The Working Groups of Cardiovascular Pharmacotherapy of the Sociedad Española de Cardiología and Cardiovascular Disease of the Sociedad Española de Diabetes have prepared a consensus document on the treatment of hypertriglyceridaemia in patients with high/very-high-cardiovascular risk with icosapent ethyl, a highly purified and stable eicosapentaenoic acid ethyl ester. This document is necessary since there are differences among the three main omega-3 fatty acids and there is large-scale clinical evidence with icosapent ethyl that demonstrates that in addition to its efficacy in lowering triglyceridaemia, it reduces the risk of cardiovascular events in both patients with atherosclerotic cardiovascular disease and in those with type 2 diabetes, with a good safety profile. The number needed to treat to avoid a major cardiovascular event is analysed, comparing it with other pivotal studies of pharmacological intervention in cardiovascular prevention, and an estimate of the Spanish population likely to be treated with ethyl icosapent is carried out. These recommendations are of interest to all clinicians who manage patients with lipid metabolism disorders, cardiovascular disease and diabetes.
Los Grupos de Trabajo de Farmacoterapia Cardiovascular de la Sociedad Española de Cardiología y de Enfermedad Cardiovascular de la Sociedad Española de Diabetes han elaborado un documento de consenso sobre el tratamiento de la hipertrigliceridemia en pacientes de alto/muy alto riesgo cardiovascular con el icosapento de etilo, éster etílico del ácido eicosapentaenoico altamente purificado y estable. Este documento es necesario al existir diferencias entre los tres principales ácidos grasos omega-3, y disponer de evidencias clínicas a gran escala con el icosapento de etilo que demuestran que, además de su eficacia para disminuir la trigliceridemia, reduce el riesgo de episodios cardiovasculares tanto en los pacientes con enfermedad cardiovascular aterosclerótica como en aquellos con diabetes mellitus tipo 2, con un buen perfil de seguridad. Se analiza el número necesario a tratar para evitar un episodio cardiovascular grave, comparándolo con otros estudios seminales de intervención farmacológica en prevención cardiovascular, y se lleva a cabo una estimación de la población española susceptible de ser tratada con el icosapento de etilo. Estas recomendaciones son de interés para todos los profesionales que tratan a pacientes con alteraciones del metabolismo lipídico, enfermedad cardiovascular y diabetes.
Cardiovascular disease (CVD), and especially coronary heart disease (CHD) and atherothrombotic ischaemic stroke, represents the main cause of mortality worldwide and one of the main contributing factors of disability. According to the 2019 Global Burden of Diseases Study update.1 the prevalence of CVD nearly doubled from 271 million in 1990 to 523 million in 2019, and the number of deaths from CVD increased from 12.1 million in 1990 to 18.6 million in 2019. Global trends in disability-adjusted life years also increased significantly, doubling from 17.7 million to 34.4 million during the same period. In Spain, according to data from the Instituto Nacional de Estadística (INE) [National Institute of Statistics],2 diseases of the circulatory system continue to be the leading cause of death in 2020 (119,853 deaths, 24.3% of total deaths). Therefore, there is an imperative need to reduce the burden of CVD through the implementation of health policies and cost-effective interventions aimed at achieving effective cardiovascular prevention.
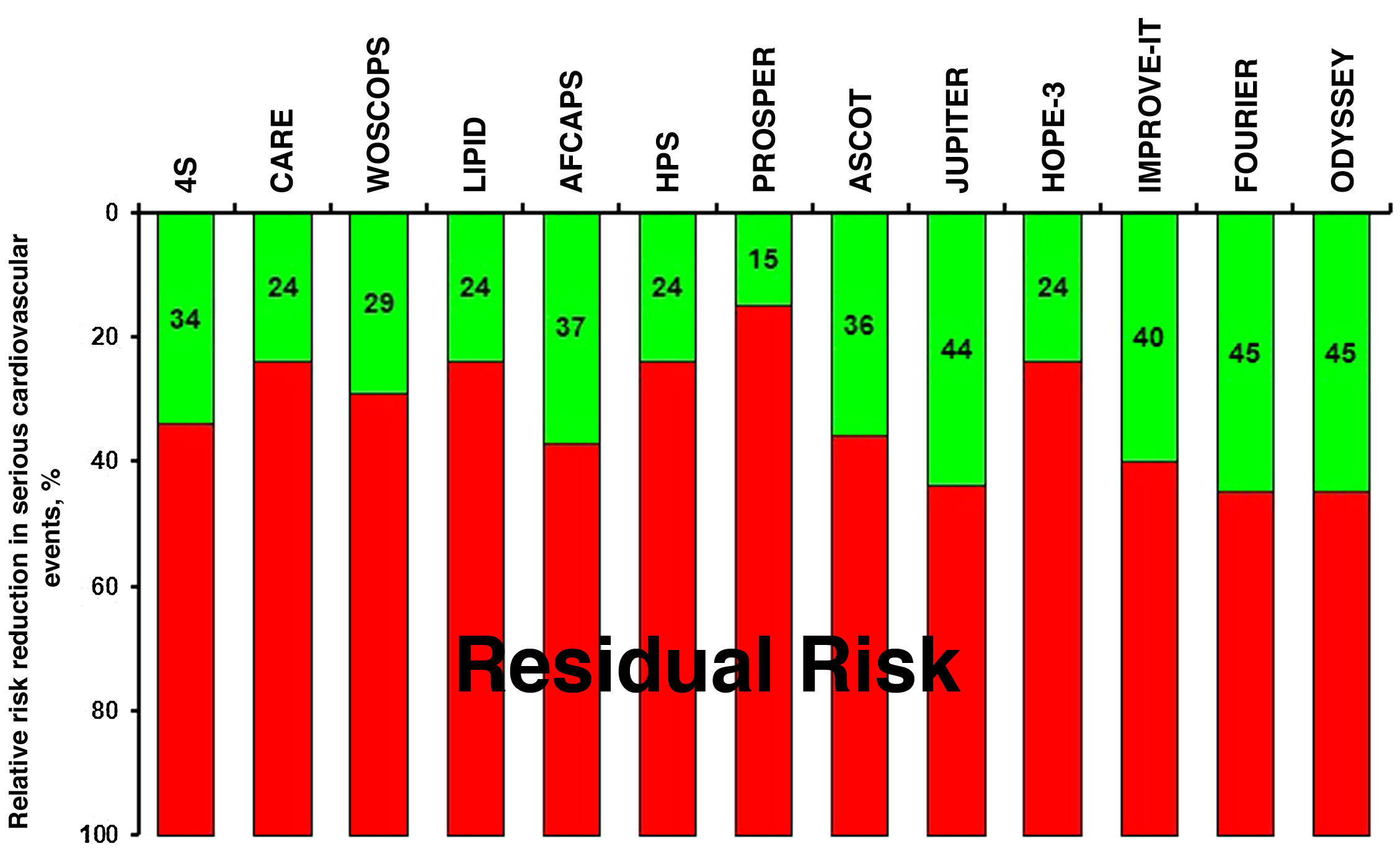
The pillars of optimal cardiovascular prevention include quitting or avoiding smoking, a heart-healthy diet, weight and stress control, regular physical exercise, and regular monitoring of blood pressure, blood glucose and lipid profile. However, despite the implementation of strategies aimed at the favourable modification of lifestyle, together with the use of pharmacological therapies based on clinical evidence with antihypertensive, antidiabetic, hypocholesterolaemic and antiplatelet agents, a high residual risk persists, especially in patients at high/very high cardiovascular risk.3–7 In this regard, clinical studies indicate that, despite treatment with statins, some subgroups of patients show a rate of cardiovascular events greater than 30% and 40% at 10 years.8 Together with the evidence from intervention studies with statins in monotherapy,9–18 the IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT),19 the Further cardiovascular OUtcomes Research with PCSK9 Inhibition in subjects with Elevated Risk (FOURIER)20 and the Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab (ODYSSEY OUTCOMES)21 have confirmed that dual statin therapies with ezetimibe or proprotein convertase subtilisin/kexin 9 (PCSK9) inhibitors, respectively, achieve greater reductions in low-density lipoprotein cholesterol (LDL-C), which implies additional reductions in cardiovascular events. However, we must bear in mind that there is still a high residual risk in these patients with very high cardiovascular risk (Fig. 1).
From what has been stated in the previous lines, it can be deduced that, although the reduction of LDL-C notably reduces cardiovascular risk, it does not eliminate it completely. In addition to LDL-C, other components of the lipid profile such as lipoproteins rich in triglycerides (TG)22,23 and lipoprotein(a) [Lp(a)]24,25 participate in atherogenesis. Epidemiological and Mendelian randomisation studies show that hypertriglyceridaemia is an independent marker of cardiovascular risk.23,26–29 Thus, in the Pravastatin or Atorvastatin Evaluation and Infection Therapy Thrombolysis In Myocardial Infarction (PROVE IT-TIMI) study,30 a TG concentration ≥150 mg/dl was a predictor of increased cardiovascular risk despite being treated with statins and having an LDL-C <70 mg/dl. However, until 2019, intervention studies with drugs that reduce plasma TG concentrations such as extended-release niacin with laropiprant31,32 and fibrates33,34 have not shown cardiovascular benefits when administered together with conventional medical treatment, including statins. The only evidence comes from post hoc analyses of patients with atherogenic dyslipidaemia, or one of its components, treated with fibrates,35–38 so its use should be reserved for cases of hypertriglyceridaemia, particularly in some subgroups of patients, especially when icosapent ethyl cannot be used. The Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in patients with Diabetes (PROMINENT) study,39 which will end in mid-2022, is evaluating the efficacy of pemafibrate (0.2 mg twice daily), a potent and selective selective modulator of peroxisome proliferator-activated receptor α (PPAR-α), in reducing the risk of cardiovascular events in 10,000 patients with diabetic dyslipidaemia (TG between 200 and 499 mg/dl [2.26 and 5.64 mmol/l] and high-density lipoprotein cholesterol [HDL-C] concentration ≤40 mg/dl [1.03 mmol/l]), who are already receiving statins.
In reference to omega-3 fatty acids, randomised studies with a mixture of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) at low doses (1 g/day), such as the Outcome Reduction with an Initial Glargine Intervention (ORIGIN) study40 with 12,536 patients, the Study of Cardiovascular Events in Diabetes (ASCEND)41 with 15,480 participants and the Vitamin D and Omega-3 Trial (VITAL)42 with 25,871 subjects, or high doses (4 g/day) in the Long-Term Outcomes Study to Assess Statin Residual Risk with Epanova in High Cardiovascular Risk Patients with Hypertriglyceridemia (STRENGTH),43 as well as a meta-analysis44 with 77,917 individuals from 10 studies, in nine of which the omega-3 fatty acid was a combination of EPA and DHA, have not shown cardiovascular protective effects in patients receiving statin therapy.
Specifically, the STRENGTH study43 was designed to assess the effect of a combination of EPA/DHA (4 g/day, in a ratio of 2.75:1) in patients with high cardiovascular risk and atherogenic dyslipidaemia treated with statins, using corn oil as placebo. The study was stopped prematurely based on an interim analysis that showed a low probability of clinical benefit from the use of this combination versus corn oil, despite observing a significant reduction in the concentration of TG (19%) and of high-sensitivity C-reactive protein (hs-CRP) (20%) in the omega-3 group versus the control group.
The Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention (REDUCE-IT) study45 has shown that a dose of 4 g/day of icosapent ethyl reduced the risk of ischaemic events, including cardiovascular death, in patients treated with statins with concentrations of LDL-C between 41 and 100 mg/dl (1.06–2.59 mmol/l) and of TG between 135 and 499 mg/dl (1.52–5.63 mmol/l), with established CVD or type 2 diabetes mellitus (DM2) with at least one risk factor. The solid findings of the REDUCE-IT study and the pre-specified sub-analyses (Fig. 2) are reflected in the indications approved on 26 March 2021 by the European Medicines Agency (EMA)46 and in the different European,47–49 American50–52 and Canadian53 clinical guidelines for cardiovascular prevention and control of dyslipidaemia.
Main findings of the REDUCE-IT study and the pre-specified sub-analyses.
aCardiovascular death, non-fatal myocardial infarction, non-fatal stroke, coronary revascularisation or unstable angina.
bNon-fatal cardiovascular death, myocardial infarction or stroke.
PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft; HR: hazard ratio; CI: confidence interval.
Different hypotheses have been proposed to explain the unequal results obtained in the trials with icosapent ethyl and EPA/DHA mixture (Table 1), including the possible negative effects of mineral oil used as placebo, the differential effects of EPA and DHA, and even the possibility that EPA and DHA counterregulate each other. However, the Food and Drug Administration (FDA), Health Canada and the EMA have all concluded that any theoretical effect from the choice of placebo on the observed 25% risk reduction in the REDUCE-IT study would have been non-significant.54
Main differences between the two large clinical studies of cardiovascular prevention with an EPA/DHA mixture or icosapent ethyl.
| STRENGTH43 | REDUCE-IT45 | |
|---|---|---|
| Patients | High CVR with atherogenic dyslipidaemia | CVD or DM2 with ≥1 CVRF treated with statins, LDL-C 41−100 mg/dl and TG 135−499 mg/dl |
| Active treatment | EPA + DHA (4 g/day) | Icosapent ethyl (4 g/day) |
| Placebo | Corn oil | Mineral oil |
| Clinical follow-up | Premature discontinuation | 4.9 years |
| LDL-C (mg/dl) | ||
| Baseline | 75 (56−99) | 74 (61.5−88) |
| Δ% with treatment at one year | 1.2 | −3.1 |
| HDL-C (mg/dl) | ||
| Baseline | 36 (31−40) | 40 (34.5−46) |
| Δ% with treatment at one year | 5.6 | −2.6 |
| Non-HDL-C (mg/dl) | ||
| Baseline | 125 | 118 |
| Δ% with treatment at one year | −6.1 | −3.6 |
| Triglycerides (mg/dl) | ||
| Baseline | 239 (192−307) | 216.5 (176.5−272) |
| Δ% with treatment at one year | −19 | −18.3 |
| Apolipoprotein B (mg/dl) | ||
| Baseline | 56.2 | 82 |
| Δ% with treatment at 2 years | −2 | −2.5 |
| hs-CRP (mg/l) | ||
| Baseline | 2.1 (1.1−4.2) | 2.2 (1.1−4.5) |
| Δ% with treatment at one year | −20 | −13.9 |
| EPA (μg/ml) | ||
| Baseline | 21.0 | 26.1 (17.1−40.1) |
| Δ% with treatment at one year | 269 | 393.5 |
| Main objective | HR 0.99 (95% CI 0.90−1.09) | HR 0.75 (95% CI 0.68−0.83) |
| p = 0.84 | p < 0.001 | |
| Secondary objective | HR 1.05 (95% CI 0.93−1.19) | HR 0.74 (95% CI 0.65−0.83) |
| p < 0.001 |
HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; DHA: docosahexaenoic acid; DM2: type 2 diabetes mellitus; CVD: cardiovascular disease; EPA: eicosapentaenoic acid; CVRF: cardiovascular risk factor; hs-CRP: high-sensitivity C-reactive protein; TG: triglycerides; HR: hazard ratio; CI: confidence interval.
Icosapent ethyl is in the process of review by the Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) [Spanish Agency of Medicines and Medical Devices], so we consider it appropriate that the Cardiovascular Pharmacotherapy Working Group of the Spanish Society of Cardiology and the Cardiovascular Disease Working Group of the Spanish Society of Diabetes involved in the control of high and very high risk patients with new cardiovascular drugs establish their indications for use.
Omega-3 fatty acidsThe three main omega-3 fatty acids involved in human physiology are alpha-linolenic acid (ALA) (derived from vegetable oils), EPA and DHA (both derived mainly from fish oils).55 New knowledge indicates that EPA and DHA differ in their effects on cell membrane structure, lipid oxidation, inflammatory biomarkers and tissue distribution. In this sense, DHA is found mainly in the membranes of the brain and retina, and EPA in the vascular membranes. EPA forms part of cell membranes, preserving their structure and the normal distribution of cholesterol, and is a precursor to some eicosanoids.56,57 In endothelial cells, EPA, unlike DHA, has the ability to reverse endothelial dysfunction and produce a greater release of nitric oxide, a potent inhibitor of platelet aggregation, of the synthesis of adhesion molecules, of chemotactic agents and of proinflammatory cytokines.58 It also exerts positive effects on blood pressure, insulin resistance, and signal transduction pathways related to inflammation. Its reducing effect on plasma TG levels is due to the decrease in the synthesis of very low density lipoproteins (VLDL) by inhibition of phosphatidic acid phosphatase and diacylglycerol-acyl-transferase, to the increased clearance of VLDL particles, and to the lower activity of apolipoprotein (Apo) C-III that leads to the activation of lipoprotein lipase (LPL).59 In addition, EPA increases serum antioxidant capacity by increasing glutathione peroxidase activity and decreasing malonyldialdehyde activity, and inhibits the oxidation of Apo B-containing particles such as LDL, VLDL and intermediate-density lipoproteins (IDL).60 Consequently, it decreases the concentrations of TG (20–50%) and VLDL (35%), without increasing those of LDL-C (which DHA does do),61 but it does increase those of HDL-C.62
Despite all of the above, the exact mechanism(s) of action of EPA remain unknown. Similarly, sodium-glucose cotransporter-2 (SGLT-2) inhibitors have shown very notable cardiovascular benefits, despite their moderate hypoglycaemic effect,63 a fact that exemplifies the possible disconnection between the selected biomarkers and the observed clinical benefits.
Cardiovascular prevention studies with omega-3 fatty acidsDietary supplements of omega-3 fatty acids have not consistently shown substantial reductions in TG, and the possible cardiovascular effects are controversial.64 The decrease in triglyceridaemia is dose-dependent, with considerable inter-individual variability, but with greater absolute risk reductions in those with higher baseline concentrations.55 Furthermore, the findings from clinical studies have not been entirely consistent, and current data do not support the routine use of omega-3 fatty acid supplements for the prevention of cardiovascular disease.65–68 For this reason, this document will focus on analysing the results with the different pharmacological formulations of omega-3 fatty acids.
In the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico (GISSI-Prevenzione) study,69 11,324 patients with a myocardial infarction were randomised to receive omega-3 fatty acids (1 g/day of EPA/DHA in a mean ratio of 1:2) or vitamin E (300 mg/day), both or neither. At 3.5 years, treatment with the EPA/DHA combination was associated with a significant reduction of the endpoint of death, myocardial infarction, or non-fatal stroke (RR 0.90; 95% CI 0.82−0.99), but not of the secondary endpoint (cardiovascular death, myocardial infarction or non-fatal stroke) (RR 0.89; 95% CI 0.80–1.01). It should be noted that <5% of the patients were on lipid-lowering therapy at the beginning of the study, given that they were recruited in the period 1993−95.
The ASCEND41 and VITAL42 studies that evaluated the use of omega-3 fatty acids (1 g/day of EPA/DHA in a mean ratio of 1.3:1) in subjects in primary prevention with and without diabetes, respectively, did not observe a significant reduction of the primary objective. The prevalence of lipid-lowering therapy in both studies was approximately 75% in the ASCEND study and 38% in the VITAL study and, therefore, much higher than in the GISSI-Prevenzione study.69 These results could be explained because the population was of low/moderate risk, the formulation used for omega-3 acids was a mixture of EPA/DHA, or because of the low dose used in one or other of the studies. Therefore, when evaluating intervention studies with omega-3 fatty acids in cardiovascular prevention, three fundamental aspects must be taken into consideration: the type of omega-3 fatty acid, the dose tested and the study population with respect to the level of risk and the presence or absence of hypertriglyceridaemia.
Studies of cardiovascular prevention with icosapent ethylFew studies have examined the efficacy of EPA alone, and specifically of icosapent ethyl. In the Japan EPA Lipid Intervention Study (JELIS),70 18,645 participants with a total cholesterol concentration ≥250 mg/dl (6.5 mmol/l) were randomised to receive 600 mg of icosapent ethyl three times daily plus statin, or statin alone. The mean statin doses were pravastatin 10 mg/day and simvastatin 5.6 mg/day. After a mean clinical follow-up of 4.6 years, the risk of a serious coronary event was reduced by 19% in the icosapent ethyl group (HR 0.81; 95% CI 0.69−0.95; p = 0.01). The results were comparable between patients with or without CHD, although they were only significant in those in secondary prevention (HR 0.81; 95% CI 0.67−0.998; p = 0.048). The reduction in concentration of LDL-C was similar in both groups (25%), but that of TG was more marked in the icosapent ethyl group (9% vs. 4%; p < 0.001). The use of stable EPA ethyl ester showed a greater relative reduction in the primary endpoint among patients with a TG concentration ≥150 mg/dl and HDL-C <40 mg/dl (HR 0.47; 95% CI 0.23−0.98; p = 0.04).71 The risk of coronary events was also correlated with plasma levels of EPA during treatment.72 It is interesting to note that the population in this study started with baseline plasma concentrations of EPA four times higher than those described in the STRENGTH and REDUCE-IT studies, a fact attributable in part to the abundant intake of oily fish in the Japanese diet, and that despite receiving only 1.8 g/day of EPA, participants reached levels similar to those of the REDUCE-IT population who received 4 g/day.73
In the Combination Therapy of Eicosapentaenoic Acid and Pitavastatin for Coronary Plaque Regression Evaluated by Integrated Backscatter Intravascular Ultrasonography (CHERRY) study,74 193 patients undergoing percutaneous coronary intervention were randomly assigned to receive icosapent ethyl 1.8 g/day plus pitavastatin 4 mg/day, or pitavastatin monotherapy. Based on coronary intravascular ultrasound findings, after six to eight months of follow-up, icosapent ethyl and pitavastatin combination therapy significantly reduced coronary plaque volume compared with statin monotherapy. Furthermore, plaque stabilisation criteria were enhanced by combination therapy, especially in patients with stable CHD.
Finally, the REDUCE-IT study45 included 8179 participants with established CVD or DM2 with one or more additional risk factors who had received a statin for ≥4 weeks, with a fasting TG concentration of 135–499 mg/dl and LDL-C of 41–100 mg/dl (mean: 75 mg/dl), and they were assigned to icosapent ethyl 2 g twice daily, or placebo. With a median of 4.9 years of follow-up, the risk of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, coronary revascularisation or unstable angina was significantly reduced in the icosapent ethyl group by 25%, as was the secondary endpoint of cardiovascular death, non-fatal myocardial infarction or non-fatal stroke.45,75 In fact, all cardiovascular endpoints were significantly improved in patients assigned to icosapent ethyl, including vascular death (HR 0.80, 95% CI 0.66−0.98; p = 0.03) and total ischaemic events (first and successive) (HR 0.70; 95% CI 0.62−0.78; p < 0.001).76,77 In addition, the beneficial effects of icosapent ethyl were consistent across all tertiles of baseline TG, including in 10% of participants with desirable TG levels, regardless of LDL-C levels.78 It should be noted that the efficacy of icosapent ethyl was greater than expected based on the 18.3% reduction in TG levels, a fact that reinforces the concept that icosapent ethyl has pleiotropic effects beyond the decrease in TG levels.
In the REDUCE-IT study, first revascularisations decreased significantly by 34%, with similar reductions in total revascularisations (first and successive), and according to whether they were elective, urgent or emergent.79 Icosapent ethyl also reduced the number of percutaneous coronary interventions (HR 0.68; 95% CI 0.59−0.79; p < 0.0001) and coronary artery bypass surgery (HR 0.61; 95% CI 0.45−0.81; p = 0.0005).79
Recently, the REDUCE-IT RENAL study,80 with a median estimated glomerular filtration rate of 75 ml/min/1.73 m2, has shown after a mean follow-up of 4.9 years that icosapent ethyl reduced the relative risk by 25% and absolute risk by 4.8% in the primary composite endpoint of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, coronary revascularisation or unstable angina. It should be noted that the reduction was more marked in patients with a filtration rate <60 ml/min/1.73 m².
Additionally, of the 8179 patients in the REDUCE-IT study, 1837 (22.5%) had a history of coronary revascularisation surgery. The REDUCE-IT patients with prior coronary artery bypass graft (CABG) surgery81 randomised to icosapent ethyl showed a significant reduction of 24% in the primary endpoint, 31% in the secondary endpoint, and in the total number (first and successive) of ischaemic events (rate ratio 0.64, 95% CI 0.50−0.81; p = 0.0002) compared to placebo.
Almost 60% of the population included in the REDUCE-IT study45 had DM2. In 2020, the American Diabetes Association Congress reported the results for this specific population. There was a 23% reduction in the primary composite endpoint of cardiovascular death, myocardial infarction, stroke, coronary revascularisation or unstable angina, and a 30% reduction in the secondary endpoint of cardiovascular death, myocardial infarction and stroke.82
The Effect of Vascepa on Improving Coronary Atherosclerosis in People With High Triglycerides Taking Statin Therapy (EVAPORATE) study83 established whether the addition icosapent ethyl 4 g/day to statin therapy modifies atherosclerotic plaque assessed by multidetector computed tomography in middle-aged hypertriglyceridaemic patients (135−499 mg/dl) with established CHD (≥1 angiographically demonstrated stenosis ≥20%). At 18 months, treatment with icosapent ethyl and statins was associated with a significant reduction in the volume and characteristics of coronary plaques, compared with the statin plus placebo group. It is noteworthy that patients in the icosapent ethyl group had a 17% (p < 0.01) relative reduction in low-attenuation plaque volume compared with the placebo group, with low-attenuation plaque volume being the strongest predictor of fatal and non-fatal myocardial infarction.84
Regarding the safety profile of icosapent ethyl, adverse effects were equally distributed between the two treatment arms, regardless of severity. The rate of major bleeding events, including haemorrhagic stroke, and other major central nervous system and gastrointestinal bleeding, was not significantly increased in the icosapent ethyl group (2.7% vs. 2.1%; p = 0.06). However, when minor bleeding was considered, all adverse bleeding events increased significantly (11.8% vs. 9.9%; p = 0.006), a fact that could be explained by the antithrombotic effect of the molecule. Furthermore, while the rate of hospitalisation for atrial fibrillation or atrial flutter was higher in the icosapent ethyl group (3.1% vs. 2.1%; p = 0.004), the risk of stroke was lower (HR 0.72; 95% CI 0.55−0.93; p = 0.01).45 We must not forget that the risk of bleeding increases when icosapent ethyl is co-administered with anticoagulants or platelet antiaggregants, so these patients should be monitored periodically. In patients with hepatic insufficiency, alanine and aspartate aminotransferase levels should be determined, at appropriate intervals, before the initiation of treatment. Icosapent ethyl is only contraindicated in patients with hypersensitivity to the active ingredient, soybeans, peanuts or any of the excipients.85
Currently, the Randomized trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy – Statin and Eicosapentaenoic Acid (RESPECT-EPA)86 has recruited 3900 patients with stable CHD treated with statins, who have been randomly assigned to receive 1.8 g daily of EPA or conventional treatment, to assess the impact on the incidence of new cardiovascular episodes.
Indications for use of icosapent ethylIcosapent ethyl (4 g/day) has confirmed its efficacy in reducing cardiovascular morbidity and mortality in patients with atherosclerotic CVD and/or DM2 with at least one risk factor and LDL-C concentrations <100 mg/dl and TG < 500 mg/dl. Given that lipid-lowering drug treatments must generally be maintained for life, we consider that this treatment should only by indicated for patients who are most likely to benefit, in order to optimise healthcare resources. As we cannot estimate the financial cost due to the lack of a reference indicator to evaluate the cost-effectiveness of the use of icosapent ethyl per disability-adjusted life year, in this document we have used <25 as a reference for the number needed to treat (NNT) to avoid a cardiovascular event at five years, as has been described in other works.87 Therefore, the objective of this recommendation is to select the groups with the lowest NNT, so that we can obtain the maximum benefit by treating the least number of patients. This is equivalent to treating patients with mild-to-moderate hypertriglyceridaemia and high or very high cardiovascular risk.
Since there is only one intervention study with icosapent ethyl with statins, we have considered it pertinent to compare it with other seminal cardiovascular prevention studies, and to estimate the population susceptible to receiving this therapeutic strategy. Table 2 shows the main characteristics and results, including the NNT, of the Scandinavian Simvastatin Survival Study (4S),9 and the Heart Outcomes Prevention Evaluation (HOPE),88 EMPA-REG89 and REDUCE-IT45 studies. It should be noted that, of all the indicated studies, the REDUCE-IT45 study started from the lowest baseline LDL-C concentration, given that all patients were treated with statins and the inclusion criterion was LDL-C <100 mg/dl, and with the lowest five-year NNT (21). Moreover, in the population exclusively with stable CVD, the NNT at five years was 14.90 It should be noted that in the analysis of coronary revascularisation79 there was a reduction in relative and absolute risk of 34% and 4.1%, respectively, with a five-year NNT of 24. Likewise, in the REDUCE-IT CABG analysis81 there was an absolute risk reduction of 6.2% (95% CI 2.3–10.2) in the first episodes, with a five-year NNT of 16 (95% CI 10–44).
Characteristics and results of the main intervention studies, especially in patients with cardiovascular disease.
| Clinical study (year, drug) | Sample size (n) | Follow-up (years) | Patients | Treatment with statins or antihypertensives | Baseline lipid profile (mg/dl) | NNT |
|---|---|---|---|---|---|---|
| 4S9 (1994, simvastatin) | 4444 | 5.4 | CHD: | Before statins | TC 260 | 30 |
| DM2 (5%) | HDL-C 45 | |||||
| HT (26%) | LDL-C 188 | |||||
| – Smokers (24%) | TG 130 | |||||
| HOPE88 (2000, ramipril) | 9297 | 5 | CHD: | Before ACEI/ARB | Hypercholesterolaemia 65% | |
| DM2 (39%) | <30% statins | Low HDL-C 18% | 56 | |||
| HT (47%) | ||||||
| – Smokers (14%) | ||||||
| EMPA-REG89 (2015, empagliflozin) | 7020 | 3.1 | DM2 and CHD | 81% ACEI/ARB | TC 163.5 | 39 |
| 77% statins | HDL-C 44.6 | |||||
| LDL-C 85.9 | ||||||
| TG 170.5 | ||||||
| REDUCE-IT45 (2019, icosapent ethyl) | 8179 | 4.9 | CVD | 100% statins | HDL-C 40 | 21 |
| DM2 + CVRF (∼60%) | LDL-C 74 | |||||
| TG 216.5 |
ARB: angiotensin II receptor blocker; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TC: total cholesterol; DM2: type 2 diabetes mellitus; CHD: coronary heart disease; CVD: cardiovascular disease; CVRF: cardiovascular risk factor; HT: hypertension; ACEI: angiotensin-converting enzyme inhibitor; NNT, number needed to treat; TG: triglycerides.
We have approached the calculation in two well-differentiated sections: first, the population with stable CVD, and then the population with DM2 and at least one cardiovascular risk factor, according to the inclusion and exclusion criteria of the REDUCE-IT study.45 According to data from the INE 2020,91 the Spanish over-45 population is 23,373,937 and the over-50 population is 19,430,931. To estimate the population likely to be treated, we used a large international registry and applied it to the Spanish over-45 population for stable CVD and over-50 population for those with diabetes in primary prevention. As a result, based on the analysis of more than 65,000 patients from 44 countries, with stable CVD or ≥3 risk factors from the Reduction of Atherothrombosis for Continued Health (REACH) registry,92 after excluding patients based on age criteria, TG < 135 mg/dl or >500 mg/dl, LDL-C >100 mg/dl and those who did not receive statins, there was an eligible population of 11.2% for stable CVD and 12.3% for DM2.
If we apply the prevalence of stable CVD of 9.2% from the national SIMETAP study,93 conducted in 64 primary care centres in the community of Madrid, to the over-45 population according to the INE 2020,91 the target population would be 2,150,402 people. If we then select 11.2% from this population, according to the data from the REACH registry,92 the result would be a population in secondary prevention eligible to be treated with icosapent ethyl of 240,845 people.
In reference to diabetes, if we focus on the over-50 population, taking the reference from the INE 202091 and the prevalence of DM of 13.8% from the Di@bet.es study,94 we are considering a total population with DM2 of 2,681,468 people. In a recent study carried out in Catalonia using the database of the Sistema d'Informació per al Desenvolupament de la Investigació en Atenció Primària (SIDIAP)95 [Information System for the Development of Research in Primary Care], the cardiovascular risk of the population with DM2 cared for in basic health areas has been evaluated. According to the risk categories of the 2019 guideline of the European Society of Cardiology in collaboration with the European Association for the Study of Diabetes,96 of the 373,185 patients with diabetes evaluated, 7% had a moderate risk, 39.6% a high risk and 26.7% a very high risk defined by the presence of target organ injury or ≥3 risk factors (excluding established CVD). Therefore, if we extrapolate the data from the study by Cebrián-Cuenca et al.95 for patients with DM and high or very high risk without CVD (66.3%), we would be talking about a DM2 population with high/very high risk of 1,777,813 people. Finally, if we apply the percentage of the eligibility criterion (12.3%) for the population with DM2 according to the results of the REACH registry,92 the over-50 population with DM2 with high/very high cardiovascular risk (without CVD) susceptible to intervention would be 218,671 people.
Based on the known inter-individual variability of plasma TG and the different clinical guidelines,48,49 we have considered the use of icosapent ethyl to be opportune only in patients with hypertriglyceridaemia between 200 and 500 mg/dl (2.3–5.6 mmol/l) (Fig. 3). Therefore, the population to be treated would be lower than the estimates made by having used the TG criterion between 135 and 500 mg/dl.
Recommendations for the clinical indication of icosapent ethyl.
*CVRFs to be considered: male ≥55 years old or female ≥65 years old; active smoker or given up <3 months previously; arterial hypertension or in antihypertensive treatment; HDL-C ≤40 mg/dl (1.04 mmol/l) in men or ≤50 mg/d (1.3 mmol/l) in women; hs-CRP >3 mg/l; renal dysfunction: glomerular filtration rate >30 and <60 ml/min; retinopathy; micro- or macroalbuminuria; ankle-brachial index <0.9 without symptoms of intermittent claudication.
LDL-C: low-density lipoprotein cholesterol; CVD: cardiovascular disease; DM2: type 2 diabetes mellitus; CVRF: cardiovascular risk factor; CVR: cardiovascular risk; TG: triglycerides.
The results of the population to be treated in Spain differ from those described in the American population, extrapolating the eligibility data from the NHANES study.97 Thus, 3,041,891 people would be eligible to receive icosapent ethyl therapy in the US, of which 1,908,781 would be the population with established CVD and 1,133,110 would come from the population with DM2 in primary prevention. In Spain, therefore, the population to be treated is six times smaller, with the population in secondary prevention and the population with diabetes being very similar quantitatively, unlike what was estimated in the NHANES study, a fact that is probably attributable to the origin of the data from the studies.
ConclusionIn situations of high/very high cardiovascular risk, patients continue to present cardiovascular complications despite conventional lipid-lowering therapy. Hypertriglyceridaemia is an important factor contributing to persistently elevated residual risk and is a strong predictor of cardiovascular events. Unfortunately, hypotriglyceride-lowering drugs have failed to demonstrate clinical benefit in the statin era. Although various combinations of EPA and DHA have been tested in high-risk patients, only icosapent ethyl reduces cardiovascular events safely and effectively in today's setting. In fact, the large REDUCE-IT45 randomised trial demonstrated significant reductions in cardiovascular events with icosapent ethyl, without an overall increase in the risk of adverse events. These clinical benefits are accompanied by a decrease in coronary plaque volume and inflammatory markers. Based on the above, Fig. 3 shows the proposed recommendations for the clinical use of icosapent ethyl at a dose of 2 g twice a day.
FundingThis study has not received any type of funding.
Conflicts of interestSee ICMJE DISCLOSURE FORM.
M. Aguilar, F. Arrieta, A. Becerra, M.M. Campos, S. Durán, J.A. Gimeno-Orna, P. Iglesias, G. Maldonado, L. Montañez, J. Navarro, J.C. Obaya, J. Pedro-Botet, A. Pérez, J.L. Pardo, J.C. Pérez, R. Petrecca, J. Ribalta, V Sánchez, J. Tébar.
M. Alameda, M.P. Anguita, G. Barón, V. Barrios, C. Bonanad, M. Bravo, A. Carro, J. Cosín-Sales, I. Egocheaga, C. Escobar, M.A. Esteve, A.J. Fernández-Romero, R. Freixa, J.Mª. Gámez, F.X. García-Moll, J.J. Gómez-Doblas, D. González-Calle, A.I. Huelmos, I. Lacambra, T. López, F. Marín, A. Martín-Santana, J. Martínez-Tur, V.H. Moreno, C. Ortiz, V. Pallarés, X. Palomer, G. Pastor, M. Pedreira, L. Pérez de Isla, C.N. Pérez-García, L.M. Rincón, T. Ripoll, L.M. Ruilope, J. Ruiz de Castroviejo, A. Saltijeral, S. Sánchez-Álvarez, J. Tamargo, J. Torres, R.C. Vidal, D. Vivas.