To analyze the results of the telemedicine screening program for diabetic retinopathy (DR) in patients with type 1 diabetes conducted by the Endocrinology and Nutrition Management Unit of Virgen del Rocío University Hospital.
MethodsThis cross-sectional study comprised patients with type 1 diabetes mellitus (DM) in our DR screening program from January 2018 to November 2020. Fundus photographs are performed by trained nurses and reviewed by a trained endocrinologist. Those suggestive of pathology are sent to ophthalmology through a telematic program for review.
ResultsOf the 995 fundus photographs evaluated, 646 (65.3%) showed no evidence of DR, 327 (33.1%) presented possible DR, and 16 (1.6%) were not gradable. The diagnosis was confirmed in 254 patients after reviewing by ophthalmology, and the screening program achieved a positive predictive value for DR of 77.7%. Seventy-three were excluded by ophthalmology due to the absence of DR (false positive rate – 22.3%). In 92.5% of the cases classified by the ophthalmologist, the degree of DR was mild or very mild.
ConclusionOur telemedicine screening program for DR in patients with type 1 DM is consistent with the literature. Effective screening for DR is performed, with patients diagnosed in the early stages. Telemedicine programs facilitate efficient communication among healthcare personnel.
Analizar los resultados del programa de cribado de retinopatía diabética (RD) en pacientes con diabetes mellitus tipo 1 (DM1) en la Unidad de Gestión de Endocrinología y Nutrición (UGEN) de Hospital Universitario Virgen del Rocío.
Material y métodosEstudio transversal de pacientes incluidos en programa de cribado de retinopatía diabética con DM1, desde enero del 2018 hasta noviembre del 2020. Las retinografías se realizan por parte de enfermería y se revisan por un endocrinólogo entrenado, aquellas indicativas de enfermedad se envían a Oftalmología por medio de un programa telemático para su revisión.
ResultadosSe valoraron 995 retinografías, 646 casos (65.3%) no presentaron datos de RD, 327 (33,1%) presentaron una posible RD y 16 (1,6%) fueron no valorables. Tras revisión por Oftalmología se confirmó el diagnóstico en 254, alcanzando el programa de cribado un valor predictivo positivo para RD del 77,7%. 73 fueron desestimadas por parte de Oftalmología, por ausencia de retinopatía diabética (tasa de falsos positivos, 22,3%). El 92,5% de los casos catalogados por el oftalmólogo como RD fueron de grado leve o muy leve.
ConclusiónEl programa de cribado de RD en DM1 es concordante con la literatura. Se realiza un cribado eficaz de retinopatía diabética, siendo diagnosticados los pacientes en fases tempranas. Los programas de telemedicina permiten una comunicación eficaz entre personal sanitario.
Diabetic retinopathy (DR) is a heterogeneous disease arising from microangiopathic changes in the retina. Alterations in the small retinal vessels cause it. Increased vascular permeability leads to lipid exudation, hemorrhages, and proliferation of neovessels. DR develops over time in individuals with diabetes mellitus (DM), progressing from milder stages, such as non-proliferative DR, to vision-threatening stages, such as proliferative DR and diabetic macular edema.1–3
DR is the most common and specific microvascular complication of DM, reaching a prevalence of 30% in patients with DM. It has a major impact on health and social care as the leading preventable cause of blindness in the working-age population. This prevalence is not constant and increases with the duration of DM and the patient's age.1 Hyperglycemia, high blood pressure, microalbuminuria, and pregnancy are also risk factors for the development of DR.
DR is a condition that meets the basic criteria for a screening program: large volume of potentially affected individuals, the potential severity of the clinical presentation, the natural course can be predicted and reversed if associated risk factors are controlled, and effective treatment is available.3,4
A high burden of care is involved in screening programs such as those for DR. Even with sufficient numbers of ophthalmologists, reliance on them for DR screening has proven to be an inefficient use of resources. This, together with improved communication technologies, has led to the implementation of telemedicine systems. These telemedicine systems are already being developed in Spain with good results.5 These programs involve obtaining images (retinal fundus photographs) which are then assessed by trained personnel who are not necessarily ophthalmologists.4,6 In addition, new artificial intelligence systems have appeared, allowing adequate screening, with similar results to manual grading.7
Given the prevalence and morbidity associated with DR, this study's aim was to analyze the results of our DR telemedicine screening program in patients with type 1 DM being followed in specialized consultation in the Endocrinology and Nutrition Management Unit (ENMU) of Virgen del Rocío University Hospital.
Material and methodsThis cross-sectional study involved patients with type 1 DM participating in the DR screening program of the ENMU at Virgen del Rocío University Hospital from January 2018 to November 2020. The study was conducted in accordance with the tenets of the Declaration of Helsinki. All patients meeting the criteria for the ENMU screening program were included in the study. These patients had a confirmed diagnosis of type 1 DM (including types 1B and the LADA subtype), with a duration of diabetes greater than 5 years, and at least one previous normal complete ophthalmic evaluation in the Ophthalmology Unit. Patients with other types of diabetes were excluded: DM2, secondary diabetes (pancreatectomy, chronic pancreatitis, post-transplant), diabetes related to other diseases such as cystic fibrosis, or diabetes associated with genetic disorders. Also, women with type 1 DM in pregnancy planning, pregnant women, and women in postpartum follow-up were excluded.
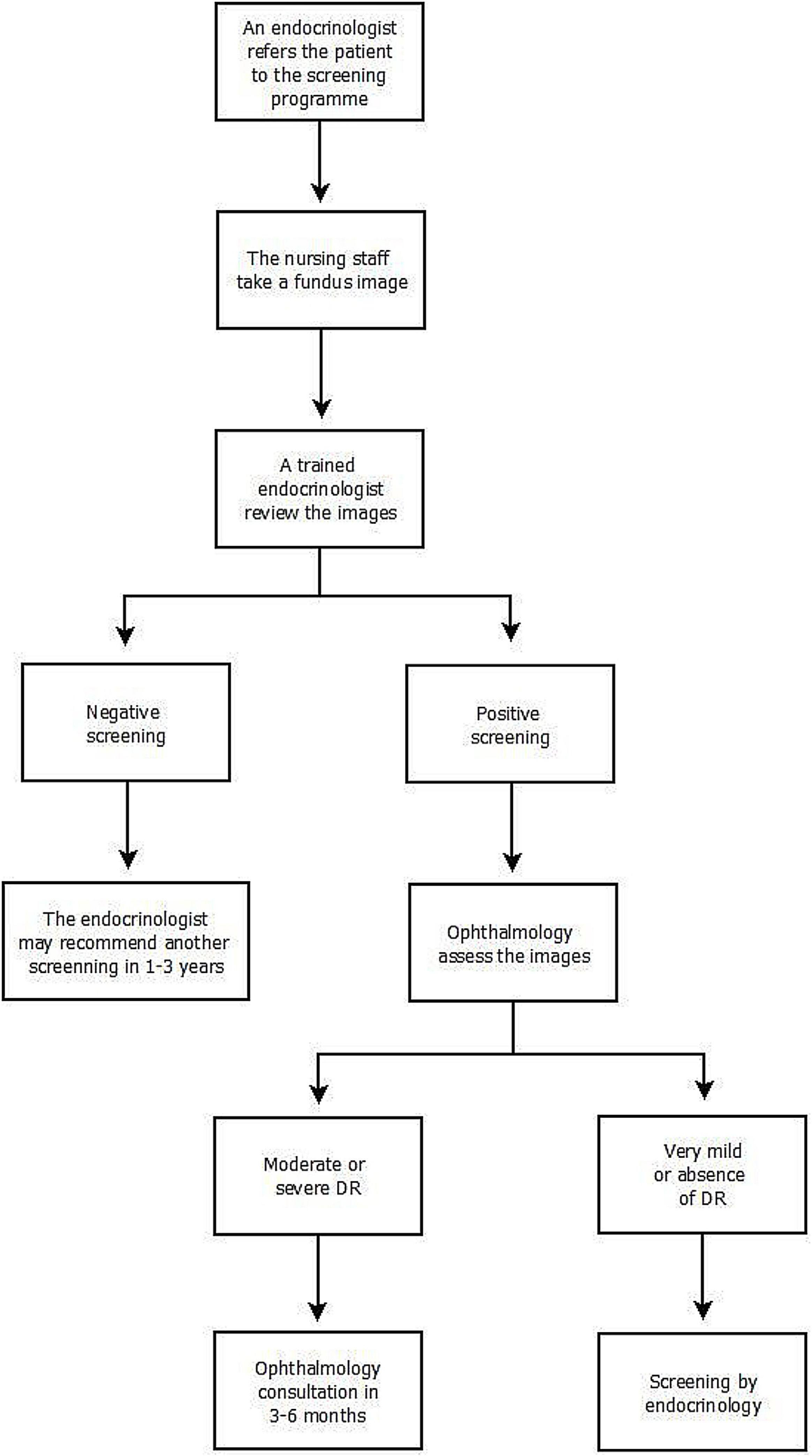
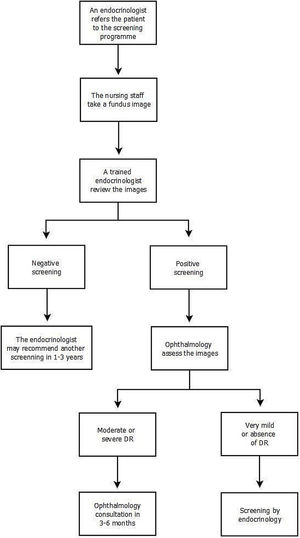
The screening program implemented in our Unit is structured so that patients who meet inclusion criteria are referred to the program by their endocrinologist. Fundus images are taken by the nursing staff using a Topcon TRC-NW200 non-mydriatic digital fundus camera in a dark room. Mydriatic drops are administered to the patient beforehand. Three images are taken of each eye, exploring the central, nasal, and temporal areas. The images are transmitted to the Comprehensive Diabetes Plan (CDP) program.
Subsequently, the retinal images are reviewed within 30 days by an endocrinologist from the ENMU trained in DR and screening for DR through the CDP program. The physician evaluates the presence of alterations compatible with DR lesions (describing the presence of microaneurysms, intraretinal hemorrhages, venous beading, retinal intravascular anomalies, and retinal neovessels) and those lesions that, without being characteristic of DR, may be considered pathological. The evaluating physician reviews the images. If the screening is negative a standardized report is issued describing the absence of DR, which is recorded in the patient's digital history. Additional retinal imaging depends on the overall assessment of the patient's characteristics. In accordance with the guidelines of the “Plan Integral de Diabetes de Andalucía” and the guide of the American Diabetes Association. Variables such as metabolic control, the time of evolution of diabetes or the existence of other cardiovascular risk factors are considered. The endocrinologist may recommend another screening in 1–3 years.
Should the screening be positive (presence of potential DR lesions or other abnormalities), the physician indicates the presence of possible DR in the platform, and the images are automatically sent to ophthalmology for further review. Once ophthalmology has assessed the images, the final diagnosis is made. The retinal images are classified according to the scale of “Global Diabetic Retinopathy project group” (Table 1). In the case of moderate–severe DR, the patient is automatically scheduled for an ophthalmology consultation in 3–6 months in accordance with the degree of DR. In the presence of very mild DR or the absence of DR, the patient is referred back to the screening program, specifying when the patient should be examined. The screening will continue to be performed by the endocrinologist (Fig. 1).
Of the 989 patients included in the study, 53.8% (532) were men. The mean age was 38.5±13.5 years. The median HbA1c was 7.8% ([Interquartile range] IQR 7.1–8.5). The median duration of diabetes was 16 years (RIQ 11–23).
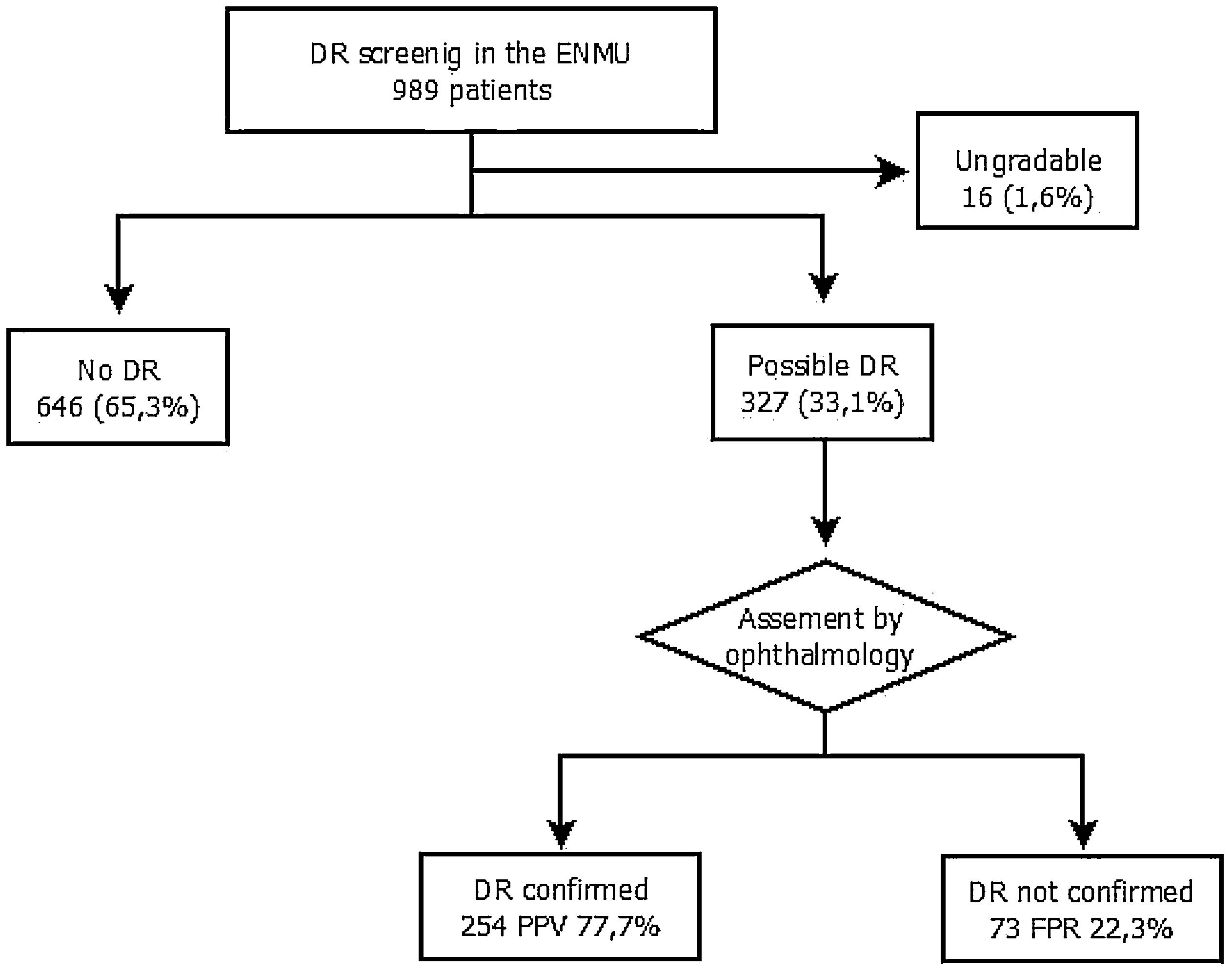
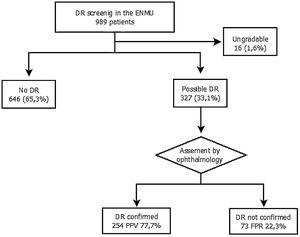
The assigned ENMU team assessed a total of 989 retinal photographs: 646 cases (65.3%) presented no evidence of DR, 327 (33.1%) presented possible DR, and 16 (1.6%) were ungradable.
After review by the Ophthalmology Unit, 73 cases were excluded due to the absence of DR (false positive rate – 22.3%) (Fig. 2).
Of the cases classified by the ophthalmologist as DR, 92.5% were mild or very mild. These patients remained in the screening program administered by the ENMU and were referred for follow-up retinal photographs within one year. Moderate DR was found in 3.5% of the patients, and 4.9% had already undergone photocoagulation and had therefore been referred erroneously to the screening program. Both groups were automatically referred for ophthalmology consultation.
DiscussionProper screening for diabetic retinopathy is essential to reduce morbidity in patients with diabetes. Our study obtained a positive predictive value (PPV) of 77.7%. The literature describes PPVs ranging from 68% to 75%.8–10 Therefore, our screening program yields slightly better results than those described. The cost-effectiveness of population-based DR screening programs depends on the frequency of retinal examinations. Screening every two to three years rather than annually in patients with diabetes without evidence of DR is cost-effective in several studies in European countries. In our screening system, the decision to lengthen the interval between screenings is made by the referring endocrinologist based on an overall assessment of the patient's characteristics, taking into consideration the duration of diabetes, current, and past glycemic control. The different risk factors for DR. Differentiation of low-risk patients from high-risk groups is essential for practical and cost-effective screening.2,4 Secondly, telemedicine systems provide cost savings increasing patients’ working ability, independent living ability, and quality of life and reducing travel costs.11 Furthermore, new automated algorithms to identify RD lesions in digital fundus images are likely to be a cost-effective adjunct to manual grading.10,12
The equipment used is a non-mydriatic digital fundus camera, which has been widely used for the last 25 years and is recommended for screening in European populations. Non-mydriatic fundus cameras are ideal for screening as they are easy to operate, do not require dilation (can be used in patients with narrow-angle glaucoma), and do not use flash, which is more comfortable for the patient. In addition, they can be used by trained technicians without needing experienced opticians. Several studies have shown these cameras to be more sensitive than or at least as efficient as direct ophthalmoscopy.13 However, in populations with darker irises, such as India, the retinal images are of poorer quality and have a low sensitivity for screening.14
Currently, there is no consensus as to the number of fundus photographs required to detect DR reliably. The established gold standard is the capture of seven 30-degree fields after mydriasis.13 However, this method is not very useful for screening as it is a time-consuming procedure with a high economic impact. Accordingly, screening with one to four captures has been proposed.15,16 Screening based on two to four images has shown sensitivities of 80–98% and specificities of 86–100%, whereas using only a single capture results in poorer sensitivity (54–78%) and specificity (88–98%).15,16 Due to its low sensitivity, this method is unsuitable for screening. In our center, three captures are performed, which is in accordance with current evidence.
Our screening system is based on telemedicine with a digital platform that facilitates the exchange of information among the different healthcare professionals involved. Telemedicine systems using retinal imaging have proven to be feasible and efficient for screening and monitoring. They may even have the potential to modify lifestyle habits, which can contribute to the control of diabetes.6 Moreover, these methods are cost-effective in urban and rural populations compared to in-person screening or no screening.17 Through the telemedicine program, we can perform assessments with little delay, providing a diagnosis of the presence or absence of lesions in less than 30 days. This allows us to refer only those patients who need further evaluation to ophthalmology, thus avoiding unnecessary ophthalmologist visits.
In conclusion, the DR screening program in patients with type 1 DM in our Unit is consistent with the published data, with a PPV of 77.7% in the period analyzed. Effective screening for DR is carried out, with most patients diagnosed in the early stages of the disease. Telemedicine programs allow efficient communication among healthcare personnel (nurses, endocrinologists, ophthalmologists), enabling safe and effective screening for DR.
FundingThe authors declare that the research was conducted with no funding.
Conflict of interestNo conflict of interest has been declared by the author(s).
The authors thank Maria Repice for help with the English language version of the text.