water was observed (Table 3). The means and SD of the pre- and post-prandial biochemical variables are shown in Table S2.
Appetite hormones and biochemical parameters after the intervention.
| Variable | Estimated mean | Standard error | t-Value | p(>|t|) |
|---|---|---|---|---|
| Ghrelin (pg/mL) | −52.1 | 35.9 | −1.452 | 0.152 |
| Cholecystokinin (pg/mL) | 76.2 | 65.9 | 1.155 | 0.254 |
| Glucose (mg/dL) | 1.7 | 6.3 | 0.276 | 0.784 |
| Triglycerides (mg/dL) | 28.5 | 13.3 | 2.144 | 0.037 |
| Total cholesterol (mg/dL) | 10.2 | 6.3 | 1.621 | 0.111 |
| HDL-cholesterol (mg/dL) | 0.5 | 1.7 | 0.319 | 0.751 |
| LDL-cholesterol (mg/dL) | 4.1 | 4.8 | 0.876 | 0.385 |
| VLDL-cholesterol (mg/dL) | 5.8 | 2.7 | 2.174 | 0.034 |
Values as presented as estimated mean and standard error. The estimated mean is the predict mean of the linear mixed model that considered fixed and random effects with its corresponding standard error. The value of the estimated mean is related to coffee intake and simple water was the reference. Comparison between interventions were analyzed with the linear mixed model. A p<0.05 was considered statistically significant, denoted in bold numbers.
In this randomized crossover clinical trial, we evaluated the effect of coffee consumption on hunger, satiety, SSD, biochemical parameters related to appetite, and dietary intake in women with overweight or obesity. The results showed that drinking coffee with 6mg of caffeine/kg of body weight increased the specific sensory desire for sweet foods, together with an increase in fructose consumption in the rest of the day compared to the water intervention.
This is the primary study that evaluates the effect of coffee on SSD, reporting for the first time that after coffee consumption there is an increased desire for sweet foods. The sense of taste has an influence on food selection4 and it is possible that the desire for sweet foods after coffee may be due to the characteristic bitter taste of coffee. The compounds responsible for this taste are alkaloids such as caffeine and phenolic compounds such as chlorogenic acids. This taste is detected by receptors coupled to the taste buds of the tongue, exactly in type II cells. When these bitter compounds bind to their receptors, a release of adenosine triphosphate (ATP) occurs and signals are sent to the brainstem and the nucleus of the solitary tract (NST) by means of peripheral nerve fibers.20 In that sense, the consumption of quinine, a bitter compound, has been reported to increase the taste for a bitter-sweet mixture,21 however; more studies are needed to corroborate our finding.
Furthermore, the result of desire for sweet foods is confirmed by the 24-h post-intervention results of the energy intake assessment. Higher fructose intake was observed when drinking coffee compared to drinking water. Fructose is found naturally in fruits and vegetables, sucrose (table sugar) and as part of the high fructose corn syrup. The flavor of fructose is sweet, so it is used in large quantities to enhance the savor of foods and make processed and ultra-processed foods and drinks more palatable.22 Hence, is possible that, when consumed coffee without sugar, as it was in this intervention, the participants tasted it in a different way and the lack of sweet taste increase the desire for foods with this sensory attribute. Adding sugar or milk masks the bitter taste of coffee and makes it more compatible with the taste, since people are born with an innate preference for sweetness.23 In addition, this study was conducted in women with overweight or obesity. In that sense, it has been described that women with overweight and obesity that rated a beverage with simple carbohydrates as more hedonic had a higher ad libitum intake of it.24
Besides, it is possible that caffeine tolerance may influence the results observed in this study. Caffeine inhibits the binding of adenosine to the A2A receptor causing an increase in the release of neurotransmitters such as dopamine. When dopamine is released, it produces a feeling of pleasure and reward that it turns favors food-seeking behavior, especially for pleasant foods.25 The women in this study had a regular consumption of coffee, and therefore may have developed a tolerance to caffeine, that is characterized by an increase of A2A receptor in the brain,25 so the release of dopamine might decrease. Hence, participants with caffeine tolerance may seek pleasant food like those with a sweet taste, but more research is needed to clarify this topic.
There was also observed a trend in the desire for fatty and savory foods after consuming breakfast with coffee. It has been reported that a low sensitivity to bitter taste is associated with a high liking for fatty foods,26 nevertheless; in this study sensitivity to bitter taste was not measured, so it will be important to evaluate it in future research.
With respect to appetite assessment, no differences were observed between feelings of hunger, fullness, satiety, desire to eat, or prospective food consumption among the test beverages; these results differ from the results of Gravieli et al. who reported that in subjects with overweight or obesity (8 women), the sensation of satiety was higher after consumption of coffee with 3 and 6mg of caffeine/kg of body weight at 15min compared with water consumption.7 In our study we did not measure the sensation of satiety at 15min after coffee intake and if the effect is at that time point, such differences were not measured. In another study also conducted by Gravieli et al. in 2011 in men only, it was observed that at 180min with the intervention of coffee with 3mg of caffeine/kg of body weight, participants reported a lower desire to eat compared to the intervention with decaffeinated coffee or water8 but, it is important to note that women respond differently to caffeine consumption.27
As for the hormones related to appetite (ghrelin and CCK), no significant changes were detected. Similar results were reported in the study by Greenberg JA et al. when drinking coffee with 6mg of caffeine/kg of body weight, there were no changes in ghrelin concentrations.28 Regarding to the results of CCK levels, in the study of Douglas B.R. et al. the consumption of 165 and 400 mL of coffee increased CCK concentration, but the amount of caffeine in the beverages was not reported.9 In addition, coffee contains several biologically active compounds such as caffeine and chlorogenic acids, so it is important that in future research these compounds be measured to know whether caffeine or other compound could contribute to the effects observed in coffee consumption.
On the other hand, triglyceride (TG) levels were higher after breakfast with coffee. In a meta-analysis of the effect coffee consumption on serum lipids levels, it was found an increase in TG levels. In a sub-analysis increased levels of TG were observed when ≥5cups/d were consumed; but not differences were identified in the preparation or type of coffee, such as boiled (filter or not), instant or decaffeinated.29 Most of the studies where there is an increase in serum lipids is due to an increase in total cholesterol and LDL cholesterol, possibly due to bioactive compounds present in coffee such as diterpenes, cafestol and kahweol.30 Besides, caffein increases lipolysis mainly through two mechanisms: caffeine is absorbed into the bloodstream and goes to the brain, where it blocks A2A receptors which leads to an increase in the release of catecholamines that stimulates lipolysis.25 The second mechanism is because caffeine increases lipolysis in the adipose tissue31 and circulating free fatty acids travel to the liver where they are used to form VLDL, hence triglyceride concentrations augment in the bloodstream.
This study had some limitations, such as not evaluating coffee consumption habits, that is, if the participants usually drank coffee with sugar, a sweetener, and/or milk (amount and type). Moreover, the amount of caffeine was not quantified with a more precise method. Therefore, more studies are needed to assess the effect of consumed a food or meal with a particular taste on the next food choices and tastes and amount of food. A further limitation of the study is the use of water as a control beverage, which has a completely different appearance and taste than coffee. For future interventions, decaffeinated coffee could be used as a control beverage to test whether caffeine is the bioactive compound that causes such effects.
ConclusionsIn conclusion, consumption of coffee with 6mg of caffeine/kg of body weight in subjects with excessive BMI may lead to higher triglycerides levels, higher intake of simple sugars, specifically fructose, through changes in the SSD, possibly triggered by the bitter taste of coffee. Nevertheless, due to this is a pilot study the findings should be taken in caution and studies with a larger sample size and with a long-time exposure are necessary.
Authors’ contributionsLisset Magaña-de la Vega, Erika Martínez-López, and Nathaly Torres-Castillo conceptualized and designed the study. Lisset Magaña-de la Vega, Tania Sanchez-Murguia, Saraí Citlalic Rodríguez-Reyes and Andrea Madrigal-Juárez participated in the recruitment of the participants. Nathaly Torres-Castillo analyzed the data. Lisset Magaña-de la Vega, Erika Martínez-López, Tania Sanchez-Murguia, Andrea Madrigal-Juárez, Saraí Citlalic Rodríguez-Reyes, Ivan Aguilar-Vega, Nathaly Torres-Castillo wrote, reviewed, and contributed to editorial changes of the manuscript. All authors read and approved the final manuscript.
FundingThis research was funded by “PRODEP” (NPTC 2020, UDG-PTC-1600) and “PRO-SNI” (Universidad de Guadalajara, number 265180, 2022) and “PIN III” (Universidad de Guadalajara, number 271723, 2023).
Conflict of interestThe authors declare no conflict of interest.
The authors of the study express their gratitude to the women who participate in it. They also thank all the people involved in the diffusion of the project. We are grateful to CONAHCYT for their support to the scholarship holder Lisset Citlalic Magaña de la Vega, with number (CVU) 1254195.