Hypoparathyroidism is the most common complication of total thyroidectomy and usually requires monitoring of calcaemia, whereby it is one of the factors that most contributes to hospital stay. The objective of the study is to evaluate the clinical usefulness of the application of our protocol for early detection, intensive treatment and control of hypoparathyroidism in the first month after thyroidectomy.
Patients and methodRetrospective observational cross-sectional study of 79 patients who underwent total thyroidectomy in whom parathormone (PTH) and calcemia determinations were performed at 6−8 h and 18−24 h post-surgery. When the PTH value was lower than inferior limit of the reference (15 pg/ml), oral treatment was started with 1000 mg of calcium and 0.25 μg of calcitriol every 8 h followed by calcemia controls.
ResultsTwenty-six cases (32.9%) of normocalcemic hypoparathyroidism were detected in whom treatment prevented their progression to hypocalcaemia, except for 3 cases that had an episode of mild asymptomatic hypocalcaemia. There were no cases of moderate/severe hypocalcaemia and only one case of asymptomatic mild hypercalcaemia. There were no readmissions due to calcium abnormalities. No case with PTH > 15 pg/ml had hypocalcaemia. The protocol allowed a hospital stay of 24 h. The prevalence of permanent hypoparathyroidism was 5.1%.
ConclusionsThe application of our protocol during the first month after thyroidectomy is very useful because it avoids the appearance of moderate/severe hypocalcaemia and hypercalcaemia, allows a short hospital stay and is associated with a low prevalence of permanent hypoparathyroidism.
El hipoparatiroidismo es la complicación más frecuente de la tiroidectomía total y habitualmente requiere la monitorización de la calcemia, por lo que es el principal factor que determina la estancia hospitalaria. El objetivo del estudio es evaluar la utilidad clínica de la aplicación de nuestro protocolo de detección precoz, tratamiento intensivo y control del hipoparatiroidismo durante el primer mes postiroidectomía.
Pacientes y métodoEstudio retrospectivo observacional transversal sobre 79 pacientes sometidos a una tiroidectomía total a quienes se les determinó parathormona (PTH) y calcemia a las 6−8 horas de la intervención y a las 18−24 horas. Cuando el valor de PTH fue menor que el límite inferior de referencia (15 pg/ml) se inició tratamiento oral con 1.000 mg de calcio y 0,25 μg de calcitriol cada 8 horas y controles de calcemia.
ResultadosSe detectaron 26 casos (32,9%) de hipoparatiroidismo normocalcémico a quienes el tratamiento impidió su evolución a hipocalcemia excepto en 3 casos, que tuvieron un episodio de hipocalcemia leve asintomática. No hubo ningún caso de hipocalcemia moderada/severa, y solo uno de hipercalcemia leve asintomática. No hubo reingresos por alteraciones del calcio. Ningún caso con PTH > 15 pg/ml tuvo hipocalcemia. El protocolo permitió una estancia hospitalaria de 24 horas. La prevalencia de hipoparatiroidismo permanente fue del 5,1%.
ConclusionesLa aplicación de nuestro protocolo durante el primer mes postiroidectomía resulta muy útil porque evita la aparición de hipocalcemia e hipercalcemia moderada/severa permitir una estancia hospitalaria corta y se asocia a una baja prevalencia de hipoparatiroidismo permanente.
Post-surgical hypoparathyroidism is the most common form of hypoparathyroidism, accounting for about 75% of all chronic hypoparathyroidism, with thyroid surgery being by far the main procedure responsible.1–4 Despite trying to preserve the parathyroid glands, post-surgical hypoparathyroidism that arises within hours or days following a thyroidectomy can occur in up to 50% of cases.5–10
Since post-surgical hypoparathyroidism is one of the most common complications of thyroidectomy and it has an acute clinical presentation that can lead to tetany, cardiac arrhythmias and altered mental status,1,11 early detection and treatment are crucial to avoid clinical complications and a prolonged hospital stay.12–15
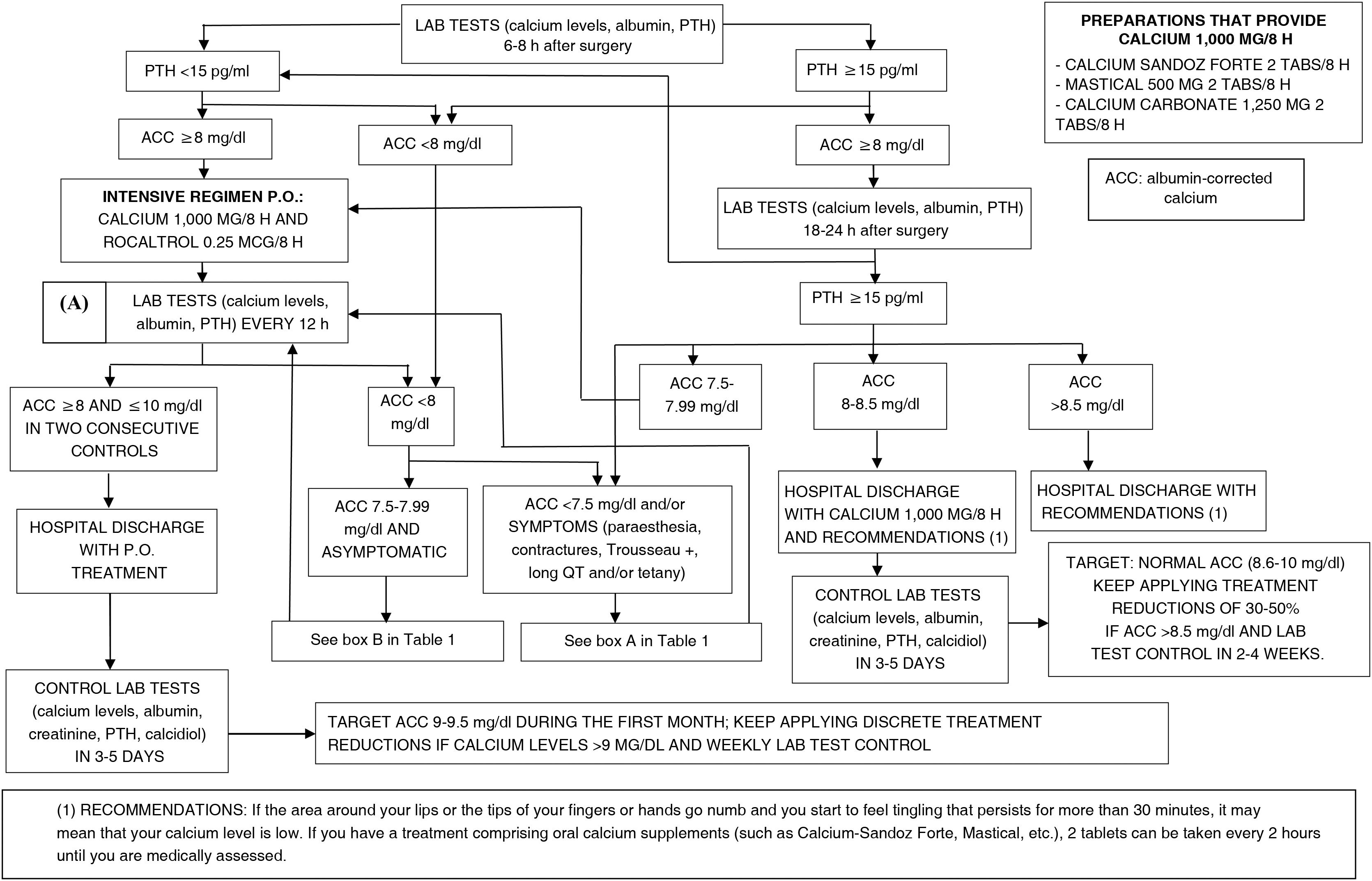
We have developed a simple protocol for early detection and control of post-surgical hypoparathyroidism after total thyroidectomy, whereby intensive treatment is prioritised based on the detection of a frankly low concentration of parathyroid hormone (PTH), in order to avoid the onset of hypocalcaemia as much as possible, and to reduce clinical complications, hospital stay and re-admissions for this reason. In turn, it requires close monitoring of possible iatrogenic hypercalcaemia. Essentially, the protocol is based on measuring PTH levels six to eight hours after surgery and its repetition at 18−24 h. If the value is below the lower limit of the laboratory reference range (15 pg/ml), oral treatment comprising 1000 mg calcium with 0.25 μg calcitriol every eight hours and repeat laboratory tests for albumin-corrected blood calcium is initiated.
The objective of this study was to evaluate the clinical usefulness of routine application of our protocol for early detection, intensive treatment and control of post-surgical hypoparathyroidism in the first month after total thyroidectomy. Specifically, the aim was to analyse the detection of episodes of normocalcaemic hypoparathyroidism in the first 24 h, episodes of hypocalcaemia and hypercalcaemia occurring during the first month of post-surgical follow-up, length of hospital stay and the prevalence of permanent hypoparathyroidism associated with the application of this protocol.
Material and methodsThis was a retrospective observational study based on routine clinical practice in a hospital setting. Patients who underwent total thyroidectomy (including hemithyroidectomies with a previous history of contralateral hemithyroidectomy) from May 2017 to May 2022 were included in our protocol for early detection, intensive treatment and control of post-surgical hypoparathyroidism. Patients with kidney failure with an estimated glomerular filtration rate of less than 30 ml/min were not included because these patients often already have altered calcium metabolism and may require different assessment and treatment. The study was approved by the Independent Ethics Committee of the Fundació Asistencial Mútua de Terrassa.
The protocol diagram is shown in Fig. 1. It is based on determining PTH and albumin-corrected calcium at six to eight hours after surgery and at 18−24 h. This schedule was established based on the historical clinical practice routines of our centre. Depending on the PTH value, bounded by the lower reference range limit, which in our centre is 15 pg/ml, and the albumin-corrected calcium as specified in the diagram, therapeutic decisions are made as soon as possible once the laboratory test results are known, usually between 30 and 60 min after taking the blood sample for analysis, and the described controls are scheduled. The exception is patients with symptoms of hypocalcaemia, in which case a confirmatory test is performed and parenteral treatment is started before the result is known. In cases with symptoms suggestive of hypocalcaemia and/or albumin-corrected calcium values below 7.5 mg/dl, an electrocardiogram is performed to assess heart rhythm abnormalities and to monitor the QT interval, every six hours if there are abnormalities, until they normalise and albumin-corrected calcium is greater than 8 mg/dl. Table 1 specifies the adjustments to treatment recommended in Fig. 1.
Treatment adjustments for post-thyroidectomy hypocalcaemia according to Fig. 1.1,19,30,35,41
| A | • Carry out confirmatory lab tests (calcium levels, albumin), and without waiting for the result, |
| • Immediately administer 20 ml of calcium gluconate 10% I.V. diluted in 100 ml of physiological saline over 20 min followed by an I.V. infusion of 80 ml of calcium gluconate 10% diluted in 500 ml of physiological saline at a rate of 40 ml/h. | |
| • If in the confirmatory lab test the albumin-corrected calcium level is greater than 8.5 mg/dl, suspend the I.V. infusion of calcium gluconate, leave the p.o. regimen with calcium 1000 mg/8 h only and return to box (A) of Fig. 1. Otherwise: | |
| • Start, if it has not been done previously, and as soon as possible, the intensive p.o. regimen with calcium 1000 mg/8 h and calcitriol 0.25 μg/8 h. | |
| • Control of calcium levels and albumin every 6 h. | |
| • Suspend the calcium gluconate infusion when albumin-corrected calcium is greater than 9.5 mg/dl and the oral calcium and calcitriol regimen is established and return to box (A) in Fig. 1. | |
| • If an albumin-corrected calcium level greater than 8 mg/dl is not achieved in 6 h, carry out the therapeutic actions in the following boxes (B and C). | |
| B | • Start, if it has not been done previously, and as soon as possible, the intensive p.o. regimen with calcium 1000 mg/8 h and calcitriol 0.25 μg/8 h. If already done: |
| • Increase the calcitriol dose by 0.25 μg every 12 h to a maximum dose of 5 μg/day, and | |
| • Increase the calcium dose by 1000 mg every 6 h up to a maximum dose of 10 g/day. | |
| • Control of albumin-corrected calcium every 6 h. When it is greater than 8 mg/dl, go to box (A) of Fig. 1. If it continues to be less than 8 mg/dl after 12 h, continue applying the above measures and consider: | |
| • Adding hydrochlorothiazide p.o. 25 mg/12 h, | |
| • See box C, and if it is not sufficient, | |
| • Teriparatide 50 μg/12 h s.c. or in continuous infusion s.c. at a starting dose of 60 μg/24 h in the form of 1 pulse every 10 min, and gradually reduce the dose by 5 μg/day until reaching 25−35 μg/day. | |
| C | • Determine plasma magnesium. For hypomagnesaemia (plasma magnesium <1.6 mEq/l), add magnesium supplements and check every 12 h to achieve a plasma concentration of 2 mg/dl: |
| • Magnesium sulfate I.V. 2 g (16 mEq) diluted in 100 cc of physiological saline to be administered over 20 min followed by an infusion of 4 g in 500 ml of physiological saline every 8 h, or | |
| • Oral magnesium 250−500 mg (20−40 mEq)/24 h divided into three doses with meals. | |
The variables collected from each patient were: a) personal data: age and sex; b) pre-operative diagnosis of thyroid disease: benign nodular goitre, nodular goitre with indeterminate cytology (Bethesda III and Bethesda IV), nodular goitre with cytology with suspicion of malignancy or malignancy (Bethesda V and Bethesda VI), hyperfunctioning nodular goitre, autoimmune hyperthyroidism (Graves-Basedow disease); c) presence of intrathoracic goitre according to information from ultrasound scans and/or computed tomography scans performed; d) type of surgery performed: total thyroidectomy, total thyroidectomy with central cervical lymphadenectomy, hemithyroidectomy with previous hemithyroidectomy; e) pre-operative treatment with calcium salts and/or calcidiol; f) pre-operative laboratory tests: plasma levels of calcium, albumin, creatinine, estimated glomerular filtration rate according to the 2009 CKD-EPI creatinine equation, PTH and calcidiol; g) laboratory tests six to eight hours after the surgery: calcium, albumin and PTH; h) laboratory tests 18−24 h after the surgery: calcium, albumin and PTH; i) post-surgical treatment administered: none, calcium salts p.o., calcium salts and calcitriol p.o., calcium I.V. with calcium salts and calcitriol p.o.; j) date of hospital admission and date of discharge; k) albumin-corrected calcium during the first month after thyroidectomy; l) hospital re-admissions for hypocalcaemia or hypercalcaemia during the first month after surgery, and m) presence of permanent hypoparathyroidism 12 months after thyroidectomy.
The primary endpoints were:
- □
Episodes of post-surgical normocalcaemic hypoparathyroidism detected in the first 24 h following surgery, defined on the basis of frankly low PTH levels (less than 15 pg/ml) with an albumin-corrected calcium level of between 8 mg/dl and less than 10 mg/dl.
- □
Episodes of hypocalcaemia during the first month following surgery:
- ͦ
Mild (albumin-corrected calcium between 7.5 and 8 mg/dl).
- ͦ
Moderate/severe (albumin-corrected calcium less than 7.5 mg/dl).
- ͦ
- □
Episodes of hypercalcaemia during the first month after surgery.
- ͦ
Mild (albumin-corrected calcium between 10 and 11.5 mg/dl).
- ͦ
Moderate/severe (albumin-corrected calcium greater than 11.5 mg/dl).
- ͦ
- □
Hospital re-admissions due to abnormal calcium levels during the first month after surgery.
The secondary endpoints were:
- □
The length of hospital stay from admission for thyroidectomy to hospital discharge.
- □
The prevalence of permanent hypoparathyroidism associated with the application of this protocol.
Calcium levels were determined using the 5-nitro-5′-methyl-BAPTA (NM-BAPTA) method; plasma albumin using bromocresol green (BCG); and intact PTH using electrochemiluminescence immunoassay. The reference values for calcium were 8.6–10.0 mg/dl, with a coefficient of variation of 0.61%, while for intact PTH they were 15.0–65.7 pg/ml, with a coefficient of variation of 3.05% and an assay sensitivity of 5.5 pg/ml.
A descriptive analysis of the variables collected was performed. Univariate analysis of the differences between continuous and categorical variables for pooled data was investigated using the Student's t-test or analysis of variance; the relationship between categorical variables was investigated with the chi-square test (χ2), and the relationship between continuous variables was evaluated using the Pearson correlation coefficient and multiple linear regression analysis (stepwise method). A binary logistic regression analysis was performed to investigate the influence of the independent variables on the primary and secondary endpoints. Statistical significance was established at p < 0.05 (two-tailed). Statistical analysis was performed using the Epidat software, version 3.1 (Servizo Galego de Saúde, Galicia, Spain).
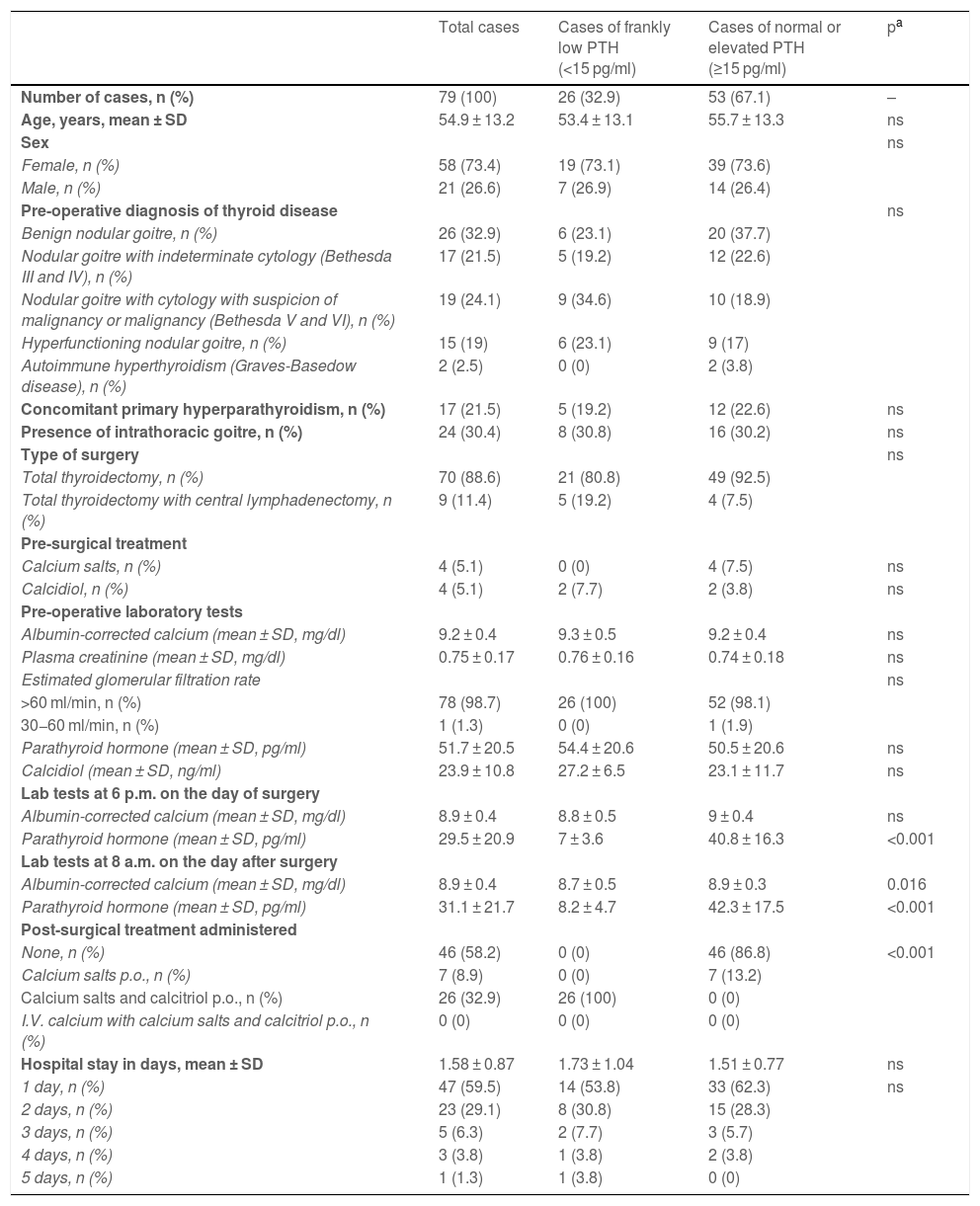
ResultsA total of 79 patients were included (58 women and 21 men), with a mean age of 54.9 ± 13.2 years. In 26 of them (32.9%), the protocol enabled the detection of post-surgical normocalcaemic hypoparathyroidism in the first 24 h after thyroidectomy, for which they began oral treatment with 1000 mg of calcium and 0.25 μg of calcitriol every eight hours. Table 2 shows the results of the variables collected. There were no discrepancies between the PTH values measured six to eight hours after thyroidectomy and those taken at 18−24 h; that is, when they were less than or greater than 15 pg/ml at one time, they were equally so at the other time.
Clinical characteristics of the patients according to the post-surgical PTH value in the first 24 h.
| Total cases | Cases of frankly low PTH (<15 pg/ml) | Cases of normal or elevated PTH (≥15 pg/ml) | pa | |
|---|---|---|---|---|
| Number of cases, n (%) | 79 (100) | 26 (32.9) | 53 (67.1) | – |
| Age, years, mean ± SD | 54.9 ± 13.2 | 53.4 ± 13.1 | 55.7 ± 13.3 | ns |
| Sex | ns | |||
| Female, n (%) | 58 (73.4) | 19 (73.1) | 39 (73.6) | |
| Male, n (%) | 21 (26.6) | 7 (26.9) | 14 (26.4) | |
| Pre-operative diagnosis of thyroid disease | ns | |||
| Benign nodular goitre, n (%) | 26 (32.9) | 6 (23.1) | 20 (37.7) | |
| Nodular goitre with indeterminate cytology (Bethesda III and IV), n (%) | 17 (21.5) | 5 (19.2) | 12 (22.6) | |
| Nodular goitre with cytology with suspicion of malignancy or malignancy (Bethesda V and VI), n (%) | 19 (24.1) | 9 (34.6) | 10 (18.9) | |
| Hyperfunctioning nodular goitre, n (%) | 15 (19) | 6 (23.1) | 9 (17) | |
| Autoimmune hyperthyroidism (Graves-Basedow disease), n (%) | 2 (2.5) | 0 (0) | 2 (3.8) | |
| Concomitant primary hyperparathyroidism, n (%) | 17 (21.5) | 5 (19.2) | 12 (22.6) | ns |
| Presence of intrathoracic goitre, n (%) | 24 (30.4) | 8 (30.8) | 16 (30.2) | ns |
| Type of surgery | ns | |||
| Total thyroidectomy, n (%) | 70 (88.6) | 21 (80.8) | 49 (92.5) | |
| Total thyroidectomy with central lymphadenectomy, n (%) | 9 (11.4) | 5 (19.2) | 4 (7.5) | |
| Pre-surgical treatment | ||||
| Calcium salts, n (%) | 4 (5.1) | 0 (0) | 4 (7.5) | ns |
| Calcidiol, n (%) | 4 (5.1) | 2 (7.7) | 2 (3.8) | ns |
| Pre-operative laboratory tests | ||||
| Albumin-corrected calcium (mean ± SD, mg/dl) | 9.2 ± 0.4 | 9.3 ± 0.5 | 9.2 ± 0.4 | ns |
| Plasma creatinine (mean ± SD, mg/dl) | 0.75 ± 0.17 | 0.76 ± 0.16 | 0.74 ± 0.18 | ns |
| Estimated glomerular filtration rate | ns | |||
| >60 ml/min, n (%) | 78 (98.7) | 26 (100) | 52 (98.1) | |
| 30−60 ml/min, n (%) | 1 (1.3) | 0 (0) | 1 (1.9) | |
| Parathyroid hormone (mean ± SD, pg/ml) | 51.7 ± 20.5 | 54.4 ± 20.6 | 50.5 ± 20.6 | ns |
| Calcidiol (mean ± SD, ng/ml) | 23.9 ± 10.8 | 27.2 ± 6.5 | 23.1 ± 11.7 | ns |
| Lab tests at 6 p.m. on the day of surgery | ||||
| Albumin-corrected calcium (mean ± SD, mg/dl) | 8.9 ± 0.4 | 8.8 ± 0.5 | 9 ± 0.4 | ns |
| Parathyroid hormone (mean ± SD, pg/ml) | 29.5 ± 20.9 | 7 ± 3.6 | 40.8 ± 16.3 | <0.001 |
| Lab tests at 8 a.m. on the day after surgery | ||||
| Albumin-corrected calcium (mean ± SD, mg/dl) | 8.9 ± 0.4 | 8.7 ± 0.5 | 8.9 ± 0.3 | 0.016 |
| Parathyroid hormone (mean ± SD, pg/ml) | 31.1 ± 21.7 | 8.2 ± 4.7 | 42.3 ± 17.5 | <0.001 |
| Post-surgical treatment administered | ||||
| None, n (%) | 46 (58.2) | 0 (0) | 46 (86.8) | <0.001 |
| Calcium salts p.o., n (%) | 7 (8.9) | 0 (0) | 7 (13.2) | |
| Calcium salts and calcitriol p.o., n (%) | 26 (32.9) | 26 (100) | 0 (0) | |
| I.V. calcium with calcium salts and calcitriol p.o., n (%) | 0 (0) | 0 (0) | 0 (0) | |
| Hospital stay in days, mean ± SD | 1.58 ± 0.87 | 1.73 ± 1.04 | 1.51 ± 0.77 | ns |
| 1 day, n (%) | 47 (59.5) | 14 (53.8) | 33 (62.3) | ns |
| 2 days, n (%) | 23 (29.1) | 8 (30.8) | 15 (28.3) | |
| 3 days, n (%) | 5 (6.3) | 2 (7.7) | 3 (5.7) | |
| 4 days, n (%) | 3 (3.8) | 1 (3.8) | 2 (3.8) | |
| 5 days, n (%) | 1 (1.3) | 1 (3.8) | 0 (0) | |
No patient with normal or elevated PTH had hypocalcaemia (less than 8 mg/dl). In seven cases (8.9%), normal PTH levels were observed in the first 24 h with a tendency towards hypocalcaemia (albumin-corrected calcium between 8 and 8.5 mg/dl), for which they were administered treatment only with calcium salts p.o.; they maintained normocalcaemia without the need to continue this treatment beyond the first month. Of these cases, one had prior hyperthyroidism, and four had decreased levels of vitamin D (8, 18, 20 and 22 ng/ml).
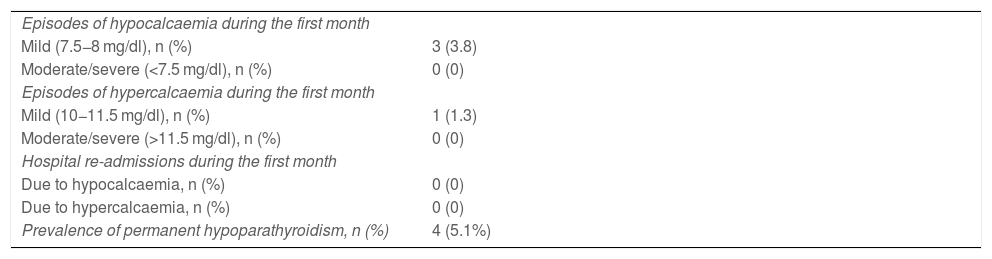
No patient with decreased PTH had hypocalcaemia (less than 8 mg/dl) in the first laboratory test carried out six to eight hours after the procedure. The clinical course of patients with normocalcaemic hypoparathyroidism treated with calcium salts and calcitriol during the first month of follow-up is shown in Table 3. There was no episode of hypocalcaemia requiring intravenous calcium salt therapy, no moderate/severe hypocalcaemia and no moderate/severe hypercalcaemia. There were three episodes of mild hypocalcaemia, asymptomatic, with albumin-corrected calcium values of 7.8 mg/dl at 22 days post-surgery, 7.7 mg/dl at 48 h post-surgery related to a 24 -h delay in the initiation of treatment in a patient with persistent sanguineous drainage, and 7.7 mg/dl in an out-of-hospital control at 72 h. The episode of mild hypercalcaemia was also asymptomatic and was detected in the out-of-hospital control on the fourth day of the post-surgery period with a value of 10.7 mg/dl. There were no re-admissions due to abnormal calcium levels. Of the 26 patients who had PTH levels less than 15 pg/ml in the first 24 h after thyroidectomy, only four (5.1%) had permanent hypoparathyroidism.
Clinical course of patients with post-surgical normocalcaemic hypoparathyroidism during the first month and prevalence of permanent hypoparathyroidism at 12 months.
| Episodes of hypocalcaemia during the first month | |
| Mild (7.5−8 mg/dl), n (%) | 3 (3.8) |
| Moderate/severe (<7.5 mg/dl), n (%) | 0 (0) |
| Episodes of hypercalcaemia during the first month | |
| Mild (10−11.5 mg/dl), n (%) | 1 (1.3) |
| Moderate/severe (>11.5 mg/dl), n (%) | 0 (0) |
| Hospital re-admissions during the first month | |
| Due to hypocalcaemia, n (%) | 0 (0) |
| Due to hypercalcaemia, n (%) | 0 (0) |
| Prevalence of permanent hypoparathyroidism, n (%) | 4 (5.1%) |
In most patients (59.5%), the length of hospital stay was one day. There was a slight tendency for a longer hospital stay in patients with normocalcaemic hypoparathyroidism, but it was not statistically significant (Table 2). In no case did the application of the protocol imply a delay in hospital discharge, and when hospital stay was longer than one day, it was due to the patient's comorbidities or haemorrhagic complications of thyroidectomy.
There were 17 patients (21.5%) who underwent thyroidectomy with concomitant normocalcaemic hyperparathyroidism: 16 due to a vitamin D deficiency and one due to a parathyroid adenoma that was resected with the thyroidectomy. They are equally distributed in both the group that had post-surgical normocalcaemic hypoparathyroidism and the group that did not. Calcium and PTH levels in the patient with resected parathyroid adenoma normalised in the post-surgical period and he did not require treatment with calcium salts or calcitriol.
In this study, no predictor of the occurrence of post-surgical normocalcaemic hypoparathyroidism has been found. Of the four patients with permanent hypoparathyroidism, one had benign intrathoracic goitre undergoing total thyroidectomy, another had intrathoracic goitre and indeterminate cytology undergoing total thyroidectomy, and two had goitre without intrathoracic expansion but with papillary carcinoma cytology undergoing total thyroidectomy, and one of them, in addition, a bilateral central cervical lymphadenectomy. The four cases had post-surgical PTH levels in the first 24 h that were lower than those of the 22 patients with normocalcaemic hypoparathyroidism who did not develop permanent hypoparathyroidism (PTH six to eight hours after surgery: 4 ± 2.35 versus 7.58 ± 3.56 pg/ml, p = 0.067; PTH 18−24 h after surgery: 3.92 ± 2.11 versus 9.04 ± 4.68 pg/ml, p = 0.046), while calcium levels in this period were similar in both groups of patients.
DiscussionOur protocol for early detection, intensive treatment and control of post-surgical hypoparathyroidism in the first month after total thyroidectomy is based on an in-depth review of the literature on the subject. Determination of albumin-corrected total serum calcium is preferable to ionised calcium concentrations, which are highly dependent on blood sampling, transport and pH.16 Although the normal reference range for albumin-corrected calcium is 8.6–10 mg/dl, most authors define post-surgical hypoparathyroidism in the first days of the post-surgical period as the combined presence of albumin-corrected serum calcium below 8.0 mg/dl with a low PTH concentration (either frankly low or inappropriately normal).2 A total calcium cut-off point of 8 mg/dl corrects the deviation due to recumbency and mild post-surgical haemodilution, and only exceptionally are symptoms of hypocalcaemia observed above this value.17 It has been recommended that oral calcium and calcitriol therapy be initiated if serum calcium falls below this level,16–21 with the aim of trying to maintain it in the upper half of the normal reference range during the first month after surgery, to minimise the incidence of permanent hypoparathyroidism.21 There is no established consensus on how to predict (or treat) post-surgical hypoparathyroidism after thyroidectomy.12,13,22–24
The determination of serum calcium levels in the post-surgical period has been the most widely used method, with some differences regarding when it should be performed and the calcium threshold considered.12,13,24–26 In one of the most recent studies carried out in Spain with serum calcium monitoring, 25–30% of episodes of mild hypocalcaemia and 1.5–4.5% of episodes of moderate/severe hypocalcaemia have been reported.24 In recent years, it has been seen that a decrease in PTH levels during the first 24 h after thyroidectomy can lead to an early diagnosis of hypoparathyroidism before serum calcium concentrations reach the range of hypocalcaemia,2,14,22,23,27–32 which does not occur until 24–72 h after the surgical procedure.14,25,28 The short half-life of PTH (3−5 min) justifies its early measurement in plasma after total thyroidectomy. Some studies have proposed that the percentage decrease in post-surgical PTH value is a good indicator of the risk of post-surgical hypocalcaemia. However, the value of this reduction in post-surgical PTH compared to pre-surgical PTH levels has not been clearly defined, since it ranges between 60% and 95%.8–10 In other studies, patients with a PTH value above the lower reference limit of 15 pg/ml, measured 20 min or more after surgery, had an extremely low risk of developing post-surgical hypocalcaemia, and they were discharged from hospital if they were stable.14,30,31 In contrast, patients with PTH lower than 15 pg/ml in the first 24 h after surgery had a very high risk of developing hypocalcaemia, at 75–100%, with a 6–46% false positive rate depending on the time the measurement was taken and where the cut-off point below 15 pg/ml was established.13,14,22,29–33
The determination of post-surgical PTH for the management of patients at risk of hypoparathyroidism has been considered cost-effective as it reduces hospital stay, especially in cases with preserved PTH in the first 24 h, since patients do not need to wait 24−72 h to confirm normal calcium levels.13,28 Some authors have used combinations of PTH and calcium to predict post-surgical hypoparathyroidism, with mixed results.22,29 However, this procedure has the drawback of the time delay that this entails by prolonging hospital stay and possibly allowing patients to become clinically hypocalcaemic.
In our protocol, PTH and calcium levels are checked twice in the first 24 h after thyroidectomy to ensure stability in cases that do not develop hypoparathyroidism and also in treated cases, and to avoid possible false negatives. In patients with vitamin D deficiency, the PTH cut-off point of 15 pg/ml could be less sensitive as a predictor of hypocalcaemia in the first few hours after thyroidectomy, but this deficiency can be circumvented by repeating the PTH test a few hours later,34 as occurred in the patients in our study with concomitant normocalcaemic hyperparathyroidism due to vitamin D deficiency, none of whom developed hypoparathyroidism that was not detected in the first 24 h after thyroidectomy. The protocol also aims to prevent the onset of hypocalcaemia as much as possible, establishing early intensive treatment with calcium salts and calcitriol in the period of normocalcaemic hypoparathyroidism, which can reduce both hospital stay and re-admissions for this reason. In turn, it requires close monitoring for possible iatrogenic hypercalcaemia.
No patient with a PTH level above the lower laboratory reference limit and concomitant albumin-corrected calcium greater than 8.5 mg/dl received hypocalcaemia treatment or had hypocalcaemia, and hospital discharge was safe after 24 h. Some patients in this study (seven cases [8.8%]) with PTH above the lower reference limit but who were prone to hypocalcaemia (albumin-corrected calcium between 8 and 8.5 mg/dl) did not have true hypoparathyroidism but a slight decrease in calcium levels due to post-surgical dilution, vitamin D deficiency, the deposit of calcium salts in bone as a complication of their previous hyperthyroidism and/or slight parathyroid dysfunction30; they were only treated in the post-surgical period with calcium salts p.o., and were able to forego the treatment within a few days.
Although we have not had any cases of hypocalcaemia (albumin-corrected calcium less than 8 mg/dl) with normal or elevated PTH, nor with decreased PTH in the first six to eight hours after surgery, which in both cases would have been very suggestive of hungry bone syndrome secondary to hyperthyroidism with or without concomitant parathyroid functional alteration,30 the protocol also provides for this possibility. However, we cannot provide conclusions in this respect.
In patients with PTH below the laboratory reference limit but still normal calcium levels (26 cases [32.9%]), oral treatment comprising calcium salts and calcitriol was administered, and they were able to be discharged from the hospital 24 h after surgery with frequent outpatient monitoring. Of these patients, only three episodes of mild hypocalcaemia and one of mild hypercalcaemia were detected, all asymptomatic, requiring only small changes in the oral treatment recommended by the protocol, and having no clinical consequences. It is possible that other episodes of mild hypocalcaemia and hypercalcaemia may have gone unnoticed because patients were asymptomatic and it occurred between lab tests. It is noteworthy that in no case were there episodes requiring parenteral treatment with calcium salts, episodes of moderate/severe hypocalcaemia, moderate/severe hypercalcaemia or re-admissions due to abnormal calcium levels.
Hypoparathyroidism is the most common complication of thyroidectomy—more common than haemorrhagic complications and recurrent nerve injury—and its monitoring based on the control of post-surgical calcium levels means it is the main determinant of hospital stay,7,12–15 which usually lasts between 2.5 and 4.5 days.13,24,25 Treatment with calcium salts and calcitriol is usually used to reverse post-surgical hypocalcaemic hypoparathyroidism.19 Calcitriol works by increasing intestinal calcium absorption and bone resorption,35 with which the normalisation of calcium levels is often delayed between three and seven days.25,35 Therefore, when hypoparathyroidism has evolved giving rise to the onset of hypocalcaemia, in addition to its clinical complications, hospital stay is extended by several days. With the application of our protocol, the hospital stay of patients undergoing total thyroidectomy is not extended beyond 24 h, since it predicts the risk of developing hypocalcaemia, albeit with false positives, and it allows intensive treatment to be initiated in advance of hypocalcaemia for safe hospital discharge. Moreover, unnecessary treatment of false positives has not resulted in clinical complications due to frequent monitoring.
The risk of post-surgical hypoparathyroidism changes depending on the extent of cervical dissection and the surgical technique used.7,36,37 It usually occurs in 10–20% of thyroidectomies,38 but this figure can increase to 50% in patients who have previously undergone cervical surgery where one or more parathyroid glands may have been removed or injured, as well as in those who have a total thyroidectomy, especially if it is extensive, as in the case of intrathoracic goitre, Graves' disease and thyroid cancer, especially when combined with bilateral lymph node dissection of the central compartment of the neck,1,5–10,15,30,31 Post-surgical hypoparathyroidism can progress to permanent hypoparathyroidism, which is generally accepted to occur in 6%–12% of patients within six to 12 months following total thyroidectomy.2,6,16,17,39 Recently, considering that the true prevalence is underestimated, as previously reported,17 a Japanese study has estimated the figure to be between 15% and 20.3% of patients undergoing total thyroidectomy.40
In this study, with the application of our protocol, a prevalence of post-surgical hypoparathyroidism of 32.9% has been calculated, based on a frankly low level of PTH with normocalcaemia within the first 24 h after thyroidectomy. This figure overestimates the true prevalence of post-surgical, non-normocalcaemic hypoparathyroidism due to the false positives that have been included in its calculation.13,14,22,29–33 In the study by Asari et al.,29 a PTH value lower than 15 pg/ml in the first 24 h after thyroidectomy resulted in a 17.4% false-positive rate in predicting post-surgical hypoparathyroidism. Taking this figure into account, in our study we could consider that post-surgical hypoparathyroidism actually occurred in about 21 patients, which represents a prevalence of 26.6%, a figure that is not outside the values referenced in the literature.
On the other hand, the prevalence of permanent hypoparathyroidism associated with the application of our protocol was 5.1%, a low percentage, probably influenced by the intention to maintain calcium levels in the upper half of normal during the first month, with intensive and early treatment administered to cases of post-surgical normocalcaemic hypoparathyroidism, as previously demonstrated.21 Although our patients exhibited some of the conditions with the highest risk of post-surgical hypoparathyroidism, in this study we have not been able to identify an association with any of them. In the only four cases of permanent hypoparathyroidism, intrathoracic goitre, cancer and central cervical lymphadenectomy were concomitant conditions, but only lower PTH levels in the first 24 h post-thyroidectomy have been predictive of those who did not develop permanent hypoparathyroidism, as previously reported.14 However, it should be noted that the study was not designed to look for markers of transient or permanent post-surgical hypoparathyroidism.
One of the limitations of the study is the fact that its retrospective nature has meant the absence of some data in the variables collected: pre-operative albumin-corrected calcium in 27 cases, pre-operative calcidiol in 17 cases and pre-operative PTH in 33 cases. However, these omissions affect the better characterisation of patients, and we believe that they do not substantially influence the outcome or conclusions of the application of our protocol. However, there may be errors or alterations in the execution of the protocol that could not be recognised in all cases, such as delay in the initiation of treatment, which could have caused a poorer or slower recovery of calcium levels. In addition, it is also possible that omissions in treatment compliance by patients during the study period have not been recognised.
In conclusion, the application of our protocol for early detection, intensive treatment and control of post-surgical hypoparathyroidism in the first month after total thyroidectomy is a safe and efficient procedure that is useful because it has detected episodes of post-surgical normocalcaemic hypoparathyroidism and prevented its progression to moderate/severe hypocalcaemia, without lengthening the mean hospital stay beyond 24 h and without causing moderate/severe hypocalcaemic or hypercalcaemic decompensations during the first month of the post-surgical period, and also because it has been associated with a low prevalence of permanent hypoparathyroidism.
Ethical considerationsProtection of people and animals. The authors declare that no experiments were performed on humans or animals for this study.
Data confidentiality. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consent. The authors declare that this article does not contain patient data.
FundingThis study has received no specific funding from public, private or non-profit organisations.
AuthorsEach author has contributed materially to the research and preparation of the article. Specifically:
Luis García Pascual: study conception and design, data acquisition and analysis, interpretation of results, critical review of the draft and approval of the final version.
Lluís García González, Xavier Lao Luque, Laura Palomino Meneses and Guillem Viscasillas Pallàs: conception and design of the study, interpretation of results, writing of the draft and approval of the final version.
Conflicts of interestThe authors declare that they have no conflicts of interest.