To report the evolution of metabolic control and to assess the clinical and metabolic factors associated with the presence of microvascular complications in patients with type 1 diabetes mellitus (T1DM).
Material and methodsThis was a retrospective, observational study analysing clinical, laboratory, and therapeutic data from a registry of patients with T1DM created in 2010.
ResultsData recorded from 586 patients (males: 50.2%; mean age: 36.1±13.5 years; T1DM duration: 18.0±12.1 years) followed for a mean of 6.0±3.1 years were assessed, and 8133 HbA1c levels (13.2±7.6 measurements/patient) were analysed, with a mean evolutionary HbA1c of 7.9%±1.2%. The mean annual HbA1c level gradually improved from 8.6%±1.6% in 2010 to 7.5%±1.4% in 2019, with 34.3% and 69.0% of patients having HbA1c levels ≤7% and ≤8% respectively. Patients with T1DM duration of <10 years and ≥20 years, non-smokers, CSII users, and those using the insulin/carbohydrate ratio had better current and evolutionary HbA1c levels. The presence of microvascular complications was independently associated with T1DM lasting ≥20 years, the presence of HBP, and evolutionary HbA1c≥7.0%.
ConclusionA progressive but still inadequate improvement in metabolic control over 10 years was seen in patients with T1DM. Poor metabolic control (mean HbA1c over 10 years ≥7%) was independently associated with the presence of microvascular complications.
Describir la evolución del control metabólico y evaluar los factores clínicos y metabólicos asociados con la presencia en la actualidad de complicaciones microvasculares en pacientes con diabetes tipo 1 (DM1).
Material y métodosEstudio observacional retrospectivo en el que se analizan los datos clínicos, analíticos y terapéuticos de un registro de pacientes con DM1 creado en 2010.
ResultadosSe han evaluado los registros de 586 pacientes (hombres: 50,2%; edad media: 36,1±13,5 años; evolución DM1: 18,0±12,1 años), seguidos un promedio de 6,0±3,1 años y se han analizado 8.133 cifras de HbA1c (13,2±7,6 mediciones por paciente) con una HbA1c evolutiva promedio de 7,9±1,2%. Se observó una mejoría progresiva del nivel promedio anual de HbA1c desde 8,6±1,6% en 2010 hasta 7,5±1,4% en 2019; en la actualidad el 34,3% y el 69,0% de los pacientes presentan cifras de HbA1c ≤7% y ≤8%, respectivamente. Los pacientes con <10 y ≥20 años de evolución, los no fumadores, usuarios de ISCI y los que utilizan la ratio insulina/hidratos presentan mejores niveles de HbA1c actuales y evolutivos. La presencia de complicaciones microvasculares en la actualidad se asoció de forma independiente con una evolución de DM1≥20 años, presencia de hipertensión arterial y HbA1c evolutiva≥7,0%.
ConclusiónDurante 10 años observamos una mejoría progresiva, pero aún insuficiente, en el control metabólico de pacientes con DM1. El mal control metabólico (HbA1c promedio durante 10 años≥7%) se asoció de forma independiente con la presencia en la actualidad de complicaciones microvasculares.
Type 1 diabetes (T1DM) is a chronic disease with a significant personal, socioeconomic and health impact. Poor chronic metabolic control in patients with T1DM induces in the long term the development and progression of chronic complications, both micro- and macroangiopathic, which are the main causes of morbidity, mortality and decreased quality of life in these patients.1
The results of the Diabetes Control and Complications Trial (DCCT) and Epidemiology of Diabetes Interventions and Complications (EDIC) studies demonstrated that intensive treatment, with the aim of keeping HbA1c levels as close to normal as possible, was associated 30 years after the start of intervention with a lower incidence of retinopathy that requires laser therapy (5% vs 45%), end-stage chronic kidney disease (0% vs 5%), clinical neuropathy (15% vs 50%), myocardial infarction (3% vs 5%), cerebrovascular accident (0.4% vs 2%), and all-cause mortality (6% vs 20%). In addition, it resulted in a gain of approximately 1.62 quality-adjusted life years and cost savings from complications of approximately $90,900 per patient.2 As a result, the American Diabetes Association establishes a glycosylated haemoglobin (HbA1c) target of lower than 7% for non-pregnant adults, stricter (lower than 6.5%) in individuals without risk of hypoglycaemia or cardiovascular disease and less strict (lower than 8%) in patients with severe hypoglycaemia, reduced life expectancy, advanced microvascular or macrovascular disorders, comorbidity, and in those for whom a stricter target is difficult to achieve despite health education, adequate blood glucose monitoring or multiple-dose insulin.3
Since the publication of the DCCT there have been numerous advances in the treatment of diabetes, such as the development of new basal and prandial insulin analogues, the implementation of advanced therapeutic education programmes and the increased use of both continuous subcutaneous insulin infusion (CSII) systems as well as continuous and flash glucose monitoring, among others.4 Despite these advances, various international5–12 and national13–19 studies indicate that in clinical practice we are a long way from achieving adequate HbA1c targets in patients with T1DM and, what is worse, in some settings metabolic control seems to have worsened in recent years.9,10 The objective of this study was to describe the evolution of metabolic control over 10 years in patients with T1DM and to evaluate the possible association between poor metabolic control and the development of microvascular complications.
Patients and methodsA retrospective observational study was carried out in a cohort of 586 patients with T1DM older than 14 years of age seen in 2019 at the T1DM specialised clinic of the Clinical Management of Endocrinology and Nutrition Unit of the Hospital Universitario Puerto Real [Puerto Real University Hospital] (Cádiz). The objectives of the study were to describe the evolution of metabolic control and assess the clinical and metabolic factors associated with current microvascular complications.
From January 2010, all patients with T1DM over 14 years of age from the hospital or who were referred from primary care or other specialties in our health district (350,000 inhabitants) were invited to attend an appointment at a specialised clinic staffed by three specialists in endocrinology and nutrition and two nurse educators. In all patients, intensive treatment with multiple-dose insulin (MDI) or with CSII was prescribed and in a complementary manner they were incorporated into a personalised therapeutic education in diabetes programme, to educate them in the management of a portion control diet and the importance of doing physical activity. Generic preprandial (80−130mg/dl), postprandial (<180mg/dl) and HbA1c (≤7%) glycaemic control targets were established. In general, with the onset of diabetes, patients were invited to appointments every four weeks for the first few months and then every three to four months for the first year of follow-up. Thereafter, patients with “acceptable” metabolic control (HbA1c <8%) attend two medical consultations a year, while those with poor metabolic control (HbA1c >8%) were seen more frequently, according to the healthcare needs in each case.
Variables analysedAll patients seen at the consultation were registered with the diagnosis “type 1 diabetes” in a database in Access format located on the hospital's intranet, with the aim of having an up-to-date registry of the patients treated at our unit and to allow for an annual evaluation of performance indicators.
The records of patients with T1DM included the following variables: a) clinical variables: year of birth, gender (male/female), year of onset of T1DM, smoker (yes/no), arterial hypertension (HT) (yes/no), macrovascular disease (ischaemic heart disease, cerebrovascular accident, peripheral vascular disease, amputation), diabetic retinopathy (presence and type of diabetic retinopathy and treatment received); presence and degree of diabetic nephropathy, presence of microvascular complication (defined as positive albuminuria, treatment with ACE inhibitors/ARBs due to previous history of albuminuria, established nephropathy or any degree of diabetic retinopathy), use of the insulin-to-carbohydrate ratio (yes/no), users of Freestyle Libre (yes/no and date), weight (kg), height (cm) and body mass index (BMI) (kg/m2); b) laboratory variables (years 2010–2019): HbA1c every four months (%) (1στ, 2νδ, 3ρδ); HbA1c annual average (%); HbA1c cumulative average (%), LDLc every four months (mg/dl) (1στ, 2νδ and 3ρδ); albumin-to-creatinine ratio (mg/g); c) imaging study variables: results of retinal scans performed and interpreted in our unit and of ophthalmology diagnoses; d) therapeutic variables: statin therapy (yes/no), antihypertensive therapy (yes/no and type), antiplatelet therapy (yes/no), therapy with other antidiabetic drugs (yes/no and type), thyroxine therapy (yes/no) and insulin therapy (MDI/CSII). The database of patients with T1DM was evaluated periodically in order to progressively analyse different indicators of intermediate results. In this study, only the data of the 586 patients (79.5%) with T1DM who attended a consultation in 2019 were evaluated, and the remaining 151 patients (20.5%) from the registry were excluded from the analysis.
Personal data protection and ethical considerationsTo analyse the information, the database was anonymised by removing personal identification variables and identifying each record with an anonymous code. The ethical requirements of the 1964 World Medical Association Declaration of Helsinki, revised in 2013, were met. The study was approved by the Cádiz Independent Ethics Committee in February 2020 and informed consent was not required to access the research data.
Statistical analysisThe evaluated database was exported to the Statistical Package for Social Science (SPSS) system version 12.0 (Chicago, IL, USA) for statistical analysis. The descriptive analysis of the qualitative variables was carried out by calculating frequencies and percentages, while for the quantitative variables mean, standard deviation and range were determined. The normality of the continuous variables was verified by the Kolmogorov-Smirnov test. Quantitative variables with normal or non-normal distribution were compared with the Student's t test and the Mann-Whitney U test, respectively, and the χ2 test and Fisher's exact test were used to compare qualitative or categorical variables. The univariate analysis was used to evaluate the association between the current presence of microvascular complications and the variables of age, gender, diabetes evolution, smoking, current HbA1c and its evolution, BMI, presence of HT and macrovascular complications. In the stepwise multivariate analysis (logistic regression), the dependent variable was the current presence of microvascular complications, and the covariates entered were those statistically associated with the presence of complications in the univariate analysis. Using ROC curve analysis, the performance of the average evolutionary HbA1c value for the detection of microvascular complications was evaluated and the sensitivity and specificity of the HbA1c ≥7.0% cut-off point was calculated. p<0.05 was considered statistically significant.
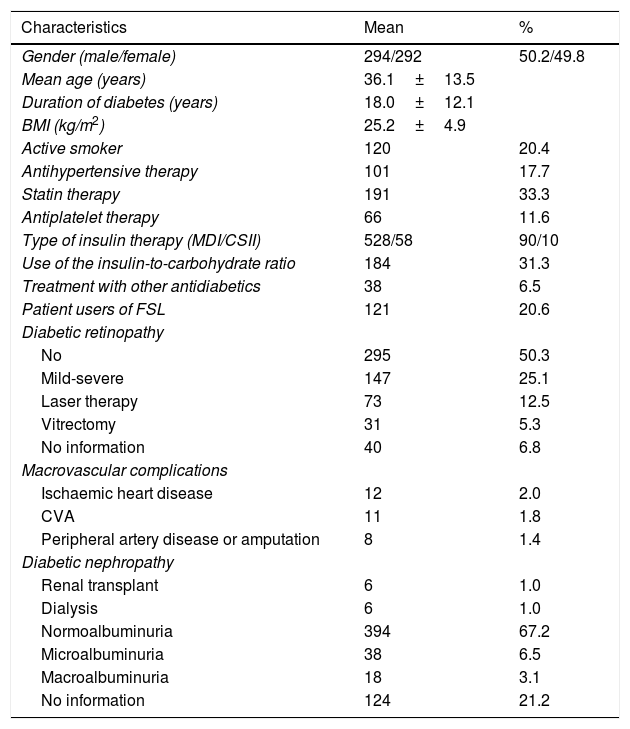
ResultsIn 2019, 586 patients with T1DM who had an average follow-up in consultations of 6.0±3.1 years (0–10 years) were seen, while 151 patients (20.5%) did not attend a check-up despite an appointment having been scheduled and despite being given the opportunity to reschedule the appointment by phone in case they could not attend the scheduled check-up. During the 10 years of consultations, an average of 19 patients a year older than 14 years of age (range of 10–28 patients/year) had onset of T1DM in our district (277,000 inhabitants over 14 years of age). Table 1 shows the main clinical characteristics of the patients with T1DM evaluated. With regard to the associated comorbidities and the treatments applied, the smoking percentage (20%), the prevalence of obesity (BMI≥30kg/m2) (10%) and the low number of patients with CSII (58 patients; 10%) are striking. Regarding complications, 5.3% of the registered patients were diagnosed with macrovascular disease, 17.8% with severe diabetic retinopathy treated with laser therapy or surgery, and 2% with diabetic nephropathy on haemodialysis or with kidney transplantation. Although the albuminuria levels for 21.2% (124 patients) are not known, almost all reported cases were for patients with a <5-year history of T1DM. Three patients died during follow-up from various causes.
Clinical characteristics of patients with T1DM treated in 2019 (n=586).
| Characteristics | Mean | % |
|---|---|---|
| Gender (male/female) | 294/292 | 50.2/49.8 |
| Mean age (years) | 36.1±13.5 | |
| Duration of diabetes (years) | 18.0±12.1 | |
| BMI (kg/m2) | 25.2±4.9 | |
| Active smoker | 120 | 20.4 |
| Antihypertensive therapy | 101 | 17.7 |
| Statin therapy | 191 | 33.3 |
| Antiplatelet therapy | 66 | 11.6 |
| Type of insulin therapy (MDI/CSII) | 528/58 | 90/10 |
| Use of the insulin-to-carbohydrate ratio | 184 | 31.3 |
| Treatment with other antidiabetics | 38 | 6.5 |
| Patient users of FSL | 121 | 20.6 |
| Diabetic retinopathy | ||
| No | 295 | 50.3 |
| Mild-severe | 147 | 25.1 |
| Laser therapy | 73 | 12.5 |
| Vitrectomy | 31 | 5.3 |
| No information | 40 | 6.8 |
| Macrovascular complications | ||
| Ischaemic heart disease | 12 | 2.0 |
| CVA | 11 | 1.8 |
| Peripheral artery disease or amputation | 8 | 1.4 |
| Diabetic nephropathy | ||
| Renal transplant | 6 | 1.0 |
| Dialysis | 6 | 1.0 |
| Normoalbuminuria | 394 | 67.2 |
| Microalbuminuria | 38 | 6.5 |
| Macroalbuminuria | 18 | 3.1 |
| No information | 124 | 21.2 |
BMI: body mass index; CSII: continuous subcutaneous insulin infusion; CVA: cerebrovascular accident; FSL: Freestyle libre; kg: kilograms; m: metres; MDI: multiple-dose insulin.
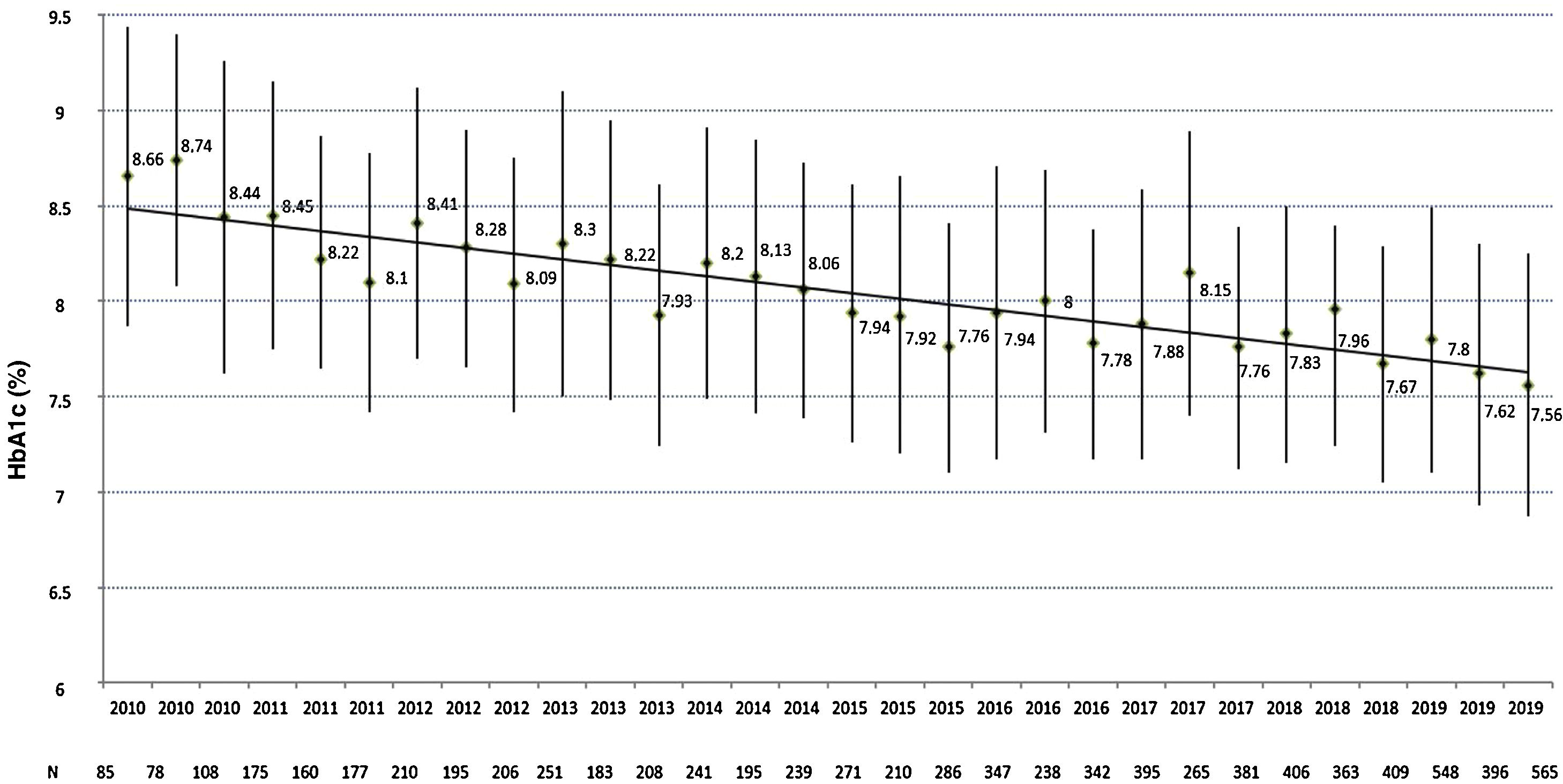
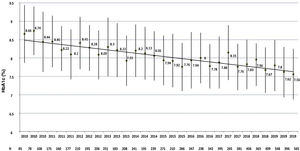
Regarding metabolic control, a total of 8133 HbA1c values were analysed (average of 13.2±7.6 measurements per patient), with an average HbA1c evolution of 7.9%±1.2%. As can be seen in Fig. 1, a progressive improvement is observed in the four-monthly HbA1c measurements from 8.7%±1.6% in the first evaluation in 2010 (n=85) to 7.5%±1.4% in the last evaluation in 2019 (n=565), in which 34.3% and 69.0% of the patients had an HbA1c level ≤7% or ≤8%, respectively. No differences were observed either in the last HbA1c measurement or in the average evolution of HbA1c between genders, age groups or users of the FSL system, while patients with <10-year history of T1DM (HbA1c 2019: 7.6±1.5 vs 7.9±1.6; p=0.025 and average evolution of HbA1c: 7.7±1.3 vs 8.2±1.3; p=0.001) and patients with >20-year history of T1DM (HbA1c 2019: 7.6±1.2 vs 7.9±1.6; p=0.008 and average evolution of HbA1c: 7.9±0.9 vs 8.2±1.3; p=0.016) exhibited better levels of both parameters compared to those with a 10−19-year history, non-smokers (HbA1c 2019: 7.6±1.4 vs 8.1±1.4; p=0.086 and average evolution of HbA1c: 7.9±1.2 vs 8.2±1.0; p=0.009), CSII users (HbA1c 2019: 7.0±1.0 vs 7.7±1.4; p<0.001 and average evolution of HbA1c: 7.6±0.8 vs 8.0±1.2; p<0.001), those who use the insulin-to-carbohydrate ratio (HbA1c 2019: 7.2±1.0 vs 7.9±1.5; p<0.001 and average evolution of HbA1c: 7.6±0.8 vs 8.2±1.3; p<0.001) and patients with normal albuminuria (HbA1c 2019: 7.6±1.2 vs 8.3±1.6; p<0.001 and average evolution of HbA1c: 7.8±1.0 vs 8.6±1.3; p<0.001) and without diabetic retinopathy (HbA1c 2019: 7.5±1.5 vs 7.7±1.2; p=0.084 and average evolution of HbA1c: 7.8±1.2 vs 8.1±1.0; p=0.003).
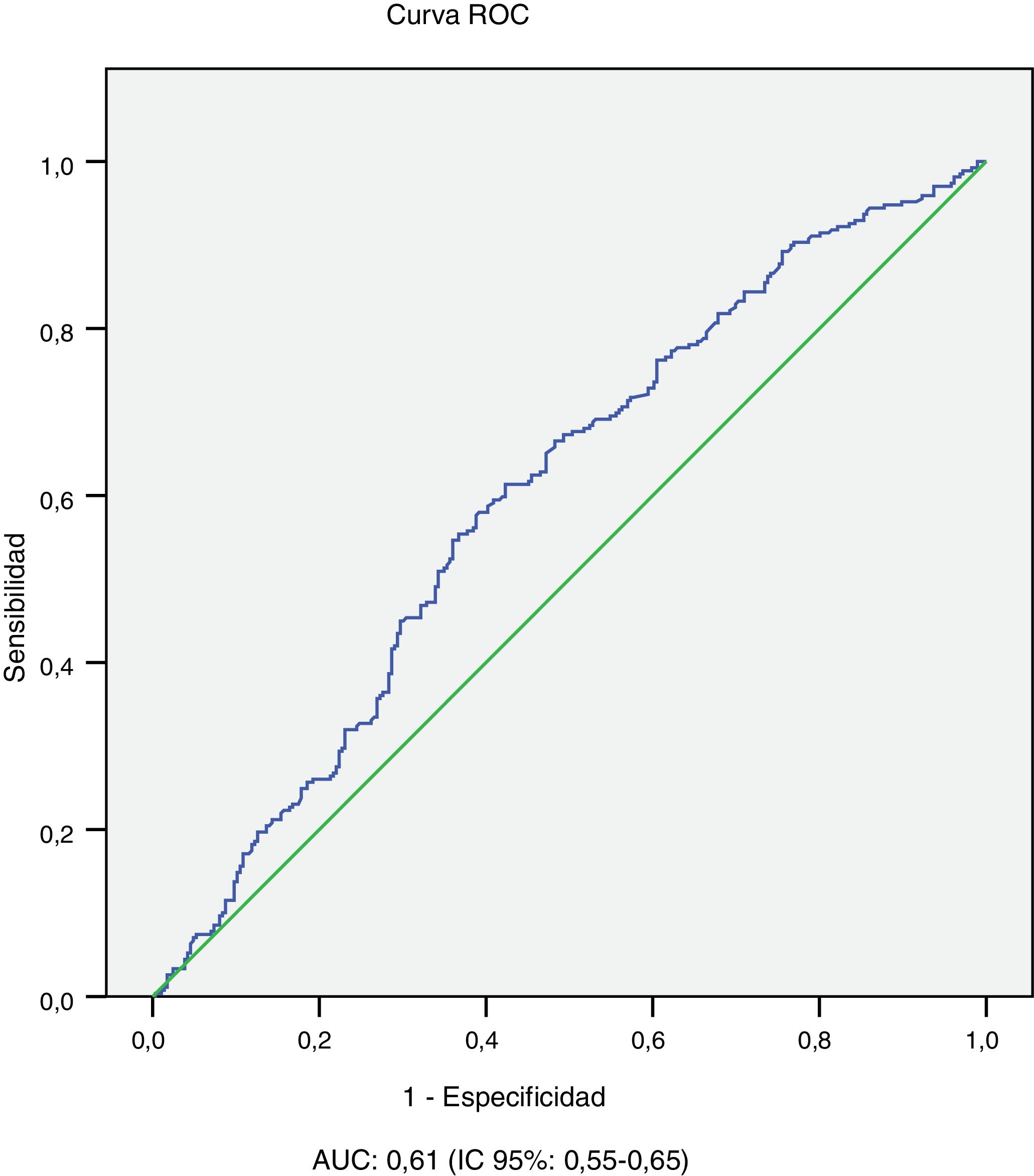
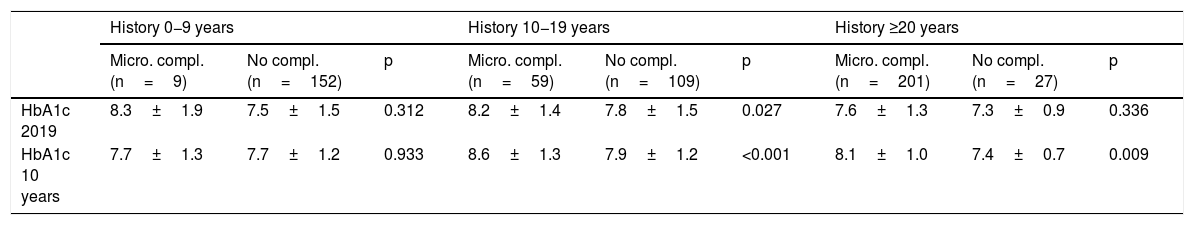
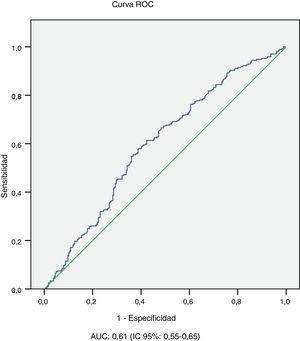
The univariate analysis shows that patients with microvascular complications (albuminuria or retinopathy) are older (43.4±11.7 vs 29.4±11.6 years; p<0.001), have a longer history of diabetes (26.6±10.3 vs 10.1±7.6 years; p<0.001), worse average evolution of HbA1c level (8.1±1.1 vs 7.8±1.2; p<0.001) and higher BMI (25.9±4.7 vs 24.7±4.8; p=0.01), and they are more frequently smokers (25.6 vs 17.4%; p=0.018) and hypertensive (32.3 vs 2.4%; p<0.001). In relation to age and history of diabetes, worse 10-year average metabolic control is observed in patients with microvascular complications in the 10−19 years and ≥20 years history of T1DM subgroups and in the age subgroups 14−25 years and 26−44 years (Table 2). In the multivariate analysis, the variables independently associated with microvascular complications (albuminuria or retinopathy) were: history of T1DM≥20 years (OR: 10.2; 95% CI: 6.87–15.15; p<0.001) HT (OR: 4.81; 95% CI: 1.91–12.14; p<0.001) and average evolution of HbA1c≥7.0% (OR: 2.29; 95% CI: 1.42–4.58; p=0.019). Finally, the area under the average evolution of HbA1c ROC curve for the detection of current microvascular complications was 0.61 (95% CI: 0.55−0.65), with a sensitivity of 90.3% and a specificity of 77.1% for an HbA1c value ≥7.0% (Table 3 and Fig. 2).
Metabolic control in 2019 and 10-year average in patients with and without microvascular complications by subgroups of age and history of diabetes.
| History 0−9 years | History 10−19 years | History ≥20 years | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Micro. compl. (n=9) | No compl. (n=152) | p | Micro. compl. (n=59) | No compl. (n=109) | p | Micro. compl. (n=201) | No compl. (n=27) | p | |
| HbA1c 2019 | 8.3±1.9 | 7.5±1.5 | 0.312 | 8.2±1.4 | 7.8±1.5 | 0.027 | 7.6±1.3 | 7.3±0.9 | 0.336 |
| HbA1c 10 years | 7.7±1.3 | 7.7±1.2 | 0.933 | 8.6±1.3 | 7.9±1.2 | <0.001 | 8.1±1.0 | 7.4±0.7 | 0.009 |
| Age 14−25 years | Age 26−44 years | Age ≥45 years | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Micro. compl. (n=13) | No compl. (n=136) | p | Micro. compl. (n=145) | No compl. (n=113) | p | Micro. compl. (n=111) | No compl. (n=37) | p | |
| HbA1c 2019 | 8.2±1.7 | 7.7±1.6 | 0.308 | 7.8±1.5 | 7.3±1.3 | 0.008 | 7.7±1.0 | 8.1±1.2 | 0.070 |
| HbA1c 10 years | 9.1±1.3 | 7.9±1.3 | 0.002 | 8.2±1.2 | 7.6±1.0 | 0.001 | 8.1±0.8 | 7.9±1.0 | 0.646 |
ave.: average; compl.: complication; micro: microvascular.
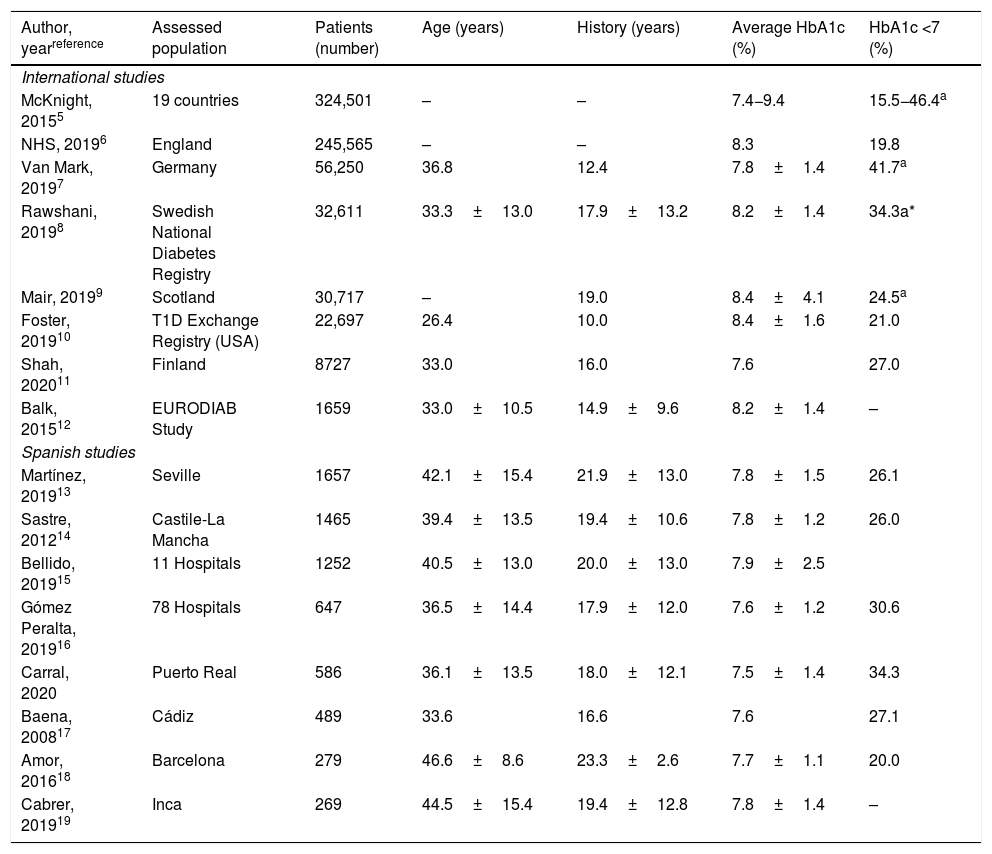
Metabolic control in patients with type 1 diabetes in national and international registries.
| Author, yearreference | Assessed population | Patients (number) | Age (years) | History (years) | Average HbA1c (%) | HbA1c <7 (%) |
|---|---|---|---|---|---|---|
| International studies | ||||||
| McKnight, 20155 | 19 countries | 324,501 | – | – | 7.4−9.4 | 15.5−46.4a |
| NHS, 20196 | England | 245,565 | – | – | 8.3 | 19.8 |
| Van Mark, 20197 | Germany | 56,250 | 36.8 | 12.4 | 7.8±1.4 | 41.7a |
| Rawshani, 20198 | Swedish National Diabetes Registry | 32,611 | 33.3±13.0 | 17.9±13.2 | 8.2±1.4 | 34.3a* |
| Mair, 20199 | Scotland | 30,717 | – | 19.0 | 8.4±4.1 | 24.5a |
| Foster, 201910 | T1D Exchange Registry (USA) | 22,697 | 26.4 | 10.0 | 8.4±1.6 | 21.0 |
| Shah, 202011 | Finland | 8727 | 33.0 | 16.0 | 7.6 | 27.0 |
| Balk, 201512 | EURODIAB Study | 1659 | 33.0±10.5 | 14.9±9.6 | 8.2±1.4 | – |
| Spanish studies | ||||||
| Martínez, 201913 | Seville | 1657 | 42.1±15.4 | 21.9±13.0 | 7.8±1.5 | 26.1 |
| Sastre, 201214 | Castile-La Mancha | 1465 | 39.4±13.5 | 19.4±10.6 | 7.8±1.2 | 26.0 |
| Bellido, 201915 | 11 Hospitals | 1252 | 40.5±13.0 | 20.0±13.0 | 7.9±2.5 | |
| Gómez Peralta, 201916 | 78 Hospitals | 647 | 36.5±14.4 | 17.9±12.0 | 7.6±1.2 | 30.6 |
| Carral, 2020 | Puerto Real | 586 | 36.1±13.5 | 18.0±12.1 | 7.5±1.4 | 34.3 |
| Baena, 200817 | Cádiz | 489 | 33.6 | 16.6 | 7.6 | 27.1 |
| Amor, 201618 | Barcelona | 279 | 46.6±8.6 | 23.3±2.6 | 7.7±1.1 | 20.0 |
| Cabrer, 201919 | Inca | 269 | 44.5±15.4 | 19.4±12.8 | 7.8±1.4 | – |
Although this study was not designed to ascertain the population prevalence of diabetes, our data show a prevalence of T1DM in those over 14 years of age of 2.7 cases per 1000 inhabitants in our area, in line with the estimated global prevalence, which ranges between 0.8 and 4.6/1000 inhabitants.20 Over the course of 10 years, 190 new cases of T1DM were recorded in patients over 14 years of age, which represents an incidence of 6.8/100,000 inhabitants per year, very similar to that reported in a recent epidemiological study carried out in Navarre.21
In the last evaluation in 2019, a third of the patients had “optimal” glycaemic control (HbA1c ≤7%), another third “adequate” control (HbA1c: 7.1–8%) and the remaining third poor glycaemic control (HbA1c ≥8%), although only 14.5% had HbA1c ≥9%, figures very similar to those reported in a multicentre study in Castile-La Mancha.14 This difference between the optimal glycaemic control targets and the results obtained in actual clinical practice is consistently repeated in the large international registries5–12 published for T1DM populations. In this regard, an international study carried out in 19 countries with data from 324,501 people with T1DM shows that, in the best of the registries (German ≥25 years), less than half of the patients achieved HbA1c levels ≤7.5%.5 Similar to that of the American Exchange Clinic Registry,10 in a study of 19 countries, the worst metabolic control was observed in the subgroup aged 15–24 years,5 an aspect not observed in our population. In Spain, the absence of a national registry of patients with T1DM prevents the making of comparisons with other countries, although several cross-sectional studies report that HbA1c ≤7.0% targets are achieved in only 26%–33% of patients with T1DM.13–19,22
In contrast to the worsening over time of glycaemic control documented in various US10 and European6,9 registries of the population with T1DM, in our cohort we observed a slow but progressive improvement in HbA1c levels (for example, −0.11% in 2018 and −0.12% in 2019), which in a decade exceeds one percentage point of HbA1c. As such, it is possible that the relatively low prevalence of microvascular complications in our population20 is due, in part, to the acceptable evolution of glycaemic control in recent years. The multivariate analysis found that the average evolution of HbA1c ≥7.0% was independently associated (OR: 2.29; 95% CI: 1.43–4.58) with albuminuria or retinopathy, similar to the results from Colom et al.22, who reported better glycaemic control in patients with T1DM without microangiopathic complications in the first 5 and 20 years of specialised follow-up.
Patients with T1DM have a marked increase in the risk of death and development of cardiovascular disease.23 The major predictors are age, HbA1c, duration of diabetes, kidney function, LDL cholesterol and systolic blood pressure.8,11 In this regard, it has been reported that for each percentage increase in HbA1c, the relative risk of mortality increases by 23% in patients with T1DM8 and that intensive insulin therapy is capable of reducing the risk of macrovascular complications in this population.1,2,24 In addition to glycaemic control, it is a priority to achieve targets in the other risk factors, especially smoking, blood pressure and lipids. In total, 20% of our patients were active smokers, in line with various national studies that report that between 16% and 35% of patients with T1DM in Spain smoke.14–19 It has been reported that tobacco use is independently associated with greater insulin resistance25 and worse metabolic control in T1DM,14,25 the latter also observed in our study. For this reason, the prevention of active smoking and the development of educational programmes aimed at quitting smoking should be part of the treatment protocols and could potentially lead to improved glycaemic control. On the other hand, both our study and several national registries14,26 suggest that many patients with T1DM have persistently high levels of LDLc, in that it has been reported that less than half of T1DM patients who meet the criteria to be treated with statins in a tertiary hospital receive this treatment.27 Furthermore, although we know that hypertension significantly increases the risk of chronic complications of T1DM,8,11 the true prevalence in this population is unknown.28 However, ambulatory blood pressure monitoring studies in Spain indicate that in patients with T1DM theoretically normotensive and normoalbuminuric, the prevalence of blood pressure abnormalities is almost 20%, and that the number of patients with a non-dipping pattern,28 an alteration associated with the development of other microvascular abnormalities and progression towards hypertension, increases to 42%.29 For this reason, it would be advisable to generalise the use of ambulatory blood pressure monitoring in these patients, since this technique makes it possible to detect subclinical blood pressure alterations, has better reproducibility, reduces “white coat hypertension” and its results correlate better with target organ damage and cardiovascular morbidity and mortality.30 Finally, in Spain, 10–28% of patients with T1DM are obese14,16,22,26 and up to 40% are overweight,16 so weight loss must be promoted in these patients since obesity in this population increases the risk of mortality and cardiovascular disease.31
This study has some limitations that we must consider. Firstly, because the information was obtained from a database completed by the staff from our unit, information from some records may be missing or there may be errors in some data. However, the registry has been active for 10 years and is periodically evaluated, by which new data are included and detected errors are corrected, which significantly minimises this bias. A second limitation arises from the selection of patients, by including in the analysis only those who attended consultations in 2019 (79.5% of those registered), who showed better cumulative HbA1c during follow-up than patients who did not attend in 2019 (7.9%±1.2% vs 8.6%±1.5%; p<0.001). In our favour is the fact that loss to follow-up is notably lower than that documented in other similar national studies, in which loss to follow-up ranges from 37% to 45%.18,19 Finally, as it is a retrospective observational study, causal relationships cannot be established between the absence of microvascular complications and the improvement in glycaemic control observed. However, in this regard, we consider that there is sufficient biological plausibility in favour of causality between the best metabolic control and the lowest prevalence of microvascular complications.1,32,33
The results of our study emphasise the need for active clinical registries for patients with T1DM in clinical practice, ideally encompassed in a single national registry similar to that available in other European countries6,7,9,11, with the objectives of identifying deficient areas and developing improvement plans aimed at avoiding or delaying the development of complications of the disease. The notable improvement in evolution of glycaemic control of patients with T1DM under active follow-up observed in our study, greater than that of patients who are lost to follow-up, obliges us to establish proactive measures aimed at “rescuing” these patients, who are generally poorly controlled,19 in an attempt to improve disease control and facilitate patient enrolment in the significant therapeutic advances that are available today4 or that will be available in the near future.34,35 In addition, and in order to reduce chronic complications, it is necessary to avoid an "exclusively glucocentric" approach to the treatment of T1DM, optimising the control of vascular risk factors, especially the control of LDLc and blood pressure, and promoting giving up smoking and weight loss when overweight or obese.
In conclusion, in our setting in the last 10 years we have observed a progressive, but still insufficient, improvement in metabolic control in patients with T1DM, associating microvascular complications with poorer evolution of metabolic control during follow-up.
FundingThis study has not been funded publicly or privately.
Authorship/collaborationsDr Carral, nurse Piñero and nurse Expósito were responsible for collecting data in the “Type 1 Diabetes” database.
Dr Carral was responsible for the design of the study, analysis and interpretation of the data and the original drafting of the article.
Dr Fernández participated in the correction of the statistical study.
Dr Ayala, Dr Tomé, Dr Jiménez and Dr García participated in the clinical care of the study patients, data collection, critical reading, editing of the article and approval of the final version for publication.
Conflicts of interestThe authors of this article guarantee that it is an original article not published or under consideration for publication in another journal. The authors have no conflicts of interest in relation to the objective or the results of this article.
Please cite this article as: Carral F, Tomé M, Fernández JJ, Piñero A, Expósito C, Jiménez AI, et al. La presencia de complicaciones microvasculares se asocia con un mal control metabólico evolutivo en pacientes con diabetes tipo 1. Endocrinol Diabetes Nutr. 2021;68:389–397.