circRNA LRP6 participates in high-glucose-regulated cellular behaviours, while its role in gestational diabetes mellitus (GDM) is unclear. Our preliminary sequencing analysis revealed the altered expression of LRP6, suggesting its potential involvement in GDM and possible clinical value. This study explored the involvement of LRP6 in GDM.
MethodsIn this study, a total of 300 pregnant women were enrolled and followed up until delivery. The occurrence of GDM and adverse outcomes was recorded. These 300 participants were grouped into high and low LRP6 level groups (n=150; cutoff=median). Occurrence of GDM and adverse outcomes were compared between the two groups. ROC curve analysis was conducted to explore the role of LRP6 expression on the day of admission in predicting GDM. Associations between LRP6 expression and adverse outcomes were analysed with the Chi-squared test.
ResultsWe observed that participants in the high LRP6 level group experienced a higher incidence of GDM during follow-up (33/150) compared to those in the low LRP6 level group (10/150). Compared to participants who developed GDM during follow-up, participants who did not develop GDM showed lower expression levels of LRP6 in plasma. ROC curve analysis showed that high expression levels of LRP6 on the day of admission effectively distinguished potential GDM patients from other participants. Interestingly, LRP6 was only closely associated with foetal malformation and intrauterine death, but not premature delivery, hypertension, macrosomia, intrauterine distress, miscarriage and intrauterine infection in all participants.
ConclusionTherefore, increased expression levels of LRP6 in GDM predicts foetal malformation and intrauterine death.
El circARN LRP6 participa en el comportamiento celular regulado por glucosa alta, pero su papel en la diabetes mellitus gestacional (DMG) no está claro. Nuestro análisis preliminar de secuenciación mostró cambios en la expresión de LRP6, sugiriendo que podría estar involucrada en DMG y podría ser clínicamente valiosa. En este estudio se examinó el papel de LRP6 en la DMG.
MétodosEn este estudio 300 mujeres embarazadas fueron seleccionadas y seguidas hasta el parto. Se registraron la aparición de DMG y resultados adversos asociados. Las 300 participantes se dividieron en grupos de niveles altos y bajos de LRP6 (n=150; corte=mediana). Se compararon la incidencia de DMG y los resultados adversos en la gestación entre los 2 grupos. El análisis de la curva ROC tiene como objetivo investigar el papel de la expresión de LRP6 en la predicción de DMG el día de la admisión. La correlación entre la expresión de LRP6 y las reacciones adversas fue analizada por la prueba Chi cuadrado.
ResultadosObservamos que los participantes en el grupo de alto nivel de LRP6 experimentaron una mayor incidencia de DMG durante el seguimiento (33/150) en comparación con los del grupo de bajo nivel de LRP6 (10/150). En comparación con los participantes que desarrollaron DMG durante el seguimiento, los participantes que no desarrollaron DMG mostraron niveles de expresión más bajos de LRP6 en plasma. El análisis de la curva ROC mostró que los altos niveles de expresión de LRP6 en el día de la admisión distinguieron efectivamente a las posibles pacientes con DMG de otras participantes. Curiosamente, la expresión de LRP6 solo se asoció estrechamente con la malformación fetal y la muerte intrauterina, pero no con parto prematuro, hipertensión, macrosomia, distrés intrauterino, aborto espontáneo e infección intrauterina en todas las participantes.
ConclusiónEl aumento de la expresión de LRP6 en DMG se asocia con malformación fetal y muerte intrauterina.
Hormonal imbalance is common in pregnancy, and it can also lead to complications during pregnancy.1 Gestational syndromes increase the risk of a diverse range of diseases, such as diabetes mellitus, cardiovascular disease, pituitary and thyroid disorders, depression, renal and liver disease, thrombosis and even cancer.1 Gestational diabetes mellitus (GDM), a major pregnancy complication, is characterised by glucose intolerance of varying severity with first recognition or onset during pregnancy as a consequence of insulin resistance caused by the hormones produced in the placenta or inflammation.1,2 During pregnancy, the development of chronic insulin resistance may cause β-cell dysfunction, leading to GDM.1,2 However, the exact pathophysiology of GDM is unclear.1,2 Incidence of GDM varies (from 1% to 45%) across the world, and increased incidence has been observed with ageing, BMI, previous GDM and certain genetic alterations.3,4 Without proper treatment, GDM may cause a series of adverse events, such as problems during the neonatal period and birth lacerations, and some of these adverse events, such as pre-eclampsia, can threaten the lives of both the baby and the mother.5 Moreover, GDM also increases the risk of diabetes in both mother and offspring.5 In the long term, GDM increases the risk of multiple severe diseases, such as cardiovascular disease and type 2 diabetes mellitus.6,7
Treatments of GDM focus on glucose blood control using different approaches, such as insulin injection and physical activities.8,9 However, early diagnosis and prevention are still the key to preventing the development of adverse events.10 Multiple biomarkers, such as sex hormone globulin, adiponectin, C-reactive protein, insulin, and glycosylated fibronectin, have been used for the early detection of GDM.11,12 However, these markers in most cases only show moderate-to-fair accuracy (AUC values around 0.8) and are usually affected by different populations.11,12 Therefore, more reliable markers are needed. Circular RNAs (circRNAs) are circularised non-coding RNAs with regulatory roles, while they are not directly involved in protein-coding.13 In GDM, differentially expressed circRNAs have been identified with critical functions.14,15 CircRNA LDL receptor related protein 6 (LRP6) (hereinafter, LRP6 for simplicity) promotes high-glucose-induced vascular smooth muscle cell migration and proliferation by regulating the miR-545-3p/HMGA1 axis,16 suggesting its potential participation in diabetes. However, its role in GDM is unclear. We performed preliminary sequencing analysis and observed altered expression of LRP6 in patients in GDM (data not shown). Early prediction of GDM and its complications is difficult. This study was therefore carried out to explore the involvement of LRP6 in GDM, with a focus on its clinical values in the prediction of GDM and its adverse events.
Materials and methodsResearch subjectsFrom May 2018 to May 2019, a total of 477 women (23–34years old, mean age 28.9±4.9years old, no history of GDM and pregnancy) trying to conceive for half a year were enrolled at Huai’an Maternity and Child Health Hospital (Jiangsu, China). The Ethics Committee of this hospital approved this study (Approval number: 69311). These participants were enrolled to study the predictive role of LRP6 in the occurrence of GDM and adverse outcomes. Out of these 477 women, pregnancy was successfully achieved in 344 cases within three months after admission, pregnancy was not achieved in 56 cases, and the remaining 77 cases were lost. Among these 344 cases with pregnancy, participants who developed any type of clinical disorder and/or received any treatments prior to pregnancy were excluded. After exclusion, a total of 300 pregnant women (23–34years old, mean age 28.4±4.3years old, no history of GDM and pregnancy) were included in the following analyses. Glucose concentrations of all 300 participants were within normal range prior to pregnancy and no comorbidity was observed. Pregnancy was detected at a gestational age of about two months. All participants signed the informed consent.
Collection of samples and a follow-up studyOn the day of admission, blood was extracted from each participant. During pregnancy, blood was extracted from each participant every month until delivery to diagnose GDM. Blood samples were transferred to plasma preparation tubes, followed by centrifugation at 1200×g for 18min to separate plasma, which was the supernatant. The detection of blood glucose was performed on all participants every month and the 50-g followed by the 100-g Glucose Tolerance Test was applied. The 100-g Glucose Tolerance Test was performed on those that showed plasma levels of glucose at 1h after a 50-g Glucose Tolerance Test higher than 140mg/dl. GDM was diagnosed after a 100-g Glucose Tolerance Test according to the following criteria (ADA recommendations for classification and diagnosis of diabetes-2021)17: (1) >95mg/dl at fasting blood glucose; or (2) >180mg/dl at 1h blood glucose; or (3) >155mg/dl at 2h blood glucose; or (4) >140mg/dl at 3h blood glucose. During follow-up through monthly outpatient visits, multiple adverse events, including maternal outcomes (hypertension, intrauterine distress, miscarriage, premature delivery and intrauterine infection) and offspring outcomes (macrosomia, foetal malformation and intrauterine death) were recorded. Plasma samples were mixed with TRIzol (Invitrogen) to a volume ratio of 10:1 and then were stored at −80°C.
RT-qPCRTo isolate total RNAs from samples, frozen mixtures of plasma and TRIzol were put on ice, followed by cell lysis on ice for at least 30min. After that, lysates were subjected to centrifugation at 12,000×g for 20min to collect supernatant containing RNA. Then supernatant samples were mixed with chloroform to purify RNA samples. After centrifugation, supernatant was collected and RNA precipitation was performed by mixing the supernatant with 0.5 volume of methanol, followed by incubation on ice for 3h and centrifugation at 14,000×g for 30min. RNA samples were washed with 70% ethanol three times and then dried and dissolved in RNase-free water. RNA quality was analysed using a 2100 Bioanalyzer (Agilent). Only those with an RNA integrity number higher than 8.5 were used in the following experiments.
ReverTra Ace qPCR RT Kit (Toyobo, Japan) was used to prepare cDNA samples through reverse transcription (RT). In each RT reaction, 1000ng RNA was used in a 10μl system. Samples of cDNA were subjected to qPCR to detect the expression of LRP6 (circBase Accession: hsa_circRNA_101014) with 18S rRNA (NCBI Accession: M10098.1) as the internal control. All qPCR mixtures (10μl) were prepared using SYBR® Green Realtime PCR Master Mix (TOYOBO). In each qPCR reaction, 0.5μl cDNA sample was used. Thermal conditions were: 2min at 94°C, followed by 10s at 94°C and 48s at 56°C. QPCRs were conducted on the CFX Opus 96 Real-Time PCR System (Bio-Rad). Three technical replicates were included in each experiment. It is worth noting that the quality of cDNA samples was tested by RT-PCR to amplify 18S rRNA. The method of 2−ΔΔCt was used to calculate relative gene expression levels18. Microsoft Excel 2016 was used. ΔCt=CtLRP6−Ct18S rRNA. The ΔCt was calculated and the sample with the biggest ΔCt value was set to the value “1”. All other samples were normalised to this sample to calculate relative gene expression levels.
Statistical analysisGraphPad Prism 8.3.0 (GraphPad software) was used to compare datasets and prepare images. Average values of two experimental groups (average values of three technical replicates) were compared with the Student's t test. To analyse the role of plasma LRP6 (average values of three technical replicates) on the day of admission in predicting GDM, ROC curve analysis was performed with the 43 cases of GDM and 257 cases of non-GDM as true positive and negative cases, respectively. GDM-free curves were plotted for both high and low LRP6 level groups (n=150, cutoff value=median plasma expression level of LRP6). Plotted curves were compared with the log-rank test. The Chi-square test was performed to analyse the associations between plasma LRP6 and participants’ adverse events. Correlations were analysed with Pearson's correlation coefficient. P<0.05 was statistically significant.
ResultsOutcomes of follow-upPrior to pregnancy, the BMI of the 300 pregnant women ranged from 18.3 to 27.1 (23.4+/5.6), and weight gain during pregnancy ranged from 7.2 to 15.8kg (10.4±3.1kg). During follow-up, 43 cases were diagnosed with GDM, accounting for 14.3% of the 300 pregnant women. A total of 13 (7 for GDM) cases of foetal malformation, 11 (6 for GDM) cases of intrauterine death, 28 (22 for GDM) cases of premature delivery, 24 (22 for GDM) cases of hypertension, 26 (16 for GDM) cases of macrosomia, 18 (10 for GDM) cases of intrauterine distress, 10 (5 for GDM) cases of miscarriage and of 10 (6 for GDM) cases of intrauterine infection were recorded. In all cases, more than half of the adverse events developed in GDM patients.
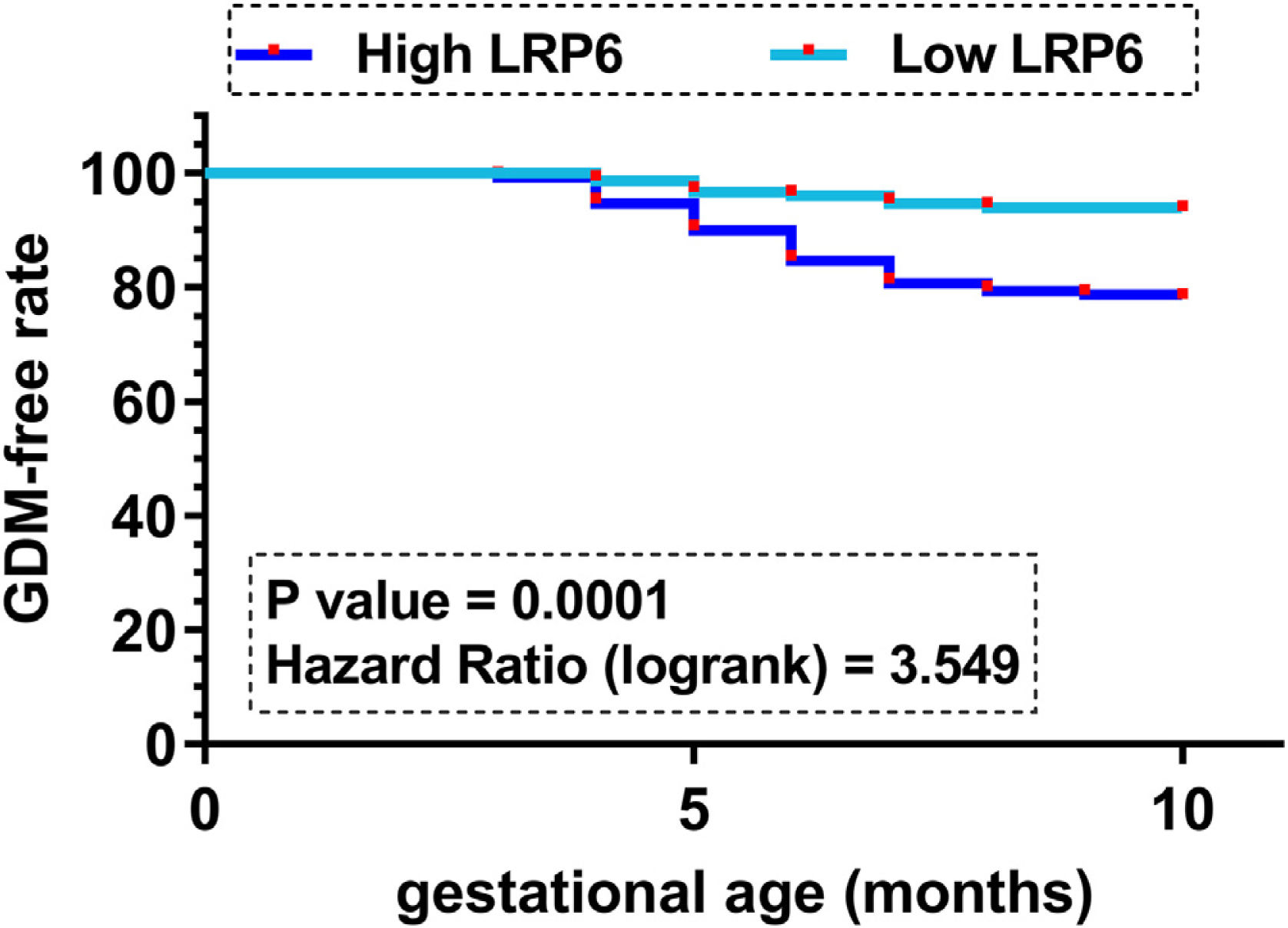
Comparison of GDM-free curves between high and low LRP6 level groupsGDM-free curves were plotted for both the high and low LRP6 level groups (n=150, cutoff value=median plasma LRP6 level). No significant differences in age and other basic clinical information prior to pregnancy were observed between these two groups. Plotted curves were compared by log-rank test. It showed that participants in the high LRP6 level group experienced a higher incidence of GDM during follow-up (33/150) compared to those in the low LRP6 level group (10/150) (Fig. 1, P=0.001). It is worth noting that multiple cutoff values, including the Youden index of the ROC curve, mean value, cutoff value and average values were used as cutoff values to perform GDM curves. Similar results were observed (data not shown).
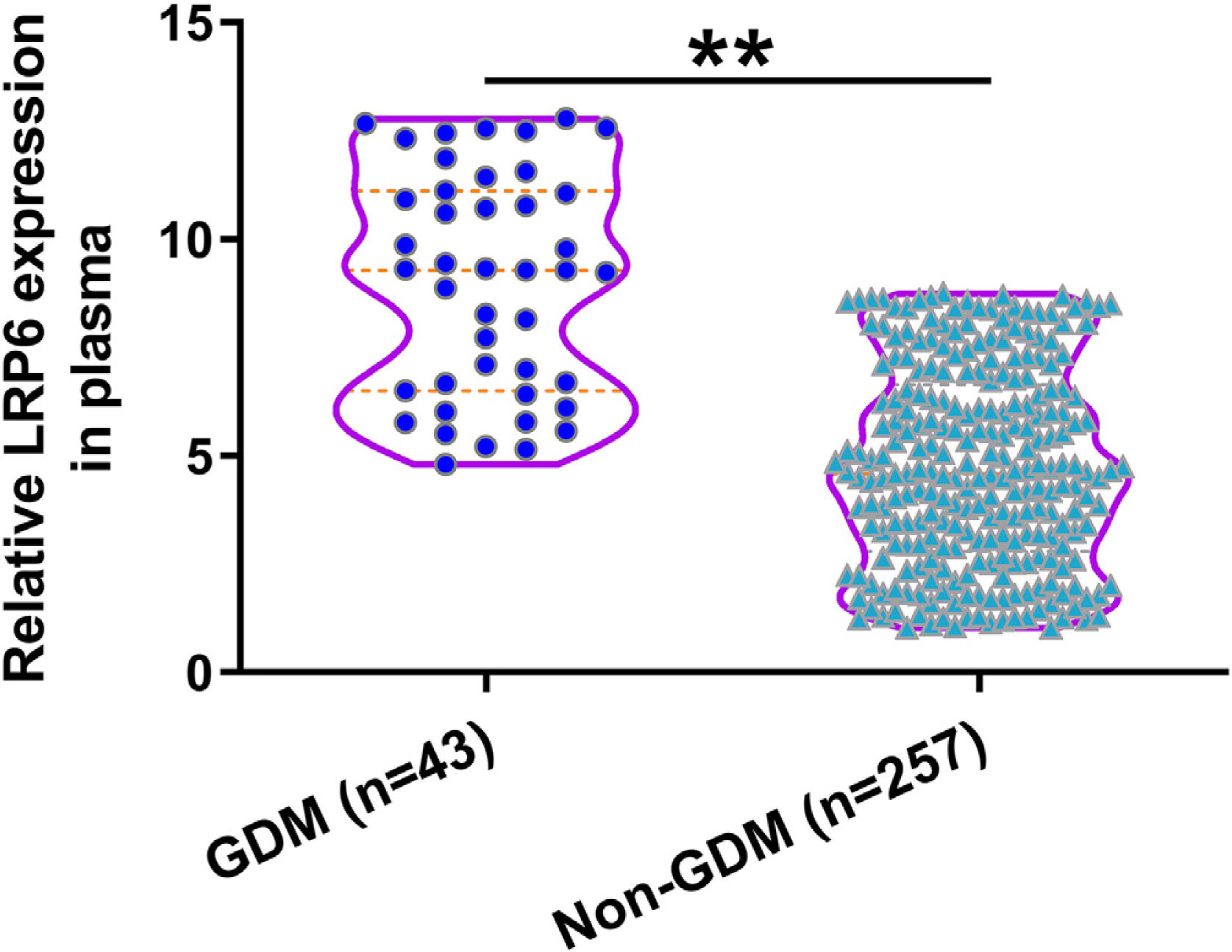
Comparison of plasma LRP6 on the day of admission between GDM and non-GDM groupsDuring follow-up, 43 cases were diagnosed with GDM and were classified into the GDM group. The remaining 257 cases were classified into the non-GDM group. Compared to those who developed GDM during follow-up, participants who did not develop GDM showed lower expression levels of LRP6 in plasma (Fig. 2, P<0.01). Pearson's correlation coefficient analysis showed that plasma expression levels of LRP6 were positively correlated with plasma levels of glucose on the day of admission prior to pregnancy (r=0.2454, P<0.01, data not shown).
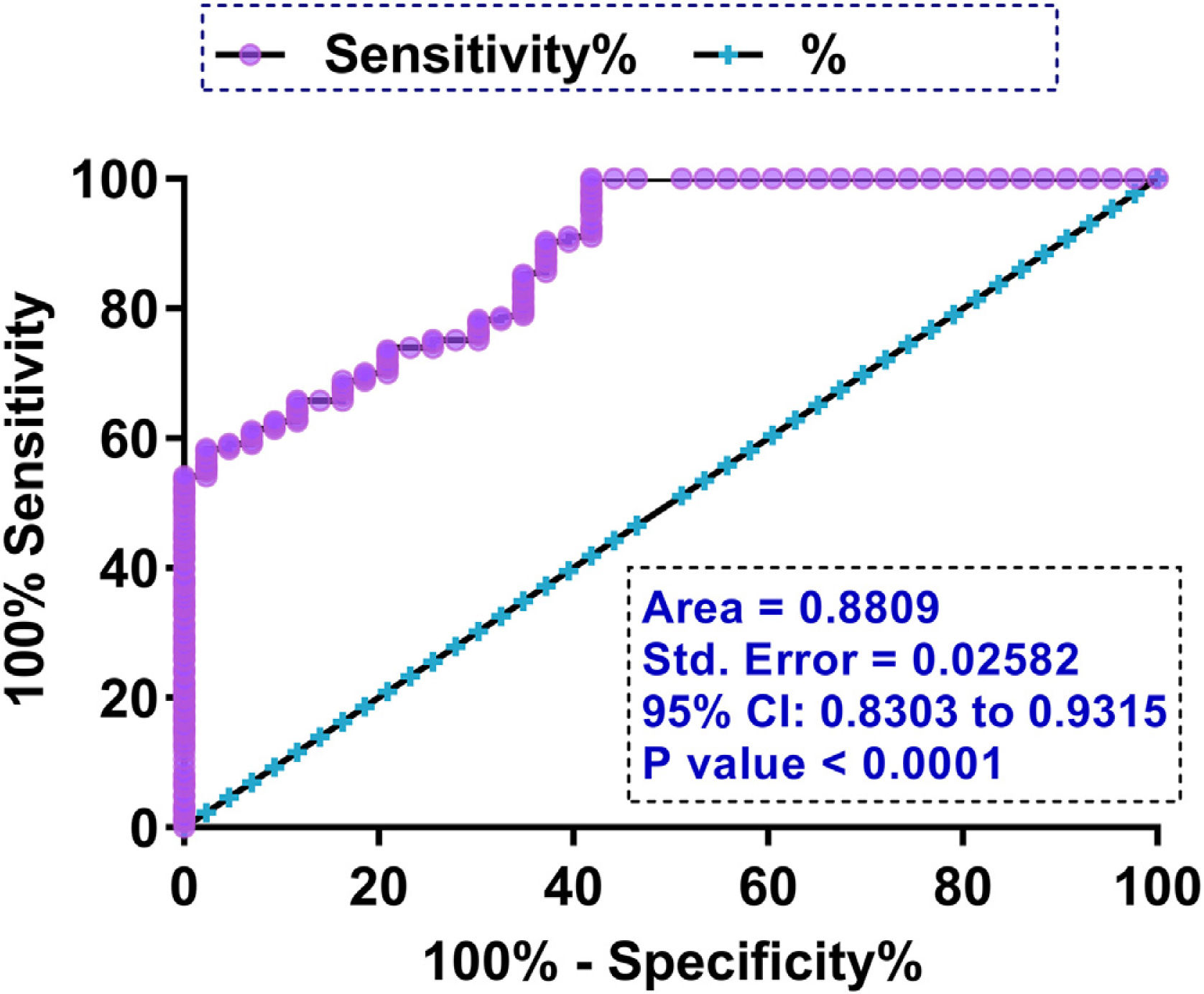
The prediction value of plasma LRP6 on the day of admission for GDMTo explore the role of plasma LRP6 on the day of admission in predicting GDM, ROC curve analysis was performed with the 43 cases of GDM and 257 cases of non-GDM as true positive and negative cases, respectively. Through ROC curve analysis using high levels of LRP6 on the day of admission as a biomarker, potential GDM patients were effectively distinguished from other participants (Fig. 3, P<0.0001, AUC=0.8809).
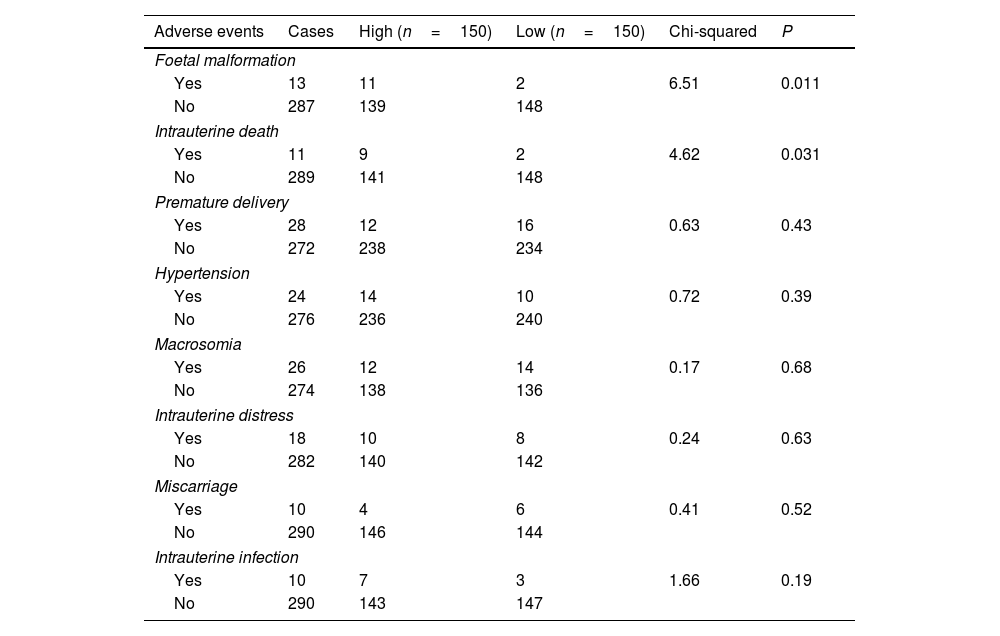
Associations between plasma LRP6 and participants’ adverse eventsThe Chi-square test was performed to analyse the associations between plasma LRP6 and participants’ adverse events. Interestingly, LRP6 was likely associated with foetal malformation and intrauterine death (Table 1, P<0.05), but not premature delivery, hypertension, macrosomia, intrauterine distress, miscarriage and intrauterine infection.
Associations between plasma LRP6 and participants’ adverse events.
| Adverse events | Cases | High (n=150) | Low (n=150) | Chi-squared | P |
|---|---|---|---|---|---|
| Foetal malformation | |||||
| Yes | 13 | 11 | 2 | 6.51 | 0.011 |
| No | 287 | 139 | 148 | ||
| Intrauterine death | |||||
| Yes | 11 | 9 | 2 | 4.62 | 0.031 |
| No | 289 | 141 | 148 | ||
| Premature delivery | |||||
| Yes | 28 | 12 | 16 | 0.63 | 0.43 |
| No | 272 | 238 | 234 | ||
| Hypertension | |||||
| Yes | 24 | 14 | 10 | 0.72 | 0.39 |
| No | 276 | 236 | 240 | ||
| Macrosomia | |||||
| Yes | 26 | 12 | 14 | 0.17 | 0.68 |
| No | 274 | 138 | 136 | ||
| Intrauterine distress | |||||
| Yes | 18 | 10 | 8 | 0.24 | 0.63 |
| No | 282 | 140 | 142 | ||
| Miscarriage | |||||
| Yes | 10 | 4 | 6 | 0.41 | 0.52 |
| No | 290 | 146 | 144 | ||
| Intrauterine infection | |||||
| Yes | 10 | 7 | 3 | 1.66 | 0.19 |
| No | 290 | 143 | 147 | ||
The early detection of GDM is a challenge in clinical practice for the prevention of GDM-related clinical disorders. The present study detected the expression of LRP6 in GDM and evaluated the potential value of LRP6 in the prediction of GDM and its related complications. We showed that analysis of plasma circulating LRP6 prior to pregnancy may aid in the prediction of GDM during pregnancy and pregnancy-related adverse events.
Through regulating the miR-545-3p/HMGA1 axis, LRP6 promotes vascular smooth muscle cell migration and proliferation under high glucose conditions.16 Therefore, it is reasonable to speculate its involvement in diabetes. Interestingly, the expression of LRP6 in diabetic patients and its role in diabetes have not been elucidated. The present study revealed increased expression levels of LRP6 on the day of admission in women who got pregnant months later. Therefore, increased expression levels of LRP6 may trigger GDM during pregnancy, and upregulation of LRP6 is unlikely a consequence of GDM. However, the role of LRP6 in GDM remains something to be explored through functional and mechanistic studies.
Although multiple biomarkers, such as sex hormone globulin, adiponectin, C-reactive and glycosylated fibronectin, have been developed to detect GDM at early stages,11,12 diagnostic sensitivity and specificity are usually low and therefore more effective biomarkers are needed. In this study we showed that high expression levels of LRP6 on the day of admission effectively distinguished potential GDM patients from other participants, and GDM-free curve analysis also showed that participants in the high LRP6 level group experienced a higher incidence of GDM during follow-up (33/150) compared to those in the low LRP6 level group (10/150). Therefore, analysis of LRP6 expression prior to pregnancy may be applied to improve the identification of women with high risk of GDM, thereby applying preventative approaches to prevent the occurrence of GDM.
Interestingly, altered plasma expression of LRP6 was only closely associated with foetal malformation and intrauterine death, but not other adverse events, while in all cases, more than half of the adverse events developed in GDM patients. Therefore, our study further confirmed the fact that GDM is a major cause of adverse events in pregnancy. LRP6 may promote foetal malformation and intrauterine death during pregnancy by increasing the risk of GDM. However, malformations may occur in the first trimester. Therefore, associations between LRP6, malformations and GDM should be further analysed. In addition, future studies may also focus on the role of LRP6 in foetal malformation and intrauterine death.
In conclusion, LRP6 is upregulated in GDM and has potential values for the prediction of GDM and pregnancy-related complications.
Informed consentInformed consent was obtained from all individuals included in this study.
Ethical approvalThis study was approved by Ethics Committee of Huai’an Maternity and Child Health Hospital. The study followed the tenets of the Declaration of Helsinki.
Author contributionsHH, QL, HZ: study concepts, study design, literature research, experimental studies, manuscript preparation and editing; HH, QL, JC: definition of intellectual content, literature research experimental studies, manuscript preparation and editing. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
FundingNot applicable.
Conflict of interestAuthors state no conflict of interest.
Not applicable.