To establish whether glycemic variability (GV) parameters used when gestational diabetes mellitus (GDM) has been diagnosed could help predict the probability that a patient will need pharmacological treatment, and to analyze the link of these parameters to the development of maternal-fetal complications.
Materials and methodsA prospective study of 87 women with GDM who underwent retrospective continuous glucose monitoring (CGM) for six days between weeks 26 and 32 of gestation, following diagnosis. The mean glycemia levels and GV variables were analyzed together with their link to maternal-fetal complications, and the need for pharmacological treatment. ROC (receiver operating characteristic) curves were developed to determine validity to detect the need for pharmacological treatment.
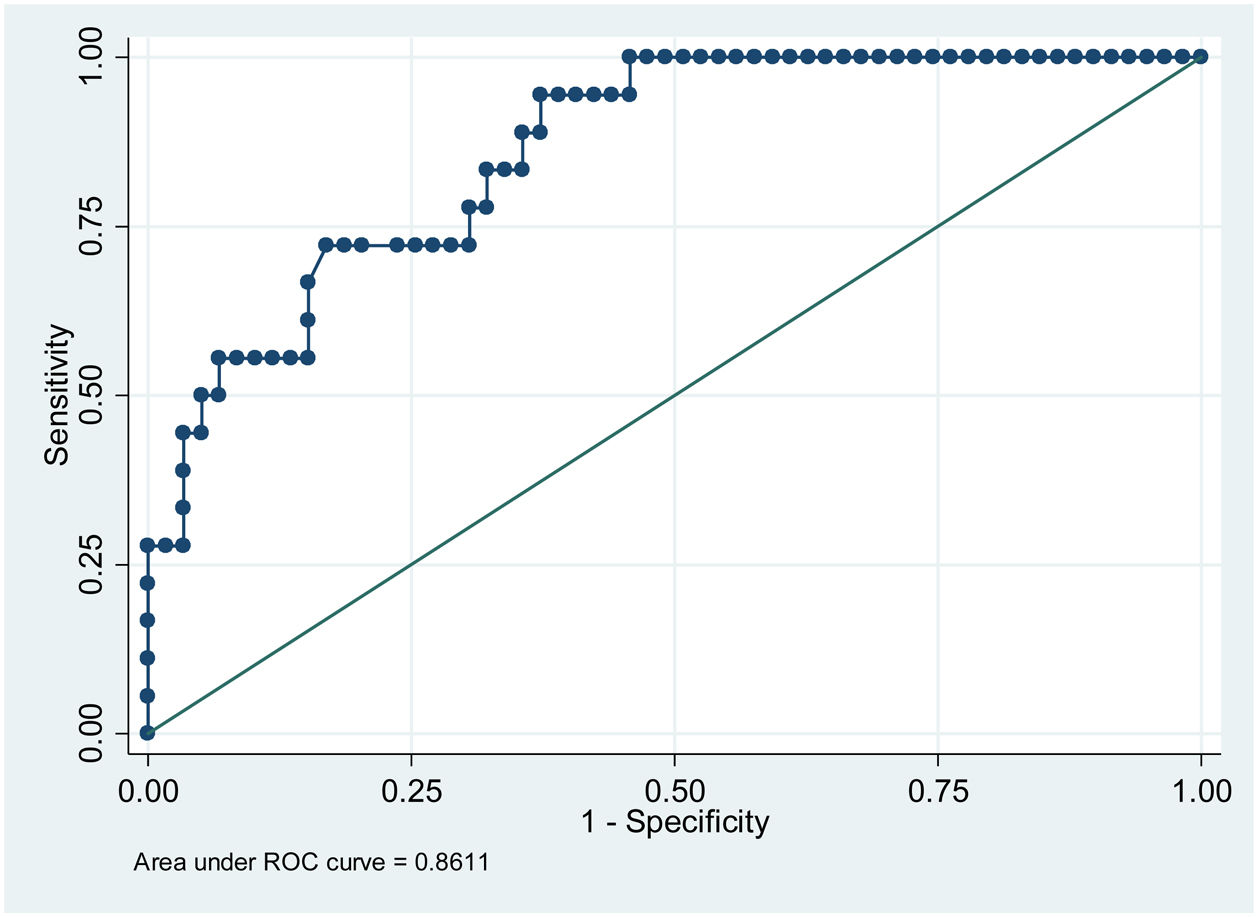
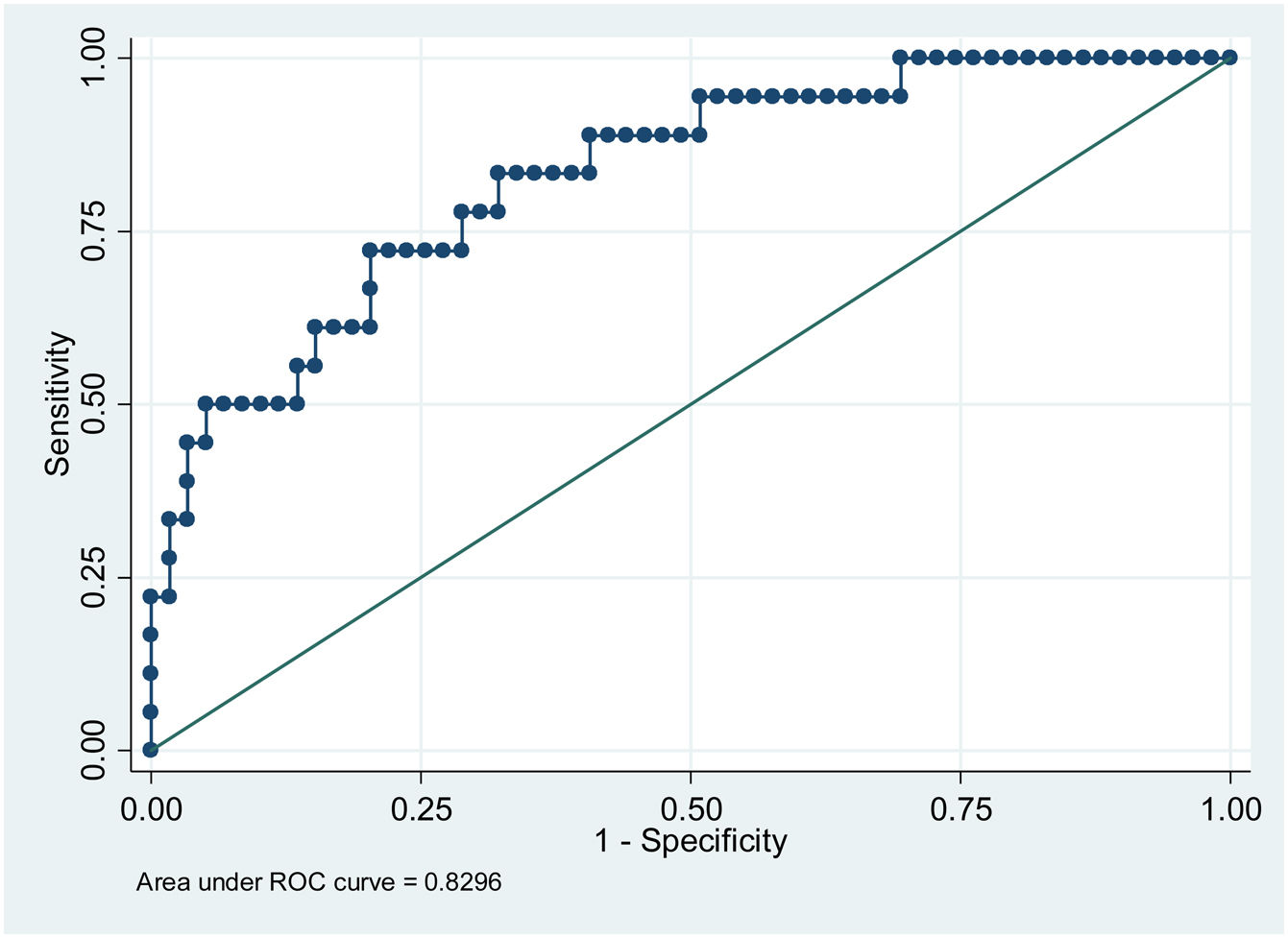
ResultsPatients with higher mean glycemia (p < 0.001) and continuous overlapping of net glycemic action in a period of n-hours (CONGAn) (p = 0.001) required pharmacological treatment. The ROC curves showed cut-off points of 98.81 mg/dL for mean glycemia, and 86.70 mg/dL for CONGAn, with 83.3% sensitivity and 67.8% specificity for both parameters. No relation between the GV parameters and development of maternal-fetal complications was observed.
ConclusionsThe use of CGM, once GDM is diagnosed, enables us to identify those patients who would benefit from closer monitoring during gestation, and facilitate a speedier take-up of pharmacological treatment. However, prospective studies involving a higher number of patients are needed, as well as a cost assessment for recommending the use of CGM following GDM diagnosis.
Establecer si determinados parámetros de variabilidad glucémica (VG) al diagnóstico de Diabetes Mellitus gestacional (DMG) podrían ayudar a predecir la probabilidad de precisar tratamiento farmacológico y analizar la relación de estos con el desarrollo de complicaciones materno-fetales.
MétodosEstudio prospectivo en 87 mujeres con DMG a las que se realiza una monitorización continua de glucosa (MCG) retrospectiva entre la 26-32 semana de gestación durante 6 días, tras el diagnóstico. Se analizan glucemia media y parámetros de VG y su asociación con complicaciones materno-fetales y necesidad de tratamiento farmacológico. Se elaboran curvas ROC para estimar la validez para detectar la necesidad de tratamiento farmacológico.
ResultadosLas pacientes con glucemia media más elevada (p < 0.001) y solapamiento continuo de la acción de la glucosa en un periodo de n-horas (CONGA) (p = 0.001) precisaron tratamiento farmacológico. A partir de las curvas ROC se obtuvieron los puntos de corte de 98.81 mg/dL para glucemia media y de 86.70 mg/dL para CONGA con una sensibilidad de 83.3%% y especificidad de 67.8% para ambos parámetros. No se observó una relación entre los distintos parámetros de VG y el desarrollo de complicaciones materno-fetales.
ConclusionesEl uso de la MCG al diagnóstico de la DMG permite identificar a aquellas pacientes que se beneficiarían de una vigilancia más estrecha durante la gestación, facilitando el inicio de un tratamiento farmacológico precoz. No obstante, son necesarios estudios prospectivos con un mayor número de pacientes y que evalúen los costes asociados para poder recomendar el uso de la MCG tras el diagnóstico de DMG.
Gestational diabetes mellitus (GDM) is an intolerance of carbohydrates diagnosed in the second or third semester of pregnancy, with no clear evidence of previous diabetes mellitus.1 It is prevalent in about 8.8% of total gestations (1%–12%).2 Hyperglycemia in pregnancy is related to greater risk of maternal complications (hypertension induced by pregnancy, pre-eclampsia, intrauterine fetal death, caesarean section birth) and fetal complications (hypoglycemia, hyperbilirubinemia, macrosomia, obstetric traumatism).3 In addition, it involves a greater risk of developing type 2 diabetes mellitus (DM) and metabolic syndrome with an increase in incidence of cardiovascular diseases.4,5
GDM treatment aims to maintain glycemia levels within a very narrow range similar to that of gestation without diabetes. It includes hygienic dietary measures and, if these have no effect, pharmacological treatment.6
In common clinical practice, glucose levels are measured by self-monitoring of capillary glycemia (SMCG), however, nowadays continuous glucose monitoring (CGM) systems can measure the glucose of the interstitial fluid using an electrochemical method that makes a good correlation between the glucose calculated at interstitial level and in the blood.7 There are also real-time, blind or retrospective sensors, the latter used by professionals as a diagnostic-therapeutic tool to modify DM patients’ treatment.8
Studies using CGM are rare in GDM, involving few patients and frequently analyzing them alongside women with both pre-gestational and gestational DM. Nevertheless, these few studies have shown that CGM systems detect hyperglycemia more frequently than SMCG.9,10 Also, the use of both real-time and retrospective CGM is associated to better glycemia control than when SMCG is applied, and to less weight gain in both mother and newborn. Yet there is no evidence of a reduction in other maternal-fetal complications related to GDM.11,12 Recent years have seen an increase in the study of GV parameters using CGM, describing higher GV in women with GDM.13,14 However, in the retrospective use of CGM in GDM, the GV data obtained as a predictive tool for the onset of obstetric and perinatal complications have provided mixed results.15
In GDM treatment, hygienic dietary measures have been shown to reduce excess weight in newborns, and there has been a drop in maternal-fetal complications when pharmacological treatment is administered.16–18 In terms of CGM in GDM and pharmacological treatment, some studies have shown that when CGM is used, more patients start pharmacological treatment than when only SMCG is used.19 However, so far no study has analyzed the use of CGM following GDM diagnosis to detect GV parameters and their relation to the probability of requiring pharmacological treatment.
The aim of our study was to use CGM, on diagnosis of GDM, to establish whether mean glycemia levels and GV parameters can help predict the probability of a patient requiring pharmacological treatment, and whether these parameters are related to the development of obstetric and perinatal complications.
Materials and methodsPatientsThis is a prospective study of GDM patients at the Endocrinology and Pregnancy Department of the University Hospital of Jerez, Spain. The inclusion period ran from February 2016 to June 2018. This hospital provides coverage to 456,000 inhabitants, with an average of 2421 term pregnancies a year.
The study included pregnant women between weeks 26 and 32 of gestation diagnosed with GDM using the two-step strategy, and an oral glucose overload of 100 g with two abnormally high values, in line with diagnostic criteria of the National Diabetes Data Group and the 3rd Workshop-Conference on Gestational Diabetes Mellitus, which are: basal glucose 105 mg/dL, 190 mg/dL at 60 minutes, 165 mg/dL at 120 minutes, and 145 mg/dl at 180 minutes.20 Excluded from the study were those patients with pre-gestational diabetes, chronic systemic illnesses, an acute infectious process and/or refusal to give informed consent.
In the first visit following GDM diagnosis, data were obtained from the patient on race, age, gestation stage, family history of DM and personal GDM antecedents, obstetric history, pre-gestational body mass index (pBMI) and the following analytical parameters: glucose, HbA1c, uric acid, triglycerides, HDL and LDL cholesterol.
After completing gestation, data were gathered on GDM treatment and maternal complications during gestation, such as hypertension induced by pregnancy (systolic arterial pressure ≥140 mm Hg or diastolic arterial pressure ≥90 mm Hg after 20 weeks of gestation), pre-eclampsia (hypertension induced by pregnancy, with proteinuria ≥30 mg/mmol creatinine), type of birth (ectopic, instrumental delivery or caesarean). The data on newborns included their weight at birth, neonatal complications such as being small for gestational age (SGA) (percentile <10), large for gestational age (LGA) (percentile >90), macrosomia (weight >4000 g), hypoglycemia (less than 40 mg/dl in the first 24 hours), hyperbilirubinemia, traumatism, infection and length of stay in ICU.
Hygienic dietary measuresIn the first visit, all patients were given advice on diet and a personalized plan based on their pBMI: if this was less than 18.5 kg/m2, the dietary allowance was between 36–40 kcal/kg/day, 31–35 kcal/kg/day at 18.5–25 kg/m2, 25–30 kcal//kg/day at 25–29 kg/m2, and 23–25 kcal/kg/day if higher than 30 kg/m2. The macronutrient distribution was 40%–50% carbohydrates, 30%–40% fat and 20% proteins.6
Monitoring of glucose and treatmentIn the first visit, according to routine clinical practice, each woman was given a capillary glycemia monitoring device (Contour® next) to perform SMCG before, and 1 hour after, breakfast, lunch and dinner. The marked glycemic targets follows the American Diabetes Association guidelines of ≤95 mg/dl before eating, ≤140 mg/dl 1 hour after.21 If this score was exceeded, pharmacological treatment was started, with insulin as the medium of choice in our setting for treatment by gestating mothers.6 Hypoglycemia was defined as a value of glucose <70 mg/dl.
The first visit also included implanting a blind or retrospective CGM device in each patient (model iPro™2) that they would wear for 6 consecutive days. The sensor was inserted into the subcutaneous tissue and linked to a transmitter attached to the skin. This device records and stores 288 glucose measurements continuously over 24 hours, and the data are downloaded for later assessment. The parameters analyzed were: mean glycemia as centralized measurement and mean GV, standard deviation (SD), coefficient variance (CV), mean amplitude of glycemic excursions (MAGE), mean of daily differences (MODD) and continuous overlapping of net glycemic action in a period of n-hours (CONGAn). CV is defined as the SD divided by the glucose mean, its interpretation expressed in percentage terms; MAGE is defined as the arithmetic mean of glucose values from the lowest to the highest points or vice versa; MODD is defined as the mean of the differences between glucose values at the same time of day over two consecutive days, allowing the analysis of GV between different days, and, CONGA, is the standard deviation of the sum of differences between a given value and the observed values in the previous hours (n), which enables the analysis of GV over a 24-h period.
This study was approved by the Research Ethics Committee of the province of Cadiz in line with the ethical principles set out in the Helsinki Declaration. Prior to inclusion in the study, all patients wishing to participate signed an informed consent form.
Statistical analysisIn the descriptive analysis, the results of the quantitative variables were expressed in terms of mean and standard deviation, and in frequencies and percentages for the qualitative variables.
A multivariate analysis was performed using logistic regression models, taking as dependent variables each result for obstetrics and newborns; independent variables were mean glycemia, SD, CV, MAGE, MODD and CONGAn, with age and pBMI used in the models as adjustment variables. Then, an analysis was made of the need for treatment, as a dependent variable, as well as using as the GV parameters mentioned as independent variables, and adjusting for age and pBMI. When the variables associated to the need for pharmacological treatment had been identified (mean glycemia and CONGAn), the area below the ROC curve was calculated for both variables. A statistically significant association was confirmed if the p-value was less than 0.05. The Stata program version 16 was used to gather and analyze the data. The GV parameters were calculated with EasyGV software.22,23
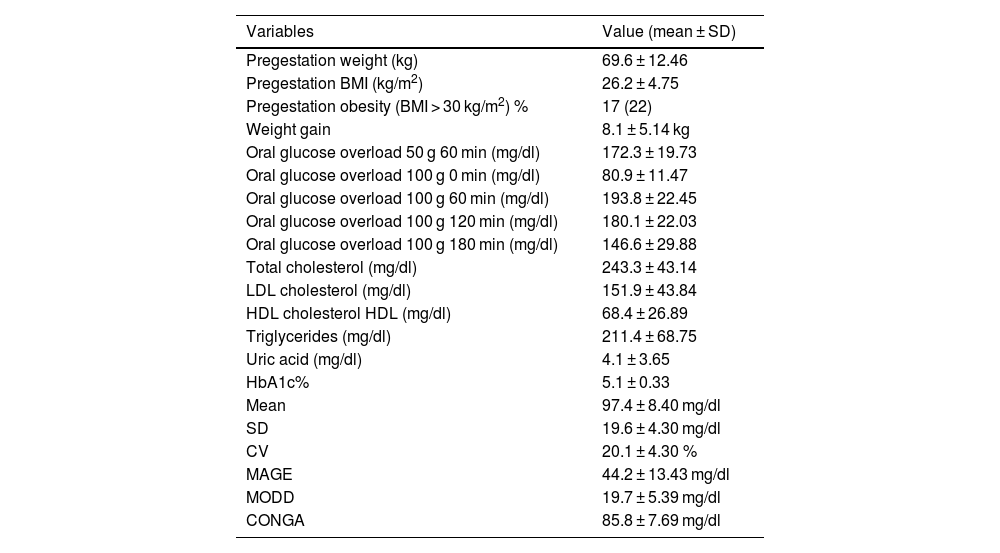
ResultsClinical characteristics, anthropometric and analytical parametersThe study initially included 87 patients with GDM, but 10 were excluded due to problems with the sensor (sensor damage or incomplete data transfer). The analysis was carried out on 77 gestating mothers, of whom 75 (97.4%) were classified as Caucasian, 1 (1.4%), Asian and 1 (1.4%) Hispanic American. The mean age was 33.5 ± 4.23 years (30 patients [38.9%] were aged 35 or over). GDM diagnosis was made at 30.2 ± 2.16 of gestation. DM family antecedents were present in 51 (66.2%) of the patients. Of all the patients analyzed, 54 (70.1%) had had one or more prior pregnancies, and of these 15 (27.7%) had presented with GDM in previous pregnancies. The anthropometric and analytical parameters are presented in Table 1.
Analytical & anthropometric parameters.
| Variables | Value (mean ± SD) |
|---|---|
| Pregestation weight (kg) | 69.6 ± 12.46 |
| Pregestation BMI (kg/m2) | 26.2 ± 4.75 |
| Pregestation obesity (BMI > 30 kg/m2) % | 17 (22) |
| Weight gain | 8.1 ± 5.14 kg |
| Oral glucose overload 50 g 60 min (mg/dl) | 172.3 ± 19.73 |
| Oral glucose overload 100 g 0 min (mg/dl) | 80.9 ± 11.47 |
| Oral glucose overload 100 g 60 min (mg/dl) | 193.8 ± 22.45 |
| Oral glucose overload 100 g 120 min (mg/dl) | 180.1 ± 22.03 |
| Oral glucose overload 100 g 180 min (mg/dl) | 146.6 ± 29.88 |
| Total cholesterol (mg/dl) | 243.3 ± 43.14 |
| LDL cholesterol (mg/dl) | 151.9 ± 43.84 |
| HDL cholesterol HDL (mg/dl) | 68.4 ± 26.89 |
| Triglycerides (mg/dl) | 211.4 ± 68.75 |
| Uric acid (mg/dl) | 4.1 ± 3.65 |
| HbA1c% | 5.1 ± 0.33 |
| Mean | 97.4 ± 8.40 mg/dl |
| SD | 19.6 ± 4.30 mg/dl |
| CV | 20.1 ± 4.30 % |
| MAGE | 44.2 ± 13.43 mg/dl |
| MODD | 19.7 ± 5.39 mg/dl |
| CONGA | 85.8 ± 7.69 mg/dl |
During gestation, 18 (23.4%) patients required pharmacological treatment with insulin, administered in accordance with our area’s action protocol.6 On average, treatment with insulin began in week 31 of gestation, requiring a total insulin dose (ID) of 16.8 ± 13.72 (IU) corresponding to 0.25 units per kilo of weight required per day (ID/kg/day). The type of insulin required was 33% basal, 33% rapid acting analog, and 33% combined basal and rapid acting analog. The most frequent time to administer insulin was before the 3 main meals of the day (n = 8, 44.4%), then at around dinner time (n = 5, 27.7%); less frequent administration occurred at breakfast and lunch (n = 2, 11.1%), lunch and dinner (n = 1. 5.5%) and breakfast (n = 1. 5.5%).
Regarding maternal complications during pregnancy, 2 (2.6%) women developed gestational hypertension but none pre-eclampsia; 44 (57.1%) patients had an ectopic birth, 8 (10.4%) an instrumental delivery and 25 (32.5%) were by caesarean.
In the perinatal results, the mean weight of the newborn was 3281.7 ± 460.68 grams (percentile 56.39 ± 30.58), 5 (6.5%) were SGA, 14 (18.2%) LGA and 10 (13%) had macrosomia. A total of 17 (22.1%) newborns experienced a hypoglycemic episode, 6 (7.8%) hyperbilirubinemia, 6 (7.8%) required oxygen therapy support, 1 (1.3%) suffered an obstetric traumatism, 1 (2.3%) was born with an infection, and 3 (3.9%) were admitted to ICU.
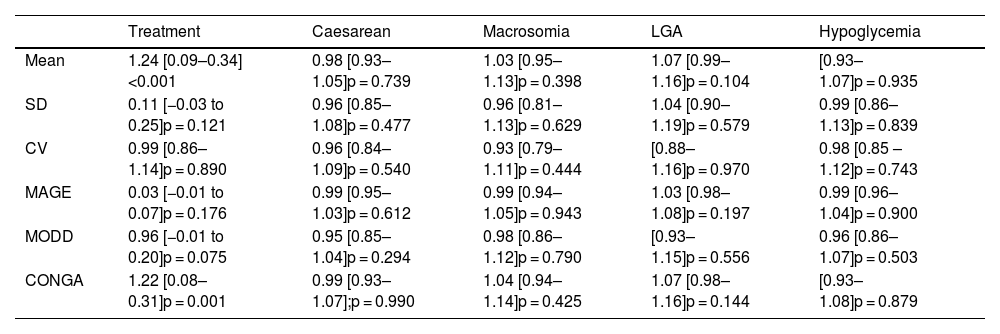
Continuous glucose monitoringTable 1 presents the GV parameters based on the results from retrospective CGM over 6 consecutive days. As observed in Table 2, no relation was found between the various GV parameters and the obstetric and perinatal results.
Logistic regression analysis of the association between GV and maternal-fetal complications and treatment with insulin. Odds ratio (confidence interval 95%), p-value.
| Treatment | Caesarean | Macrosomia | LGA | Hypoglycemia | |
|---|---|---|---|---|---|
| Mean | 1.24 [0.09–0.34]<0.001 | 0.98 [0.93–1.05]p = 0.739 | 1.03 [0.95–1.13]p = 0.398 | 1.07 [0.99–1.16]p = 0.104 | [0.93–1.07]p = 0.935 |
| SD | 0.11 [−0.03 to 0.25]p = 0.121 | 0.96 [0.85–1.08]p = 0.477 | 0.96 [0.81–1.13]p = 0.629 | 1.04 [0.90–1.19]p = 0.579 | 0.99 [0.86–1.13]p = 0.839 |
| CV | 0.99 [0.86–1.14]p = 0.890 | 0.96 [0.84–1.09]p = 0.540 | 0.93 [0.79–1.11]p = 0.444 | [0.88–1.16]p = 0.970 | 0.98 [0.85 – 1.12]p = 0.743 |
| MAGE | 0.03 [−0.01 to 0.07]p = 0.176 | 0.99 [0.95–1.03]p = 0.612 | 0.99 [0.94–1.05]p = 0.943 | 1.03 [0.98–1.08]p = 0.197 | 0.99 [0.96–1.04]p = 0.900 |
| MODD | 0.96 [−0.01 to 0.20]p = 0.075 | 0.95 [0.85–1.04]p = 0.294 | 0.98 [0.86–1.12]p = 0.790 | [0.93–1.15]p = 0.556 | 0.96 [0.86–1.07]p = 0.503 |
| CONGA | 1.22 [0.08–0.31]p = 0.001 | 0.99 [0.93–1.07];p = 0.990 | 1.04 [0.94–1.14]p = 0.425 | 1.07 [0.98–1.16]p = 0.144 | [0.93–1.08]p = 0.879 |
When assessing the relation between pharmacological treatment and GV parameters obtained from the CGM, we observe that when mean glycemia and CONGAn levels are high, the probability of the patient requiring pharmacological treatment increases with OR = 1.24 for mean glycemia (95% confidence interval [110–1.40]; p < 0.001]) and OR = 1.22 for CONGAn (95% confidence interval [1.09–1.37]; p = 0.001]). Thus, if we describe the data in terms of averages, we observe that for each mg/dL increase in mean glycemia and CONGAn, there is a rise of 24% and 22% respectively in the probability of the patient requiring pharmacological treatment (Table 2).
In terms of the capacity to predict the need for pharmacological treatment based on CGM data, the cut-off points for mean glycemia and CONGAn can be calculated with greater sensitivity (S) and specificity (E), with scores of 98.81 mg/dL (S 83.3%, E 67.8%) and 86.70 mg/dL (S 83.3%, E 67.8%), respectively (Figs. 1 and 2).
ConclusionThe GV parameters obtained from CGM quantify the amplitude, frequency and duration of glycemic fluctuations in a way that provides very useful information to add to that acquired by classic methods such as preprandial and postprandial glycemia readings from SMCG and HbA1c. For this reason, GV is increasingly important in the control of type 1 and 2 DM, as it has demonstrated that the time and amplitude of glucose fluctuations imply greater risk of hyperglycemia and hypoglycemia. GV itself is also known to represent a risk factor for diabetes-related complications.24,25
In recent years, CGM studies on GDM have also begun measuring GV parameters and analyzing their relation to maternal-fetal complications. Given the scarcity of such studies, there are still no established standardized GV reference values for GDM as there are for DM.26–30 Another drawback is disparity in the results and the difficulty in comparing them, as they do not always analyze the same GV parameters26–28; in addition, the parameters are determined at different stages of gestation and different software programs are used in the calculations23; our study used the EasyGV software.22,23 Yet although it is difficult to compare our results to those in other published studies, it can be stated that the majority present GV values that are higher than those obtained in our work.26–30
Dietary treatment is the first therapeutic step, and has been shown to reduce excess weight in newborns associated to GDM.31 Adequate glycemic control is achieved in 70%–85% of cases, thus avoiding the need for pharmacological treatment. The results in our study show that 23.4% of gestating mothers needed pharmacological treatment with insulin, similar to that described in the literature.6 In patients who failed to reach the glycemia objectives by dietary treatment, pharmacological treatment helps to reduce maternal-fetal complications,31 so it is essential to identify as soon as possible those patients who will probably need to it.
According to the latest data, there are still no studies that aim to assess the relation between mean glycemia and GV after the diagnosis of GDM and the probability of requiring pharmacological treatment during the pregnancy. Our study is the first to show that mean glycemia, as a centralized measurement, and CONGAn, as a GV parameter, both obtained by CGM after the diagnosis of GDM, can help predict the need for pharmacological treatment. In this way, we observe that for each mg/dL increase in mean glycemia and CONGAn, there is a rise of 24% and 22% respectively in the probability of the patient requiring pharmacological treatment.
The results of our work enable us to establish cut-off points for mean glycemia and CONGAn with a high degree of sensitivity and specificity (Figs. 1 and 2). GDM patients require highly sensitive tests in order for medical professionals to intensify therapeutic measures quickly to prevent or minimize maternal-fetal complications. Those patients detected with GDM via CGM with a mean glycemia above 98.81 mg/dL and/or CONGAn higher than 86.70 mg/dL should be monitored more closely. Thus, it is possible to predict from diagnosis which patients will go on to require pharmacological treatment.
Another important finding in this work is the absence of any relation between the data for mean glycemia and GV obtained in GDM diagnosis and the development of maternal-fetal complications. Several works have analyzed GV in GDM with different results. One study of women with GDM in the third trimester corroborates our results, as it did not find that CGM was a predictor of the development of complications in the mother or newborn, though the number of patients included in the study was less than in ours.28 By contrast, another study shows a direct relation between GV and fetal growth in patients with type 1 diabetes and GDM (at different stages of gestation) but it analyzed only a small number of patients, thus interpretation of the results is fairly inconsistent.27 A more recent prospective observational study of GDM patients between weeks 30–32 of gestation showed a direct proportional relation between mean glycemia and LGA.30 In our study, CGM was applied directly after diagnosing GDM, and the patients received appropriate pharmacological treatment during monitoring, with the consequent optimization of glycemic control yielding a reduction of the effect on the fetus and development of complications.
The novel aspect of this work is to demonstrate the potential usefulness of CGM on diagnosis of GDM as a tool that can identify a specific group of patients who would benefit from closer monitoring of mean glycemia and CONGAn, and the cut-off points with high sensitivity. So far, there is no published work that assesses GV via CGM in the meaning presented in this study. It also has the added value of homogeneity in the sample characteristics and the acceptable number of patients included. The results obtained can be transferred to populations with characteristics similar to those in our sample, although to extrapolate them would require future studies to recruit more patients.
One of the limitations of this study is the small number of patients included, which can undermine the strength of this work to assess the potential relation to low-incidence obstetric and newborn results, though this is still stronger than in the majority of works in the literature. Another potential weakness is that compliance with dietary measures was irregular among the patients, which could have affected the measurements as it is difficult to assess adherence to dietary advice. Nevertheless, this is unlikely to have had a significant effect since this is a highly homogeneous population with similar dietary habits marked by custom and place of residence.
In conclusion, the use of CGM after GDM diagnosis can predict the probability of requiring pharmacological treatment in patients who present higher mean glycemia and CONGAn scores. In this way, CGM could identify those patients who would benefit from closer monitoring during gestation, using SMCG in order to start pharmacological treatment as soon as a possible. It would be interesting for future prospective studies to assess the efficiency of CGM, that is, to be able to show a reduction in maternal-fetal complications and associated costs in order for its use to be recommended following GDM diagnosis.
Conflict of interestThe authors declares that they have no conflict of interest.