To evaluate the adequacy of TSH suppression therapy (TSHst) at the first disease assessment and the last follow-up visit.
MethodsRetrospective observational study of those patients under follow-up of DTC in a reference hospital.
Results216 patients (79.2% women) were evaluated, with a mean age 59.0 ± 13.1 years-old and a mean follow-up of 6.9 ± 4.3 years. 88.4% were papillary carcinomas.
At diagnosis, 69.2% had a low risk of recurrence (RR) compared to 13.6% with a high RR. Dynamic risk stratification (DRS) classified patients at first disease assessment and the last visit as excellent response (ER) in 60.0% and 70.7%, respectively.
Those patients with ER in the first and last follow-up control maintained TSHst in 30.7% and 16.3% of the cases, respectively (p < 0.001).
The factors associated with maintaining TSHst at the last control were younger age, higher RR at diagnosis, DRE at follow-up, presence of multifocality and histological vascular invasion (p < 0.05).
In a logistic regression analysis adopting tsTSH at follow-up as the dependent variable, exclusively age (β = −0.062; p < 0.001), RR at diagnosis (β = 1.074; p < 0.05) and EDR during follow-up (β = 1.237; p < 0.05) maintained statistical significance.
ConclusionsDespite the current recommendations, 30.7% of patients with low RR and initial ER are under TSHst. This percentage reduced to 16.3% in those patients with ER after a mean follow-up of 6.9 years. Age, baseline RR, and DRE during follow-up were associated to maintaining tsTSH.
Evaluar la adecuación de la supresión de TSH (tsTSH) al diagnóstico y en la última visita de seguimiento.
MétodosEstudio observacional retrospectivo en aquellos pacientes en seguimiento por CDT en un hospital de tercer nivel.
ResultadosSe evaluaron 216 pacientes (79,2% mujeres), edad media 59,0 ± 13,1 años y un seguimiento medio de 6,9 ± 4,3 años. Un 88,4% fueron carcinomas papilares.
Al diagnóstico el 69,2% presentaban un riesgo bajo de recurrencia (RR) frente a un 13,6% de RR elevado. La estadificación dinámica del riesgo (EDR) clasificó a los pacientes, inicialmente y en la última visita, como respuesta excelente (RE) en un 60,0% y un 70,7%, respectivamente.
Aquellos pacientes con RE en el primer y último control de seguimiento, mantuvieron tsTSH en un 30,7% y 16,3% de los casos, respectivamente(p < 0,001).
Los factores asociados a mantener la tsTSH en la última visita de control fueron la menor edad, mayor RR al diagnóstico, la EDR en el seguimiento, la presencia de multifocalidad e invasión vascular (p < 0,05).
En un modelo de regresión logística adoptando la tsTSH en el seguimiento como variable dependiente, exclusivamente la edad (β = −0,062; p < 0,001), el RR al diagnóstico (β = 1,074; p < 0,05) y la EDR en el seguimiento (β = 1,237; p < 0,05) mantuvieron la significación estadística.
ConclusionesA pesar de las recomendaciones actuales, un 30,7% de los pacientes con bajo RR y RE inicial se encuentran bajo tsTSH. Este porcentaje se redujo al 16,3% en RE tras un seguimiento medio de 6,9 años. La edad, el RR inicial y la EDR durante el seguimiento se relacionaron con mantener la tsTSH.
Despite differentiated thyroid carcinoma (DTC) being the most common malignant endocrine neoplasm, its prevalence in the Spanish population is less than 1%. It primarily affects middle-aged women, and its prognosis is generally excellent.1 In this context, international clinical guidelines have been amending their protocols for the diagnosis, treatment and follow-up of thyroid nodules and DTC in favour of less aggressive monitoring measures.2,3 TSH suppression therapy with levothyroxine (TSHst) has not been exempt from this change.
Classically, the long-term management of DTC after total thyroidectomy includes treatment with supraphysiological doses of levothyroxine to inhibit pituitary TSH release.2 The underlying theoretical concept was based on the premise of suppressing circulating TSH levels and their inhibition as a growth and proliferation factor of those follicular cells still present after completing DTC therapy.4 However, clinical studies have shown ambiguous outcomes in patients with a high or intermediate risk of DTC recurrence5,6 and negative outcomes in patients with low-risk DTC.7 Moreover, TSHst is not exempt from the risk of developing long-term cardiovascular or skeletal complications, including increased heart rate and left ventricular mass, atrial fibrillation and osteoporosis, etc.8
In this regard, the 2015 American Thyroid Association's management guidelines (2015 ATA guideline) recommend TSHst, to achieve TSH values <0.1 mIU/l in patients with high-risk DTC, TSH between 0.1 and 0.5 mIU/l in patients with intermediate-risk DTC, and from 0.5 to 2 mIU/l in low-risk patients. The ATA also recommends amending TSHst goals in accordance with DTC treatment response, following the dynamic risk stratification (DRS) criteria: incomplete structural response or incomplete biochemical response: TSH < 0.1 mIU/l; indeterminate response: TSH between 0.1 and 0.5 mIU/l; and excellent response: 0.5–2.0 mIU/l.
Despite the clinical guideline recommendations and scientific evidence, studies conducted and surveys administered on actual clinical practice have shown that TSHst is being overused, particularly in patients with low-risk DTC.9 However, no study to date has analysed changes in TSH-level therapeutic targets according to the initial risk of recurrence and evolution of the DRS in clinical practice.
This study aimed to assess TSHst in a cohort of patients with DTC according to their initial risk of recurrence and throughout follow-up using DRS in an endocrinology high-resolution thyroid unit (HRTU), as well as to analyse the clinical factors associated with TSHst maintenance.
Material and methodsThis was a retrospective cohort study in patients diagnosed with DTC in follow-up during 2020 in the Department of Endocrinology and Nutrition's HRTU of a tertiary hospital, with a minimum follow-up of two years after completing the initial treatment (total thyroidectomy ± radioiodine therapy).
The following clinical data were collected: age at diagnosis; year of cancer diagnosis; duration of clinical course in months from the diagnosis of the disease to the last visit; and type and characteristics of cancer diagnosed: size in centimetres, multifocality, vascular spread and TNM staging. In addition, the following were also recorded: risk of recurrence following diagnosis (excluding molecular markers) and dynamic risk stratification at 18 months of follow-up after completing the initial treatment and in the last monitoring visit, according to the criteria of the 2015 American Thyroid Association (ATA) guidelines, in patients who underwent total thyroidectomy and radioactive iodine ablation2 and modified dynamic risk stratification in patients with total thyroidectomy who did not undergo radioactive iodine ablation.10
The laboratory parameters required for DTC control were measured using electrochemiluminescence immunoassay (ECLIA) (Roche Diagnostics, Geneva, Switzerland), with this same method used throughout follow-up. Specifically: second-generation plasma thyroglobulin, antithyroglobulin antibodies, and TSH levels 12 months following the initial treatment and at the last follow-up visit. TSH levels were stratified following the 2015 ATA management recommendations2: TSH < 0.1 mIU/l; TSH between 0.1 and 0.5 mIU/l and TSH greater than 0.5–2.0 mIU/l.
Histology was classified according to the World Health Organization (WHO) classification11 into two main categories: papillary carcinoma and follicular carcinoma. The latter includes both minimally and widely invasive variants, the clear cell variant and Hurthle cell carcinoma (or the oncocytic variant). Histological variants of papillary carcinoma were grouped into three categories: classic papillary carcinoma, the follicular variant of papillary carcinoma and the more aggressive histological variants (diffuse sclerosing, solid/trabecular, tall cell and columnar cell subtypes). Tumours were classified at diagnosis according to the eighth edition of the TNM classification of the American Joint Committee of Cancer.12
Statistical analysisThe results are expressed in terms of mean and standard deviation (SD). The normal distribution of the variables was analysed using the Kolmogorov–Smirnov test. Quantitative variables with a normal distribution were analysed using a two-tailed Student's t-test. Non-parametric variables were evaluated using the Mann–Whitney U test Qualitative variables were expressed in percentages (%) and analysed using the χ2 test (with Fisher's correction when necessary). Finally, a logistic regression model was used that included as independent variables the effect of age, gender, risk of recurrence at diagnosis and the DRS at the last follow-up visit, as well as multifocality and histological vascular invasion, on TSH levels <0.1 mIU/l at the last follow-up visit as a dependent variable.
The SPSS statistical software package, version 17.0 (SPSS Inc., Chicago, IL, United States), was used for analysis. The accepted level of statistical significance was 5% (p < 0.05). The study was approved by the hospital's Independent Ethics Committee (IEC).
ResultsIn total, 216 patients (79.2% women) were evaluated, with a mean age of 59.0 ± 13.1 years and a mean follow-up since diagnosis of 6.9 ± 4.3 years. Mean tumour diameter was 1.6 ± 1.2 cm. The predominant histology was papillary carcinoma, 49.8% multifocality and 8.8% histological vascular invasion. Evaluation of the risk of recurrence at diagnosis in patients with DTC revealed that 69.2% were at low risk vs 13.6% at high risk. DRS classified 60% of patients upon completion of the initial treatment, and 70.7% of patients at the last follow-up visit, into the excellent response category (Table 1).
Characteristics of patients in follow-up for differentiated thyroid carcinoma.
| Mean (SD)/Percentage | |
|---|---|
| Patients | 216 (79.2% female) |
| Age at diagnosis (years) | 59.0 (13.1) |
| Years since onset | 6.9 (4.3) |
| Mean tumour size (cm) | 1.6 (1.2) |
| Multifocality | 49.8% |
| Vascular invasion | 8.8% |
| Treatment with I131 at diagnosis | 87.4% |
| Histological classification | |
| Papillary | 88.4% |
| Classic | (54.4%) |
| Follicular variant | (34.6%) |
| Aggressive variants | (11%) |
| Follicular | 11.6% |
| Oncocytic variant | (75.9%) |
| All other variants | (24.1%) |
| Risk of recurrence (2015 ATA guideline) | |
| Low | 69.2% |
| Intermediate | 17.3% |
| High | 13.6% |
| 8th edition AJCC staging | |
| I | 81.9% |
| II | 5.6% |
| III | 11.1% |
| IV | 1.4% |
| Dynamic risk stratification | Baseline | Follow-up |
|---|---|---|
| Excellent response | 60.0% | 70.7% |
| Indeterminate response | 15.3% | 8.8% |
| Biochemical incomplete response | 9.3% | 7.9% |
| Structural incomplete response | 15.3% | 12.6% |
AJCC: American Joint Committee of Cancer; 2015 ATA guideline: 2015 American Thyroid Association guideline; SD: standard deviation.
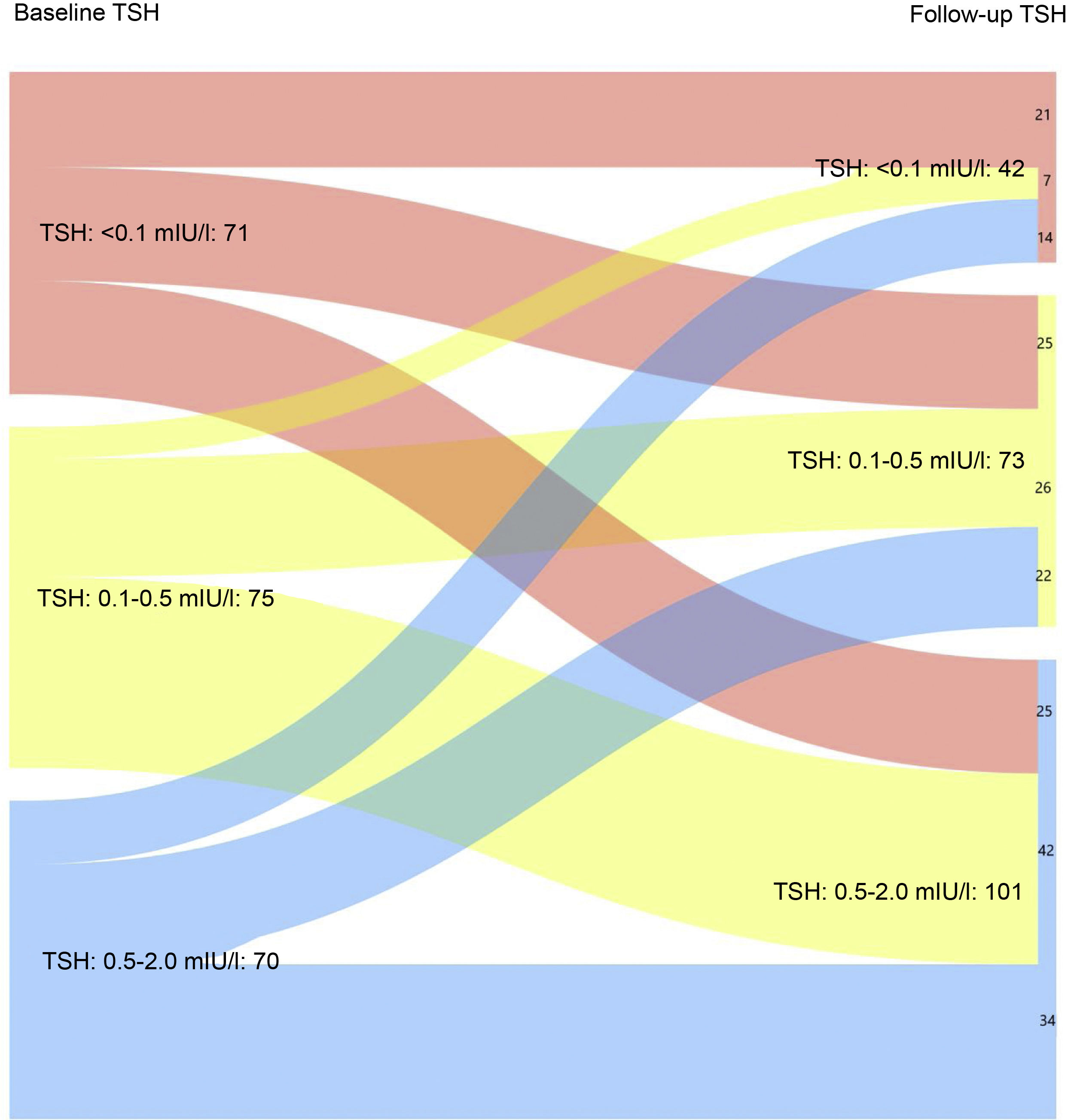
Fig. 1A shows the evolution of TSH levels in the entire sample analysed (216 patients). It can be seen that 50% of patients with TSH < 0.01 mIU/l after completing the initial treatment maintained suppressed TSH levels at the last follow-up visit (p < 0.05). Similarly, 56% and 48.6% of patients with baseline TSH levels of 0.1−0.5 mIU/l and 0.5–2 mIU/l, respectively, achieved target TSH levels of 0.5–2 mIU/l at the last follow-up visit (p < 0.05).
Evaluation of the patients by relative risk (RR) revealed that 29.1%, 37.8% and 44.8% of patients at low, intermediate and high risk of recurrence, respectively, had TSH values <0.1 mIU/l after completing the initial treatment vs 16.2 %, 18.9% and 34.5% at the last follow-up visit (p < 0.05).
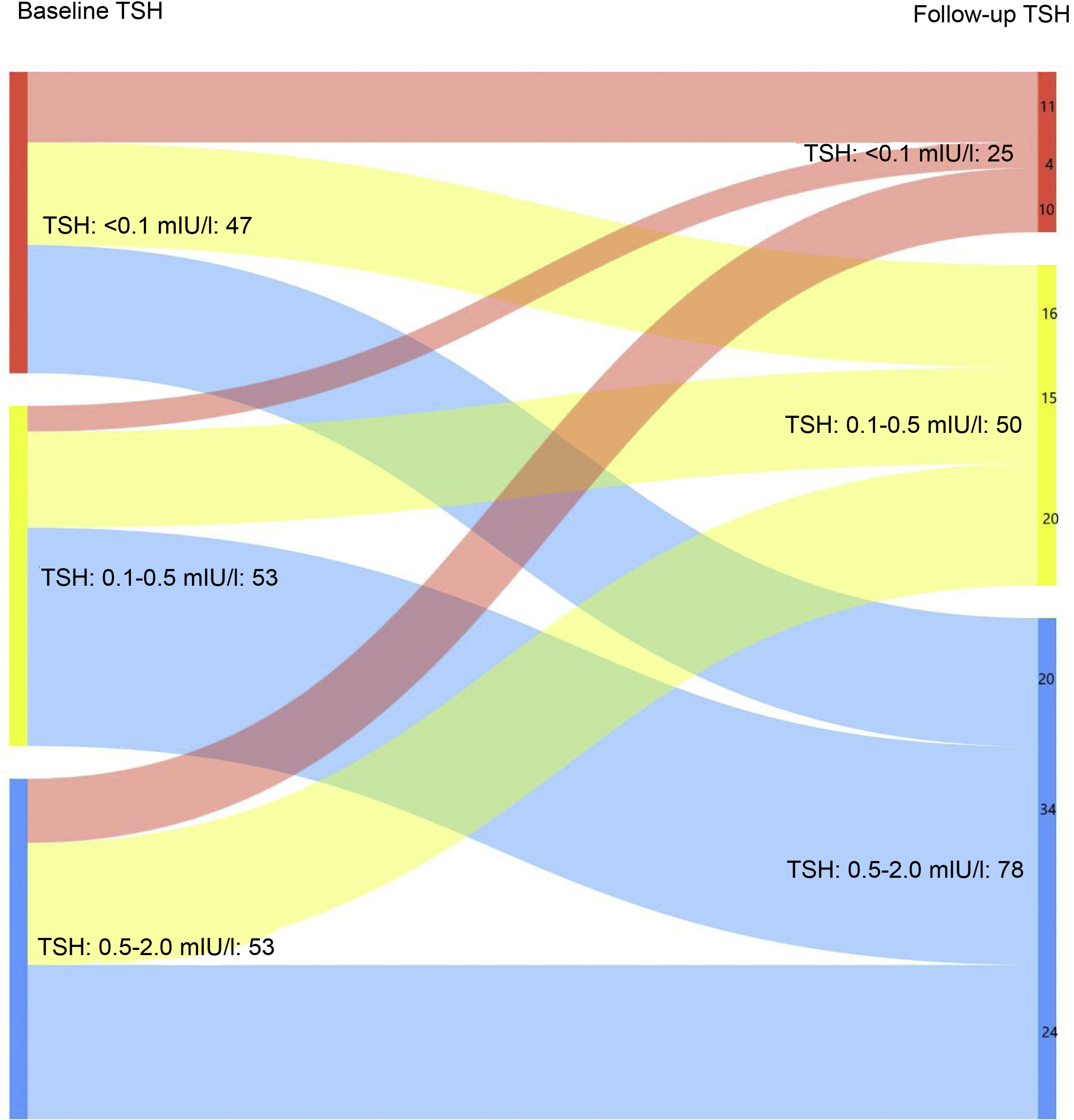
When only those patients with excellent response at the last follow-up visit were analysed, it was found that 16.3% maintained suppressed TSH levels at the last follow-up visit vs 30.7% at the first visit after completing the initial treatment (Fig. 1B). In turn, 51% had TSH levels of 0.5–2 mIU/l at the last control vs 34.6% at diagnosis (p < 0.05).
Similarly, when only those patients with an incomplete structural response or incomplete biochemical response were analysed, it was found that 22.7% and 36.4% had TSH levels <0.1 mIU/l or TSH levels of 0.1−0.5 mIU/l at the last follow-up visit, respectively. However, at the first visit after completing the initial treatment, 37.5% of patients achieved suppressed TSH levels, while 50% recorded TSH levels of 0.1−0.5 mIU/l (p < 0.05).
The characteristics associated with maintaining TSH levels <0.1 mIU/l at the last follow-up visit were: age at diagnosis (45.6 ± 13.1 vs 53.2 ± 14.3 years) –p < 0.001–, the risk of recurrence at diagnosis (2015 ATA guideline), DRS at the last control, as well as the finding of multifocality or histological vascular invasion (p < 0.05) (Table 2). When only those patients with excellent response at the last follow-up visit were analysed, younger age at diagnosis (43.9 ± 13.0 vs 51.0 ± 13.3 years) –p < 0.001–, multifocality (64% vs 44.4%) and histological vascular invasion (16 vs 4.8%) (p < 0.05) were associated with TSH levels <0.1 mIU/l. However, when those patients with structural incomplete response or biochemical incomplete response at the last follow-up visit were analysed, no clinical or histological variable was associated with maintaining suppressed TSH levels.
Comparison of the clinical, histological, risk of recurrence and dynamic risk stratification between patients with and without TSH suppression therapy.
| TSH ≤ 0.1 mIU/l | TSH > 0.1 mIU/l | p | |
|---|---|---|---|
| Gender (female) | 71.4% | 81.0% | ns |
| Age at diagnosis (years) | 45.6 ± 13.1 | 53.2 ± 14.3 | p <0.001 |
| Years since onset | 7.2 (6.0) | 6.9 (4.8) | ns |
| Mean tumour size (cm) | 1.7 (1.1) | 1.5 (1.3) | ns |
| Multifocality | 63.4% | 46.5% | <0.05 |
| Vascular invasion | 17.1% | 7.0% | <0.05 |
| Treatment with I131 at diagnosis | 92.9% | 86.1% | ns |
| Histological classification | |||
| Papillary | 90.5% | 87.6 | ns |
| Classic | (47.4%) | (56.2%) | |
| Follicular variant | (42.1%) | (32.7%) | |
| Aggressive variants | (10.5%) | (11.1) | |
| Follicular | 9.5% | 12.4% | ns |
| Oncocytic variant | (75.0%) | (76.2%) | |
| All other variants | (25.0%) | (23.8%) | |
| Risk of recurrence (2015 ATA guideline) | <0.05 | ||
| Low | 58.5% | 71.7% | |
| Intermediate | 17.1% | 17.3% | |
| High | 24.4% | 11.0% | |
| Final dynamic risk stratification | <0.05 | ||
| Excellent response | 59.5% | 73.4% | |
| Indeterminate response | 4.8% | 8.7% | |
| Biochemical incomplete response | 19.0% | 11.0% | |
| Structural incomplete response | 16.7% | 6.9% | |
2015 ATA Guideline: 2015 American Thyroid Association guideline; ns: not significant.
In a logistic regression analysis that adopted suppressed TSH levels in follow-up as a dependent variable and which included age, gender, risk of recurrence at diagnosis and DRS at the last follow-up visit, as well as multifocality and histological vascular invasion as independent variables, the only variables that maintained statistical significance were age (β = −0,062; p < 0.001), high risk of recurrence at diagnosis (β = 1.074; p < 0.05) and final DRS (β = 1.237; p < 0.05).
DiscussionTreatment with supraphysiological doses of levothyroxine to achieve suppressed circulating TSH levels has for decades been considered an essential part of DTC treatment, irrespective of risk level or evolution of treatment.13,14 However, current recommendations classify the target TSH level in accordance with the risk of initial recurrence and DRS evolution throughout follow-up.2,3 In fact, scientific evidence supporting suppression therapy in patients with a high or intermediate risk of recurrence is subject to much debate.5–7 At the same time, there is practically no support for its use in low-risk cases.7,15
Despite the published scientific evidence and current clinical guideline recommendations, according to the findings of clinical surveys evaluating the attitude of specialists responsible for monitoring patients with DTC in various scenarios, overtreatment continues to be the norm.9 The recent study by Papaleontiou et al. found that improper maintenance of suppressed TSH levels was associated with an overestimated risk of recurrence and less experience in DTC follow-up.9 Moreover, overtreatment is not unique to DTC but is also typical of hypothyroidism treatment (particularly subclinical), irrespective of the underlying cause, according to recently published findings.16 In both cases, goals are pursued without sufficient scientific evidence: reduced risk of recurrence/mortality in DTC and the supposed improvement in quality of life, depressive symptoms, fatigue or cognitive function in treating subclinical hypothyroidism.17 Both approaches also share a marked underestimation of the risks related to the possibility of developing iatrogenic subclinical hyperthyroidism, especially if maintained indefinitely over time.8
The results of this study objectify the risk of overtreatment in patients with DTC, including in specialist units. In fact, 16.3% of the population evaluated with excellent response maintained TSH levels <0.1 mIU/mL after an average of almost seven years of follow-up. This is of particular concern given that current scientific evidence is clearly against TSHst in patients with excellent response and low or intermediate risk of recurrence, including within the first years of diagnosis.7,15
It is also clear that the percentage of patients with TSHst after completing the initial treatment is statistically higher both in terms of excellent response (30.7%) and when evaluating the RR at diagnosis. In fact, a marked reduction was observed in the percentage of patients receiving TSHst after the initial treatment versus the last follow-up visit, irrespective of the initial risk category or DRS evaluated. Moreover, even though the clinical guideline targets were not met in almost a fifth of the patients evaluated with excellent response, there was a clear trend to reduce TSHst during follow-up in all patients.
Explaining why TSHst is maintained, even in patients with an excellent prognosis2 is complex and cannot be attributed to any one single factor. Previous studies have associated this persistence of suppression therapy with an overestimated risk of recurrence by specialists9,18 and a lack of scientific evidence in certain categories of the DRS (incomplete biochemical response or indeterminate response), even in the face of favourable progression towards an excellent response in a high percentage of patients.19 In addition, there is no doubt that certain recommendations based exclusively on low-quality studies or expert consensus are an obstacle to implementing clinical guidelines and promoting therapeutic inertia.20 They also delay the implementation of changes recommended in the guidelines, sometimes for years.21 Lastly, although centres with less experience in DTC management could record even higher TSHst rates9 this reality is not alien to those centres with a high volume of complex patients in follow-up, as in our study.
It must be mentioned that these results may be partially biased due to the smaller number of subjects in the higher-risk categories in our cohort. Strikingly, our results show a reduction in suppression therapy throughout follow-up, similar to those patients with excellent responses. However, the percentage of patients with TSH ranges of 0.5–2 mIU/l at the last follow-up visit is lower in the incomplete structural response and biochemical incomplete response subgroup than in the excellent response subgroup (40.9 vs 51.0%, respectively). It must be mentioned that these results may be partially biased due to the smaller number of subjects in the higher risk categories in our cohort. However, it is important not to forget the heterogeneous evolution, particularly in the biochemical incomplete response category.19 Furthermore, the current clinical guidelines recommend target TSH levels of 0.1−0.5 mIU/l in the biochemical incomplete response category in the absence of clear signs of progression,2 which would at least partially explain the results of this study.
Lastly, when trying to evaluate those clinical, histological or progression characteristics of DTC that could be associated with TSHst, it was found that clinical criteria (age), classic criteria of histological aggressiveness22,23 (multifocality and vascular invasion), as well as risk stratification (risk of recurrence after diagnosis and DRS during follow-up), played a key role when it came to maintaining therapy, both as a whole and in those patients with excellent response in follow-up. However, the multivariate analysis revealed that only age, risk of recurrence at diagnosis and DRS during follow-up were associated with TSHst maintenance. Therefore, the essential variables driving the decision to maintain TSHst in this study, even beyond the current recommendations, were based on the DRS classification at the last follow-up visit, as well as on the initial risk of recurrence primarily based on criteria of histological aggressiveness at diagnosis.2 That is to say, although DRS at the last follow-up visit should be the only criterion on which to base target TSH levels in a DTC cohort with a minimum follow-up of two years, the baseline histology continues to play an essential role in establishing the target TSH value, even after an average seven years of follow-up, in violation of current recommendations.2 In other words, the histological aggressiveness of DTC at diagnosis is a barrier to TSHst relaxation, even in patients with excellent response years after its onset.
The fact that younger age was related to TSHst maintenance, irrespective of the risk of recurrence or DRS, deserves special mention. In this study, it is striking that DTC is the only cancer that includes age in its staging.11,24 Moreover, thanks to their better prognosis, patients under the age of 55 years cannot be classified beyond stage II, even if it has spread to the lymph nodes or there is metastasis.11 The results obtained from this study go against this evidence: those patients with TSHst in follow-up were significantly younger (45.6 vs 53.2 years), despite a similar time since disease onset (seven years). This could be due to several reasons: an overestimated risk of recurrence in those patients with a longer life expectancy; or an underestimated risk of the onset of complications associated with iatrogenic subclinical hyperthyroidism, some of which would be directly related to the TSHst maintenance time (osteoporosis).8
Our study has certain limitations. Firstly, the results obtained are from a single HRTU led by endocrinologists with extensive experience in DTC follow-up. As such, the results may not be comparable to other centres or units or even to physicians of other specialties.9 Moreover, even though the levothyroxine dose's stability was considered when measuring TSH levels, these levels correspond to a single value. In addition, the narrow target range of TSH levels in DTC follow-up is not always easy to achieve and maintain (poor compliance, malabsorption, etc.)25 and can sometimes be modified by the specialist due to associated comorbidities not evaluated in this study (such as osteoporosis, cardiac arrhythmia, etc.).2 Finally, in the categories of biochemical incomplete response and structural incomplete response, the small number of patients means there is insufficient statistical power for a detailed analysis of the results in this group. However, the study also has a number of strengths. Firstly, it is one of the first studies to evaluate the achievement of target TSH values in a specialist Thyroid Unit not just at diagnosis but also during long-term follow-up, according to current recommendations2 and DRS. Secondly, the results are consistent and in line with the few existing publications on this topic.
In conclusion, despite the current recommendations, almost one-fifth of patients at low risk and with excellent response received levothyroxine suppression therapy during DTC follow-up. The variables associated with maintaining TSHst were age, initial risk of recurrence and DRS during follow-up.
FundingThis project did not receive funding of any kind.
Conflicts of interestThe authors declare that they have no conflicts of interest.