Testicular adrenal rest tumours (TART) are rare cancers mainly associated with congenital adrenal hyperplasia in its classic form, with a prevalence of up to 40% of patients, particularly in those with inadequate control of their illness.1 Although rare, they can also occur in other diseases associated with high ACTH concentrations.2–5
The lesions can be confused with Leydig cell tumours, for which patients may end up having a gonadectomy. It is therefore important to be aware of the possibility of TART, as treatment should be conservative with the objective being to preserve fertility.
We present this case of TART in a patient with Addison's disease; the second to be reported in the literature.
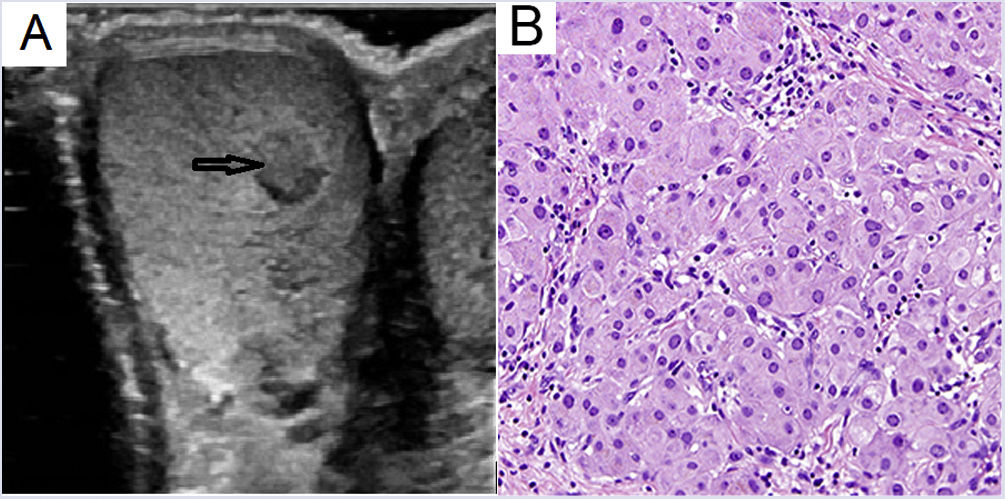
Case reportThis was a 36-year-old male with Addison's disease diagnosed at the age of 21 years who was on treatment with 20 mg/day of hydrocortisone (15 mg at 9.00 a.m. and 5 mg at 4.00 p.m.) and 0.1 mg/day of fludrocortisone. He was referred to Urology after self-detection of a small mass in both testicles. On examination he was found to have solid, mobile tumours in both testes. Several hypoechoic focal lesions were identified by ultrasound in the testicular parenchyma, the largest measuring 6 × 6 mm in the right testicle (Fig. 1A). Increased vascularisation and ectasia of the rete testis was also observed. Excision of this lesion was performed with a right epididymectomy.
Pathology examination identified a well defined, firm, yellowish mass of 2 cm in size. Under the microscope, the mass was clearly demarcated from the epididymis and did not have histological features of malignancy. It consisted of polygonal cells with abundant eosinophilic cytoplasm and round nuclei (Fig. 1B). Also noted was the presence of lipofuscin in some cells and an absence of Reinke crystals. Immunohistochemistry was positive for vimentin, inhibin A, calretinin, and CD56; weakly positive for pancytokeratin, synaptophysin and androgen receptor; and negative for S100 and chromogranin A.
Although difficult to differentiate from Leydig cell tumours, we were able to diagnose TART in our case based on the histopathological data, so orchiectomy was not performed. The multifocal and bilateral nature of the lesions, combined with the patient's history of Addison's disease, which causes chronic exposure to high concentrations of ACTH, were determining factors.
DiscussionTesticular adrenal rest tumours are benign tumours that develop as a consequence of the chronic stimulation of high plasma concentrations of ACTH on adrenal stem cells in the testes. They have been reported primarily in patients with congenital adrenal hyperplasia, but could theoretically occur in other diseases that involve persistent elevation of ACTH.1,2 Although these are exceptional cases, they have been reported in five patients with Nelson's syndrome,3 recently in two patients with primary adrenal insufficiency associated with adrenoleukodystrophy linked to the X chromosome,4 and in one patient with Addison's disease of autoimmune origin.5 The case we report here is the second to be published associated with Addison's disease.
TART express adrenal gland-specific genes, such as ACTH-MCR2, and actual gonadal genes, such as LH-CGR, HSD17B3 and INSL3.6 Markers routinely tested for in sex cord stromal tumours, such as Leydig cell tumours, are absent in TART. However, specific markers of androgen-producing cells, in addition to adrenocortical markers including adrenal steroidogenic proteins, are found in TART.7
It has been suggested that the origin of TART is in pluripotent cells of the adrenogonadal primordium or urogenital ring, where ACTH is their main stimulus.7 For that reason, patients with a higher level of ACTH and early exposure would be more likely to develop it. There has been no study of the mechanism by which adrenal rests might not be exposed to the antibodies present in Addison's disease.
Conservative surgery has been described in small cohorts of patients, without significant improvements in gonadal function.1 The therapy of choice is the intensification of glucocorticoid treatment to, as far as possible, suppress exposure to high ACTH concentrations and avoid steroid overdose. This is based on the reduction in tumour size and the improvement in testicular function achieved in some patients.8 Corticosteroids with a longer half-life could be used, such as low-dose prednisone or dexamethasone. In our patient, we added low-dose nocturnal prednisone and achieved significant slow-down of the release of ACTH, as was to be expected, going from an initial concentration of 1208 pg/mL to 291.7 pg/mL after one month of treatment.
In terms of drug treatment, glucocorticoid release patterns that are more physiological are required to keep ACTH under control, along with the development of drugs that antagonise the ACTH receptor, or the CRF-1 receptor, which are already being investigated in the treatment of congenital adrenal hyperplasia.9
ConclusionsWe have presented this case because of its exceptional nature. The development of a testicular mass in a patient with Addison's disease should alert to the possibility of TART. The ultimate goal of treatment is to preserve fertility, avoid unnecessary gonadectomy and offer cryopreservation, if appropriate.
Ethical responsibilitiesFor the drafting of this article, our workplace's protocols on publication of patient data, which include obtaining patient consent for said data to be published, were followed, and the subjects' privacy was respected.
FundingNo funding was received.
Conflicts of interestNone.