Therapeutic inertia (TI) is the lack of initiation or intensification of treatment when indicated. It contributes to the fact that more than a third of people with type 2 diabetes mellitus (T2D) do not have adequate metabolic control. We set out to analyze the impact of TI during 4 years of follow-up in a cohort of T2D and its possible variables.
Materials and methodsProspective cohort study of a cohort of 297 TD2 patients. We considered TI when treatment was not modified during the 4 years, despite poor control. We contemplate uncontrolled those that did not meet their individualized HbA1c target.
ResultsUncontrolled patients: 87; age: 62.2 ± 9.2; 58.7% men. We consider TI in 41.6% of the patients. Average HbA1c 8.22% in patients with treatment intensification of which 43.1% achieved their HbA1c goal, 29.8% were on monotherapy at the beginning, 29.8% double, 36.2% triple and 2,1% in quadruple therapy. There was more change in treatment in people with obesity (67.6 vs. 34.6%; P < 0.01) and the 6 of the study patients with cardiovascular events (P < 0.05). Metformin was part of the treatment in 97.1% of IT cases (vs. 76.6%; P < 0.01). Achievement of the HbA1c target was higher in patients receiving iSGLT2 (0 vs. 68.4%; P < 0.001).
ConclusionsIn 2 out of 5 uncontrolled T2D patients, the treatment was not changed; this was more evident in those patients treated with metformin. Patients with obesity and presence of cardiovascular events seem to protect against IT. Those who were on iSGLT2 have an advantage in meeting their HbA1c target.
La inercia terapéutica (IT) es la falta de inicio o de intensificación del tratamiento cuando está indicado; contribuye a que más de un tercio de las personas con diabetes de tipo 2 (DM2) no tenga un adecuado control metabólico. Nos planteamos analizar el impacto de la IT durante 4 años de seguimiento en una cohorte de DM2 y sus posibles variables asociadas.
Materiales y métodosEstudio de cohortes prospectivo de una cohorte de 297 pacientes con DM2. Consideramos IT cuando no se modificó el tratamiento durante los 4 años, a pesar del mal control. Clasificamos no controlados a aquellos que no cumplían su objetivo individualizado de HbA1c.
ResultadosPacientes no controlados: 87, con una edad de 62,2 ± 9,2; el 58,7% eran hombres. Consideramos IT en el 41,6% de los pacientes. La HbA1c media fue de 8,22% en pacientes con intensificación de tratamiento, de los cuales el 43,1% consiguieron su objetivo de HbA1c; el 29,8% estaban al inicio en monoterapia, el 29,8% en doble, el 36,2% en triple y el 2,1% en cuádruple terapia. Hubo más cambios de tratamiento en pacientes con obesidad (67,6 vs. 34,6; p < 0,01) y en los 6 pacientes con episodios cardiovasculares (p < 0,05). La metformina formaba parte del tratamiento en el 97,1% de los casos de IT (vs. 76,6%; p < 0.01). La consecución del objetivo de HbA1c fue mayor en los pacientes en tratamiento con iSGLT2 (0 vs. 68,4%; p < 0,001).
ConclusionesEn 2 de cada 5 pacientes con DM2 no controlados no se cambió el tratamiento; esto fue más evidente en pacientes tratados con metformina. La obesidad y presentar un episodio cardiovascular protegen frente a IT. Los pacientes en tratamiento con iSGLT2 tienen la ventaja de cumplir su objetivo de HbA1c.
Good control of blood glucose and cardiovascular risk factors in people with type 2 diabetes mellitus (T2DM) is linked to a decrease in macrovascular and microvascular complications.1
For a few years, the main clinical practice guidelines have recommended personalised metabolic targets depending on patient characteristics and time since onset of T2DM: glycosylated haemoglobin (HbA1c) under 7% in the majority, stricter (<6.5%) in select individuals with no risk of hypoglycaemia and less strict (up to 8%) in patients with a history of severe hypoglycaemia, reduced life expectancy or advanced microvascular or macrovascular complications.2
However, the degree of control of T2DM is very far from desirable, despite the availability of numerous antidiabetic drugs. Some studies have indicated that at least one out of every three patients does not achieve their personalised HbA1c target. Special mention must be made of patients with obesity and T2DM, who account for more than half of patients with diabetes and usually have worse blood glucose control.3,4
The reasons for not achieving suitable control are many and complex. They include so-called therapeutic inertia (TI), which is defined as an unexplained delay in starting or intensifying treatment in patients who, according to the guidelines, do not achieve the established control targets. Some studies have estimated that professionals usually take one to three years to intensify treatment and up to six to eight years to start insulin therapy in patients with uncontrolled diabetes.3,5–8
TI largely depends on the professional (specialisation, years of experience, degree of training, clinical interviewing skills, type of work contract, etc.) and on the patient (age, socioeconomic status, knowledge of their disease and its management, fear of adverse drug effects, rejection of medication for injection, lack of treatment adherence, etc.). The characteristics of the healthcare system also influence TI (public or private, limitations on drug prescription such as negative incentives and bureaucratic obstacles to their funding, etc.).3,5,6,9
The objective of this study was to evaluate the prevalence of TI, during four years of follow-up, in a cohort of patients with T2DM who did not previously reach their personalised HbA1c target. It also sought to study which factors might be linked to TI or treatment intensification, as well as which antidiabetic agents were used in this intensification. Finally, it examined the impact of treatment intensification and analysed which factors were associated with achievement of HbA1c targets.
Material and methodsWe conducted a prospective, longitudinal, fixed-cohort study based on a prior descriptive study,10 with the objective of assessing degree of blood glucose control in patients with T2DM in our health area. This included two clinics serving an urban population of 18,481 people over 18 years of age. The study was approved by the Comité de Ética Asistencial Metropolitano [Metropolitan Healthcare Ethics Committee] and complied with the ethical requirements expressed in the Declaration of Helsinki and its subsequent amendments, as well as the Spanish data protection law.
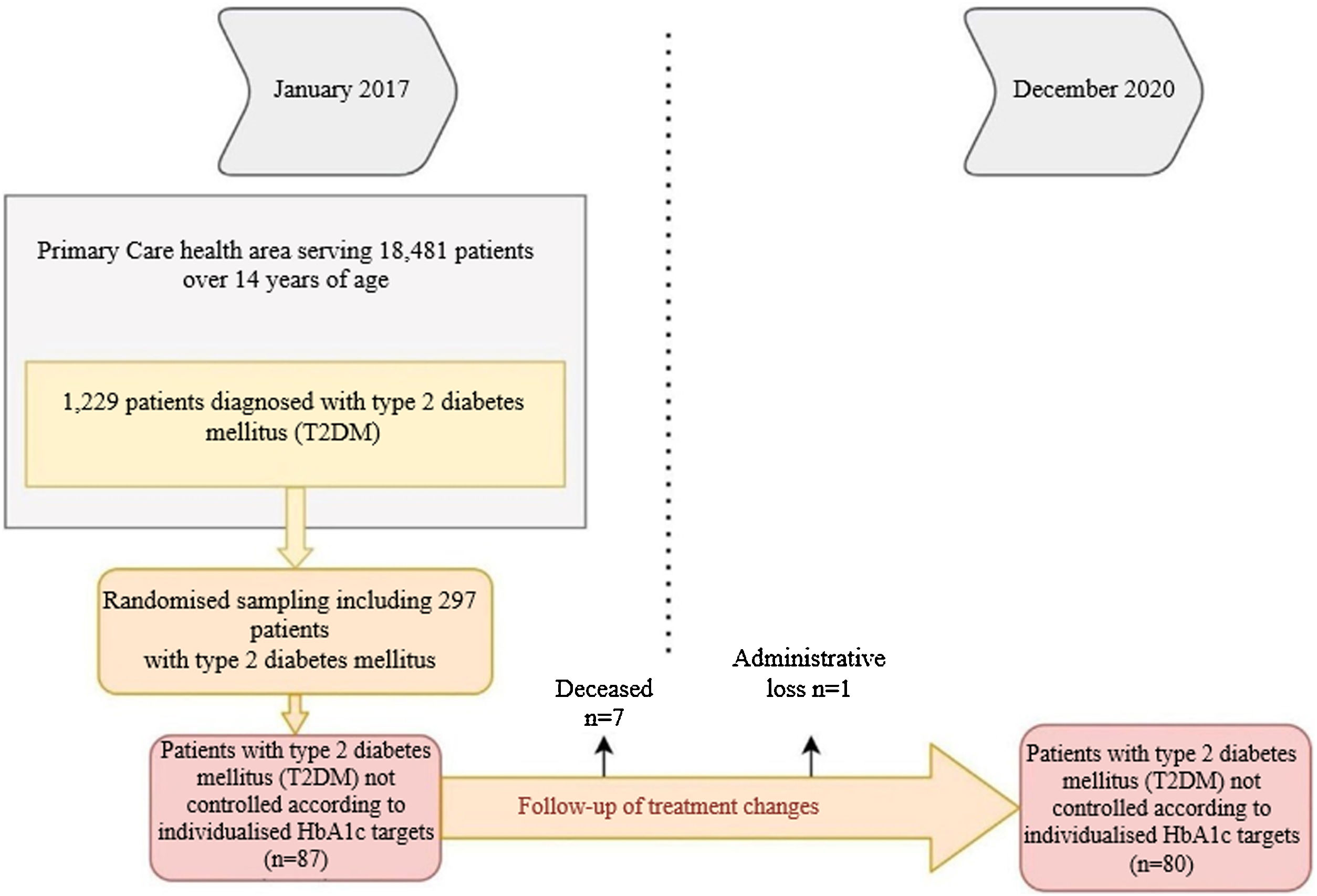
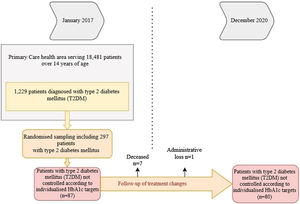
We considered data for patients with T2DM recorded in January 2017 who were reassessed in December 2020, 48 months later. Of the 1229 patients with T2DM, 297 were randomised (95% confidence interval; margin of error <5%), and we selected those who were uncontrolled according to personalised HbA1c targets2 at the start of the study (n = 87). Deaths (n = 6) and losses to follow-up (n = 1) were excluded. In the remaining 80, we analysed whether there had been any changes in their treatment during the four years of follow-up (Fig. 1).
The study variables collected in the second slice of the electronic record were: demographic data, HbA1c, body mass index (BMI), glomerular filtration rate (GFR), time since diabetes onset and prescription of antidiabetic drugs. To calculate GFR, we used the MDRD/CKD-EPI equation, defining chronic kidney failure as GFR < 60 ml/min/1.73 m2. Patients were considered to be obese if they had a BMI ≥ 30 kg/m2, and to have a cardiovascular episode if they had a myocardial infarction or stroke documented in their medical record.
We divided patients with out-of-range HbA1c into two groups: the first included those with any treatment changes during follow-up, and the second included those with no treatment changes (this was considered the TI group).
A case report form was prepared and volunteer physicians were trained. In addition to the variables from the prior study,10 we assessed drug treatment and current degree of metabolic control.
We recorded quantitative variables with their mean, standard deviation (SD) and range (minimum-maximum), and qualitative variables with the numbers of patients and frequencies. To compare quantitative variables, we used Student’s t test after confirming its applicability with the Lilliefors test for normality and Levene’s test for equality of variances. To analyse independent qualitative variables, we used the χ2 test and Fisher’s exact test. For dependent qualitative variables, on the other hand, we used McNemar’s test. We used the R statistics software package (R Foundation for Statistical Computing, Vienna, Austria), specifically Rcmdr 4.0.3. For all hypothesis comparisons, an alpha (α) risk of 0.05 was set.
ResultsTable 1 shows the baseline demographic and clinical data for the people with T2DM at the start of the study. It should be noted that the mean age of the patients studied was 62.2 ± 9.2 years (range: 37−83). Some 40% were ≥65 years of age, with a predominance of men (58.7%). The mean time since diabetes onset was 9.9 ± 5.3 years. During the four years of follow-up, 7.6% of patients had a cardiovascular episode and 3.8% had at least one admission for heart failure. It should be noted that not all patients had records for all study variables; weight was notably under-recorded (21.25%).
Variables linked to treatment intensification.
| Factor | Overall n = 80 | Group with treatment changes n = 33 (41.25%) | Group in which treatment was intensified n = 47 (58.7%) | p |
|---|---|---|---|---|
| Sociodemographic data | ||||
| Age in years, mean ± SDa(minimum-maximum) | 62.2 ± 9.2 (37−83) | 62.9 ± 8.2 (48−83) | 61.7 ± 9.9 (37−82) | 0.68 |
| Age groups, n (%) | ||||
| <65 years | 32 (60) | 20 (60.6) | 28 (59.6) | 0.92 |
| ≥65 years | 48 (40) | 13 (39.4) | 19 (40.4) | |
| Sex, n (%) | ||||
| Male | 47 (58.7) | 19 (57.6) | 28 (59.6) | 0.85 |
| Female | 33 (41.2) | 14 (42.4) | 19 (40.4) | |
| Clinical conditions | ||||
| Years since diabetes onset ± SD | 9.9 ± 5.3 | 10.7 ± 5.5 | 9.3 ± 5.1 | 0.25 |
| Prescribing physician with teaching background | ||||
| Yes | 22 (27.5) | 7 (21.2) | 15 (31.9) | 0.29 |
| No | 58 (72.5) | 26 (78.8) | 32 (68.1) | |
| Uncontrolled hypertension, n (%) | 13 (25.5) | 6 (27.3) | 7 (24.1) | 0.79 |
| HbA1c target | ||||
| <6.5 | 5 (6.25) | 2 (6.1) | 3 (6.4) | 0.46 |
| <7 | 52 (65) | 22 (66.7) | 30 (63.8) | |
| <8 | 17 (21.3) | 5 (15.2) | 12 (25.5) | |
| <8.5 | 6 (7.5) | 4 (12.1) | 2 (4.3) | |
| Out-of-target LDL, n (%) | 18 (31.6) | 8 (25.8) | 14 (29.8) | 0.70 |
| CKF, n (%) | 15 (19.0) | 6 (18.8) | 9 (19.1) | 0.96 |
| Tobacco use, n (%) | 9 (11.2) | 1 (3) | 8 (17) | 0.07 |
| Obesity, n (%) | 34 (54.0) | 8 (32.0) | 26 (68.4) | <0.01 |
| BMI ± SD kg/m2 | 31.3 ± 5.4 | 29.5 ± 5.9 | 32.3 ± 4.8 | <0.05 |
| Cardiovascular episode | 6 (7.5) | 0 (0) | 6 (12.8) | <0.05 |
| Admission for heart failure | 3 (3.8) | 0 (0) | 3 (6.4) | 0.27 |
| Number of antidiabetic agents at the start of the study | ||||
| 1 | 21 (26.25) | 7 (21.2) | 14 (29.8) | 0.32 |
| 2 | 28 (35) | 16 (48.5) | 12 (25.5) | |
| 3 | 27 (33.75) | 8 (24.2) | 19 (40.4) | |
| 4 | 6 (7.5) | 2 (6.1) | 4 (4.3) | |
| Prior antidiabetic treatment, n (%) | ||||
| Metformin | 68 (85) | 32 (97.1) | 36 (76.6) | <0.01 |
| Sulphonylureas | 26 (32.5) | 9 (27.3) | 17 (36.2) | 0.40 |
| DPP4 inhibitors | 26 (32.5) | 10 (30.3) | 16 (34.0) | 0.72 |
| SGLT2 inhibitors | 9 (11.2) | 3 (9.1) | 6 (12.8) | 0.72 |
| GLP-1 analogues | 6 (7.5) | 2 (3) | 4 (10.6) | 0.39 |
| Insulin | 35 (43.7) | 17 (51.5) | 18 (38.3) | 0.26 |
BMI: body mass index; CKF: chronic kidney failure; DPP4 inhibitors: dipeptidyl peptidase-4 inhibitors; GLP-1 analogues: glucagon-like peptide-1 analogues; HbA1c: glycosylated haemoglobin; HDL: high-density lipoproteins; LDL: low-density lipoproteins; SD: standard deviation; SGLT2 inhibitors: sodium–glucose cotransporter-2 inhibitors.
Of the 80 patients who were uncontrolled at the start of the study, 58.7% (n = 47) had their treatment intensified, whereas 41.3% (n = 33) maintained the same treatment throughout the follow-up period. We found higher numbers of obese patients in the treatment intensification group (67.6% versus 34.6%; p < 0.01; BMI: 32.2 versus 29.9; p < 0.05), with a relative risk (RR) of 0.42 (95% CI: 0.22−0.80). In addition, this group included all patients with a cardiovascular episode (n = 6; 12.8%) (p < 0.05).
Moreover, in the group with no treatment changes, prescription of metformin was higher than in the other group (97.1% versus 76.6%; p < 0.01), with a RR of 5.65 (95% CI: 1.2–26.5) (Table 1). Of all patients with uncontrolled glycosylated haemoglobin (n = 80), 17.5% (n = 14) were being treated with metformin alone. Of these, 42.4% (n = 6) had no treatment changes.
In the group of patients with treatment intensification, we found no differences between the sexes. The mean age was 1.2 years younger and the time since DM onset was 1.4 years shorter. In addition, prescribers with a teaching background in the group with treatment changes were more numerous than in the group with no treatment changes. We found no significant differences in any of these variables (p > 0.05).
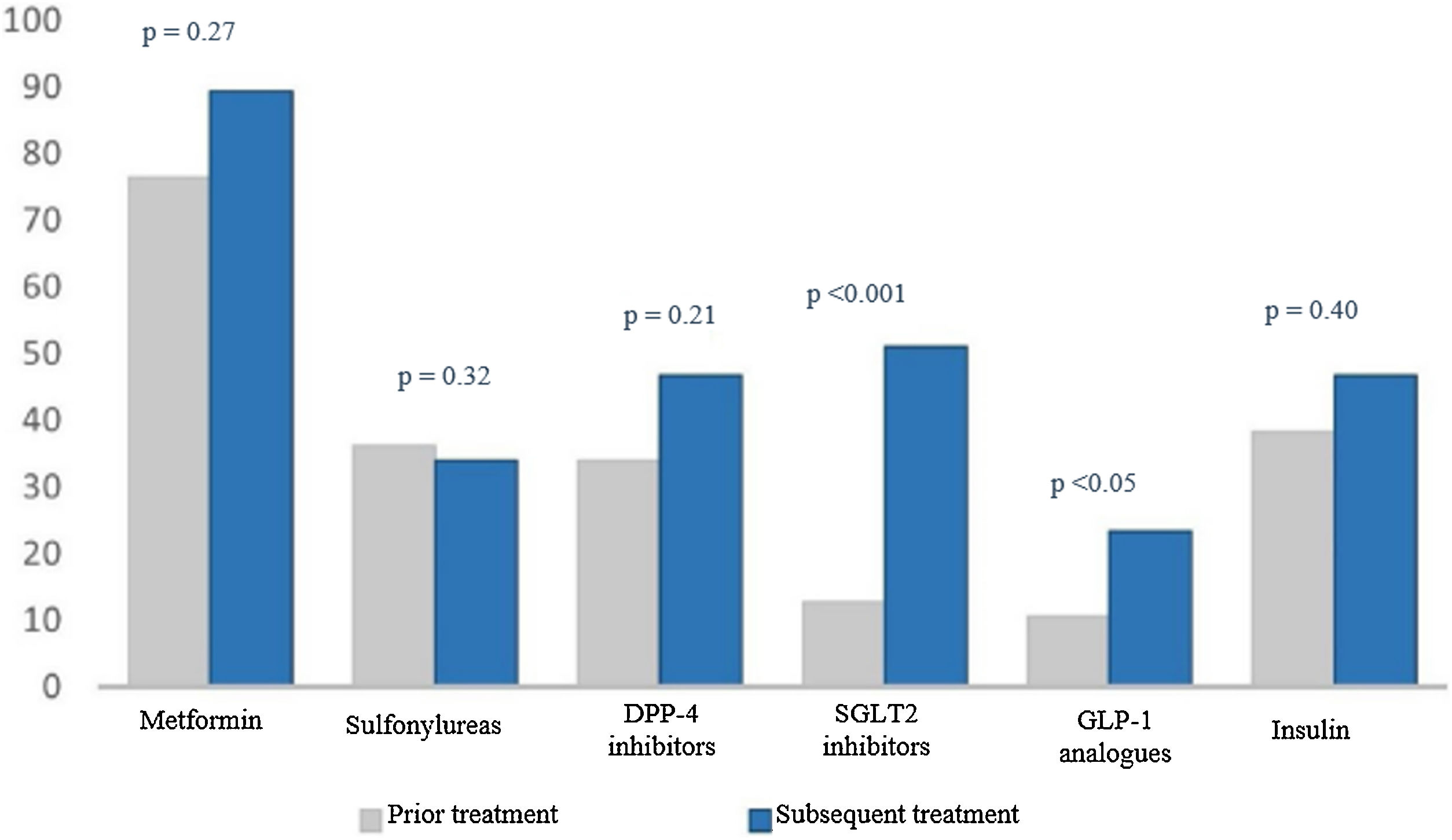
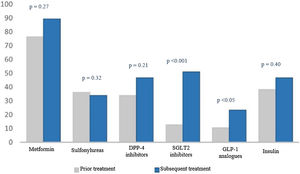
When we analysed treatment changes during the four years of follow-up in the intensification group, we found a significant increase in the prescribing of two specific drug groups: sodium-glucose co-transporter-2 (SGLT2) inhibitors (9.1% versus 51.1%; p < 0.001) and glucagon-like peptide-1 (GLP-1) analogues (3% versus 23.4%; p < 0.05) (Fig. 2).
Percentage of drugs prescribed at the start and end of the study.
The numerical value (as a percentage) of prescribed antidiabetic drugs is represented on the Y axis. The different drug groups at the start and end of the study are represented on the X axis.
DPP4 inhibitors: dipeptidyl peptidase-4 inhibitors; GLP-1 analogues: glucagon-like peptide-1 analogues; SGLT2 inhibitors: sodium–glucose cotransporter-2 inhibitors.
To evaluate the impact of intensification (Table 2), the group without treatment changes was compared to the group with treatment intensification, and it was found that the degree to which targets were achieved was not very different between them: mean HbA1c at the end of the study (8.22 ± 1.8 versus 8.12 ± 1.5; p = 0.79) and reduction in HbA1c during the study (0.54 ± 1.9 versus 0.66 ± 1.5; p = 0.82). The group of patients with greater achievement of targets after intensification were those with a target HbA1c of <8% (8.3% versus 42.1%; p < 0.05) and those being treated with SGLT2 inhibitors (0% versus 68.4%; p < 0.001).
Impact of treatment intensification and achievement of personalised targets.
| Factor | Group without treatment modification n = 33 (41.25%) | Group with treatment intensification n = 47 (58.7%) | p |
|---|---|---|---|
| Impact on HbA1c | |||
| Mean HbA1c at the end of the study ± SD | 8.22 ± 1.8 | 8.12 ± 1.5 | 0.79 |
| Reduction in HbA1c ± SD | 0.54 ± 1.9 | 0.66 ± 1.5 | 0.82 |
| Personalised HbA1c target achieved | 12 (37.5) | 19 (41.3) | 0.73 |
| HbA1c target, n (%) | |||
| <6.5 | 1 (8.3) | 0 (0) | <0.05 |
| <7 | 6 (50) | 10 (52.6) | |
| <8 | 1 (8.3) | 8 (42.1) | |
| <8.5 | 4 (33.3) | 1 (5.3) | |
| Number of antidiabetic agents at the start of the study, n (%) | |||
| 1 | 5 (41.7) | 3 (15.8) | 0.23 |
| 2 | 4 (33.3) | 6 (31.6) | |
| 3 | 2 (16.7) | 9 (47.4) | |
| 4 | 1 (8.3) | 1 (5.3) | |
| Antidiabetic treatment at the end of the study, n (%) | |||
| Metformin | 11 (91.7) | 16 (84.2) | 0.49 |
| Sulphonylureas | 2 (16.7) | 6 (31.6) | 0.35 |
| DPP4 inhibitors | 6 (50) | 6 (31.6) | 0.30 |
| SGLT2 inhibitors | 0 (0) | 13 (68.4) | <0.001 |
| GLP-1 analogues | 0 (0) | 5 (26.3) | 0.052 |
| Insulin | 4 (33.3) | 10 (52.6) | 0.29 |
DPP4 inhibitors: dipeptidyl peptidase-4 inhibitors; GLP-1 analogues: glucagon-like peptide-1 analogues; HbA1c: glycosylated haemoglobin; SD: standard deviation; SGLT2 inhibitors: sodium–glucose cotransporter-2 inhibitors.
TI has a major impact on blood glucose control in T2DM; consistent with other studies, we saw that it had repercussions for more than 40% of patients.3 In addition, in our case, it was maintained for four consecutive years, unlike other publications that analysed it for only 12 months.3,11 In Europe, the GUIDANCE12 and PANORAMA13 studies found that just 53.6% and 62.6% of patients achieved a HbA1c ≤7% (in our study, 53.64%).10 The delay in intensifying treatment had direct repercussions on the onset of cardiovascular diseases and on the increased mortality in these patients.14
We found that 42.8% of patients being treated with metformin alone did not have their treatment changed. On this point, there are data linking metformin to TI 5.65 times higher than in patients not prescribed metformin.11,15 The clinical practice guidelines recommend intensifying treatment if out-of-target HbA1c levels persist after three months of taking metformin.16 However, TI in this patient group prolonged this period and, therefore, pushed back achievement of good control. This finding could indicate that professionals know, use and prescribe metformin safely, but face certain barriers and limitations in the use and prescription of other antidiabetic drugs.9
SGLT2 inhibitors were the most commonly used drugs when intensifying treatments in patients with uncontrolled T2DM. This could be due, among other reasons, to their ease of use as they are administered orally (unlike GLP-1 analogues and insulin analogues, which are injected and require prior learning), and their safety — specifically their low rate of hypoglycaemia17 (in contrast to sulphonylureas, which, for example, were not used in treatment intensification in our patients).
Other very commonly used drugs were GLP-1 analogues, which, along with SGLT2 inhibitors, have seen their use increase in recent years. Both have proven safe for use in patients with high cardiovascular and renal risk, as they reduce episodes and complications associated with T2DM.17–19 These data could have promoted a “fashion effect” and fostered an increase in their prescription among professionals. According to data provided by our health district, in the province of Granada during the four years of follow-up studied, prescription of GLP-1 analogues doubled and prescription of SGLT2 inhibitors quadrupled.
Obese patients with T2DM showed a lower rate of TI. Specifically, they had a 58% lower risk of TI than non-obese patients. The use of SGLT2 inhibitors and GLP-1 analogues motivated treatment intensification in this patient group, since both showed special benefits in patients with T2DM with an elevated BMI as they not only reduced the development of cardiovascular complications but also promoted weight loss.17,20
We did not detect TI in patients with a cardiovascular episode. All patients with T2DM admitted to hospital for a major cardiovascular episode benefited from intensification of their antidiabetic treatment on discharge. González-Clemente et al.21 obtained similar results and concluded that patients in primary prevention were undertreated and had worse control than patients in secondary prevention.
Patients with T2DM and a less strict target HbA1c level (<8.5%) seem to show a certain trend towards TI. In another study conducted in Spain, 18.1% of patients with HbA1c >8% were in TI, with no changes to their treatment for more than four years.15 This could be due to a conservative attitude on the part of the professional when treating a fragile patient, which would lead to maintaining very high HbA1c levels, even above 9%.11,22 It should be remembered that such high figures not only increase chronic complications but could also promote the development of acute and severe complications, such as ketoacidosis, hyperosmolar coma and the possibility of suffering from serious infections, including those associated with SARS-CoV-2.23,24
Endocrinologists appear to have lower rates of TI than primary care physicians, since they usually take more drastic measures when intensifying treatment in patients with T2DM.15,25,26 Although our results indicated lower TI in primary care professionals with a teaching background (resident tutors), we found no prior studies analysing this variable. One possible explanation could be that these professionals tend to meet a number of specific requirements, such as participating in training, refresher and quality-improvement activities, which promotes stricter adherence to clinical practice guideline recommendations in terms of treatment intensification in uncontrolled patients.27
Patients who had their treatment intensified had better HbA1c levels and achieved their targets to a greater degree (41.4% versus 37.5%; p = 0.73) than those affected by TI (although statistical significance was not attained). Specifically, patients with a less strict target (HbA1c < 8%) and patients treated with SGLT2 inhibitors were those who most often successfully achieved their target HbA1c after this intervention.
One of the main limitations of our study was its small sample size, which probably prevented other statistically significant results from being obtained. A lack of documentation, perhaps another form of inertia, was an important determining factor to take into account (for example, one out of every five patients did not have their weight documented). Another limitation that we would like to point out is that we did not know how many patients were treated with the maximum dose of each drug and at what point during follow-up this dose was reached, since we could not confirm this data as treatment intensification. According to our study, more than half of patients in whom treatment was not intensified received insulin therapy (51.3% versus 38.3%; p = 0.28). Had our study analysed an increase in insulin dose or addition of a rapid-acting insulin regimen to basal therapy and included it as treatment intensification, it probably would have yielded different results (for this reason, we could have considered excluding this patient group from our study). The study's small sample size precluded making adjustments for other variables, such as chronic kidney failure, and analysing them properly, considering that this condition hinders the addition of other existing treatment options.
A distinguishing feature of our study was that it considered blood glucose control based on personalised target HbA1c, unlike most of the studies on T2DM reviewed, which indicated a single target under 7%.3 We also attempted to avoid other types of bias that might have been introduced had the professionals felt watched.
It could be interesting, as a continuation of this work, to study and analyse the impact of a lack of treatment adherence on TI, as it is one of the fundamental causes of a lack of treatment intensification. Research could be conducted on how restrictions in health systems influence new antidiabetic drugs (negative incentives and bureaucratic obstacles to their prescription, as with GLP-1 analogues, which require a pharmaceutical inspection permit and having a BMI > 30 represents a limitation on their funding). It would also be useful to analyse the impact of TI in patients with T2DM in terms of control of blood pressure and lipid levels, since, according to other studies,3 these data are more significantly affected by TI than those related to blood glucose control.
ConclusionIn summary, two out of every five patients with diabetes without suitable metabolic control maintained the same treatment during the four years of follow-up. Patients in whom treatment was intensified achieved a better degree of control than those with no treatment changes. TI was more evident in patients being treated with metformin, perhaps due to certain barriers and limitations faced by professionals in using and prescribing other antidiabetic drugs. By contrast, obesity and having had a cardiovascular event appeared to have a protective effect on TI. SGLT2 inhibitors and GLP-1 analogues were the most commonly used drugs in treatment intensification. TI appeared to be lower in professionals with a teaching background (resident tutors). Target HbA1c achievement was greater in patients being treated with SGLT2 inhibitors and in patients with T2DM with a target >8%. These results should spark reflection on the importance of TI and the mechanisms to combat it.
Key findings
- •
Two out of every five patients with uncontrolled diabetes suffered from therapeutic inertia.
- •
Obese patients and patients with a past cardiovascular episode had less therapeutic inertia.
- •
Professionals with a teaching background seemed to have less therapeutic inertia.
- •
SGLT2 inhibitors and GLP-1 analogues were the antidiabetic agents most commonly prescribed for treatment intensification.
This study received no external funding.
Conflicts of interestThe authors declare that they have no conflicts of interest in relation to this study.
Authorship/collaborationManuscript concept and design: Abraham Hidalgo Rodríguez, Juan Carlos Aguirre Rodríguez and David Martín Enguix.
Data collection: Juan Carlos Aguirre Rodríguez, David Martín Enguix, Abraham Hidalgo Rodríguez and María Sánchez Cambronero.
Data analysis and interpretation: David Martín Enguix and Juan Carlos Aguirre Rodríguez.
Drafting, review and approval of the manuscript submitted: Juan Carlos Aguirre Rodríguez, David Martín Enguix, Abraham Hidalgo Rodríguez and María Sánchez Cambronero.
To compare changes over time in prescribing for patients in our sample to those in our province, we requested data on prescribing of antidiabetic agents for 2017 and 2020 from the provincial pharmacy unit. These data revealed that during these four years, the established daily dose doubled in GLP-1 analogues and quadrupled in SGLT2 inhibitors.