To analyze the clinical and histopathological features of patients with thyroid cancer in the southwest Madrid area and to identify poor prognostic factors in the subgroup with differentiated thyroid carcinoma (DTC) of the follicular epithelium.
Patients and methodsA retrospective cohort study of patients diagnosed with thyroid cancer at our hospital from 1998 to 2009 was carried out. Significant clinical, surgical, and histopathological variables were included in Cox proportional hazard and logistic regression models to identify baseline factors which predict death, recurrence, and persistent disease in DTC.
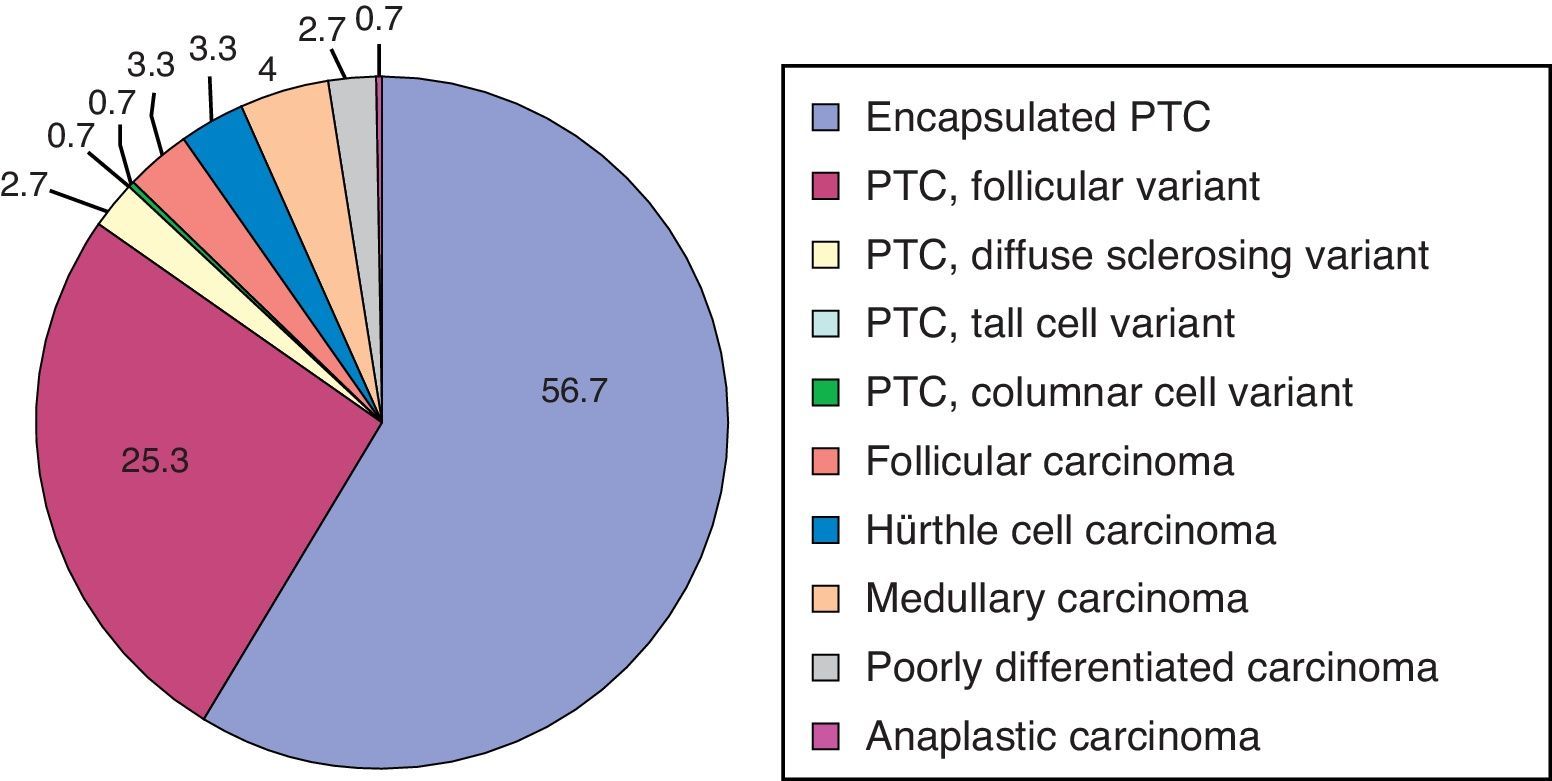
ResultsA total of 150 patients with a median age of 49 years and a median follow-up of 5.4 years were enrolled. Histological subtypes were: papillary carcinoma (86%), follicular carcinoma (6.6%), medullary carcinoma (4%), poorly differentiated carcinoma (2.7%), and anaplastic carcinoma (0.7%). At the end of the study, 68% of patients were cured, 3.3% had died (disease-specific mortality, 1.3%), 1.3% were lost to follow-up, 6.7% had persistent biochemical disease, and 2.7% had persistent clinical disease, while 18% of patients were pending assessment. The best prognostic model for DTC recurrence was TNM staging (stage II–IV vs I: HR 5.9, 95% CI 1.3–26.6), while the best model for persistent disease or death was ETA clinical staging (high risk vs low or very low risk: OR 9.2, 95% CI 2.6–33.2).
ConclusionsIn our study, disease-specific mortality and persistent clinical disease were low. Classification of DTC patients based on ETA staging after initial treatment was a good predictor of persistent disease or death.
Análisis de las características clínicas e histopatológicas de los pacientes con cáncer de tiroides en el área suroeste de Madrid e identificación de los factores de mal pronóstico en el subgrupo de carcinoma diferenciado de tiroides (CDT) del epitelio folicular.
Pacientes y métodosEstudio retrospectivo de una cohorte de cáncer de tiroides de nuestro hospital entre 1998-2009. Las variables clínicas, quirúrgicas e histopatológicas significativas se incluyeron en modelos de regresión de Cox y logística para la identificación de factores pronósticos de muerte, recidiva y persistencia de enfermedad.
ResultadosSe incluyeron 150 pacientes con mediana de edad 49 años y mediana de seguimiento de 5,4 años. Los subtipos histológicos fueron: carcinoma papilar (86%), carcinoma folicular (6,6%), carcinoma medular (4%), carcinoma pobremente diferenciado (2,7%) y carcinoma anaplásico (0,7%).
Al final del estudio: 68% curación, 3,3% muertos (mortalidad por cáncer tiroideo 1,3%), 1,3% pérdida de seguimiento, 6,7% con enfermedad bioquímica persistente, 2,7% con enfermedad clínica persistente y 18% pendiente de evaluación. El mejor modelo pronóstico para recidiva de CDT fue el estadiaje TNM (estadio ii-iv frente a i: HR 5,9, 95% IC 1,3-26,6) y para persistencia de enfermedad o muerte el estadiaje clínico de la ETA (alto riesgo frente a bajo/muy bajo riesgo: OR 9,2, 95% IC 2,6-33,2)
ConclusionesEn nuestro estudio la mortalidad y persistencia clínica de enfermedad fueron bajas. La clasificación de pacientes con CDT según estadiaje de la ETA fue un buen factor predictor de enfermedad persistente o muerte.
Thyroid carcinoma (TC), although a rare neoplasm (accounting for less than 1% of all cancers), is the most common endocrine cancer.1 Over the past decades, the worldwide prevalence of TC derived from follicular epithelium (differentiated thyroid cancer, DTC) has markedly increased,2 especially at the expense of the papillary histological variant.3 This increase in DTC has been attributed both to advances in diagnostic procedures and potential changes in the biological behavior of TC, and the debate has not yet been definitively closed.2
Ever since 2000, the availability of better diagnostic procedures, the need for a multidisciplinary approach to treatment, and changes seen in DTC presentation have promoted the development of different guidelines and expert consensuses, which have been generally accepted and followed by Spanish endocrinologists.4 Although the number of studies related to TC published in Spain has increased in recent years, few reports of historical series of patients with long-term follow-up are available.5–7
The purpose of our study was to analyze the clinical and histopathological data from patients with TC diagnosed in our area during the 1998–2009 period, and to identify course predictors in the subgroup of patients with DTC arising from follicular epithelium.
Patients and methodsA retrospective study of a cohort of patients diagnosed with TC in our hospital from March 1998 to December 2009 was carried out. Patients who were diagnosed at another center or followed up at our hospital for less than six months were excluded from the study. A protocol for diagnosis, treatment, and follow-up of TC, partly based on the 2002 British Thyroid Association guidelines, was implemented in our unit in 2004.8 The histological type of tumor was defined based on the WHO classification.9 Two prognostic scales were used to classify patients with DTC into risk categories: the sixth edition of the TNM scale (stages I, II, III, IVA, IVB, and IVC)10 and the scale proposed by the European Thyroid Association (ETA) consensus (very low risk, low risk, and high risk categories).11 In 2007, a protocol for the diagnosis, treatment, and follow-up of DTC based on the ETA consensus was implemented in our unit.11 Remission criteria were defined based on this consensus.11 In patients with a very low risk treated with lobectomy, remission was defined as thyroglobulin (Tg) levels less than 10ng/mL on suppressive treatment with negative neck ultrasound examination. In low-risk patients (T1N0M0 or T2N0M0 with no risk histology or multifocality) treated with total thyroidectomy and ablation with 131I, remission was defined as hrTSH-stimulated Tg levels at less than 0.5ng/mL and undetectable annual Tg levels under suppressive treatment. In patients at moderate to high risk (any T3 and T4 or any T, N1 or any M1), remission was defined as the concomitant occurrence of at least one negative whole body scan (WBS) with Tg levels less than 2ng/mL without suppressive treatment or after stimulation with hrTSH and annual undetectable Tg levels under suppressive treatment.
In patients who achieved remission, annual Tg measurements and ultrasound examinations of the neck were performed.
TSH suppressive therapy was defined as the achievement of TSH levels less than 0.1μIU/mL with the administration of thyroxine (FT4), which was continued in patients with persistent disease. Patients at moderate to high risk continued on suppressive therapy for five years, and when they no longer had evidence of disease, the same treatment was administered as for low risk patients. In low risk patients in whom a cure had been achieved, the chance of recurrence was low, and the dose of FT4 was therefore decreased to achieve TSH levels in the lower limit of normal (from 0.5 to 1.0μIU/mL).
Tg-off was defined as Tg levels reached after FT4 withdrawal for four weeks with concomitant TSH levels higher than 30μIU/mL.
WBS after thyroid surgery was performed one week after an ablation dose of 131I administered to high and low risk patients. High risk patients underwent two WBS, a new WBS at one year and a second additional WBS at the second year, and a negative result was considered to be a criterion for cure.
In patients with positive Tg antibodies (anti-Tg), undetectable Tg levels were not considered as a marker of disease remission, and follow-up therefore consisted of regular WBS and neck ultrasounds. If distant disease was suspected, other imaging procedures were performed, including CT, magnetic resonance imaging, and fluorodeoxyglucose positron emission tomography.
The following variables were collected concerning:
- (a)
Diagnosis
- -
Clinical characteristics, including demographic data and form of presentation. The finding of a thyroid nodule in any imaging test requested for a reason other than thyroid disease or the diagnosis of TC in the histological study of the specimen from thyroidectomy performed for thyroid disease not suspected to be malignant was defined as incidental presentation.
- -
Fine needle aspiration (FNA) cytology: defined as not done, diagnostic, benign, indeterminate, and inadequate.
- -
Histological type, largest diameter, and extension of the tumor.
- -
- (b)
Treatment
- -
Types of thyroid and lymph node surgery.
- -
Postoperative complications: permanent hyperthyroidism and recurrent nerve injury.
- -
Treatment with 131I. Treatment with external radiotherapy.
- -
- (c)
Status at the last visit:
- -
Dead from TC or other cause, alive with criteria of disease persistence, recurrence, or cure, lost to follow-up. Patients with a scheduled visit within six months of closure of variable collection were defined as pending evaluation.
- -
Serum Tg levels were measured by chemiluminescence by using an Immulite 2000® analyzer (Siemens), with a limit of detection of 0.2ng/mL and intra- and inter-assay coefficients of variation of 3.7% and 4.6%, respectively. Serum anti-Tg and TSH levels were quantified by chemiluminescence using an Advia-Centaur® analyzer (Siemens), with limits of detection of 30U/mL and 0.008μIU/mL, respectively, and intra- and inter-assay coefficients of variation of 3.7% and 5.1% for anti-Tg and 3.1% and 4.2% for TSH.
Most of the WBSs were performed at the same nuclear medicine unit using a gamma camera with high-energy collimators after an oral dose of 131I.
Quantitative variables are given as the median and its interquartile range (IQR), and qualitative variables as percentage. Survival curves were estimated by using the Kaplan–Meier procedure, and a Mantel–Cox (Log-Rank) test was used to compare the curves.
Predictors of death, recurrence, and persistent disease in DTC were searched using two multivariate models: a Cox regression predictive model using time to recurrence as the time-dependent variable, and a logistic regression predictive model where the outcome variable was death or disease persistence. The complete initial models included clinical, surgical, and histopathological variables with p<0.1 in the univariate analysis. If the categorical variables resulted in unstable estimators due to the low number of patients in some of the groups, they were recoded into new variables with a lower number of categories. After constructing all the potential submodels from the maximum model, the criterion for selecting the best predictive model was Atkinson's R2 value in Cox regression and Mallows’ Cp value in logistic regression.
SPSS 15.0 statistical software was used for statistical analysis.
ResultsOne hundred and fifty patients with TC with a median age of 49 years were included. Of these, 139 (92.6%) had DTC. Median follow-up time was 5.4 years (IQR, 2.0–8.4).
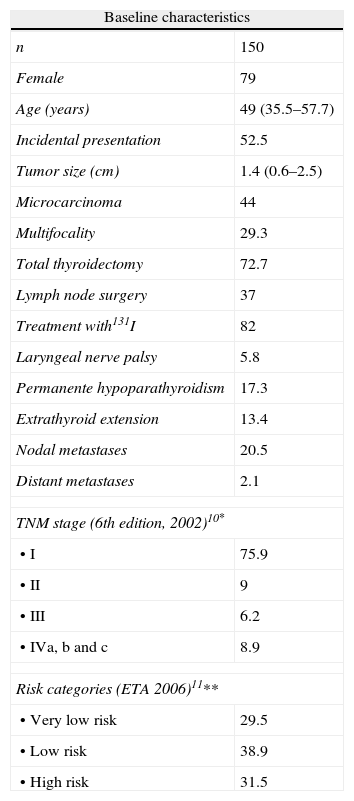
Table 1 shows the baseline characteristics of patients. In 52.5% of patients the condition was incidentally found in an imaging test requested for reasons other than thyroid disease (19.5%) or in the specimen from a thyroidectomy performed for thyroid disease not suspected to be malignant (normofunctioning multinodular goiter, hyperfunctioning goiter) (33%). In the remaining patients, the forms of presentation were goiter (18%), palpable thyroid nodule (24%), and palpable cervical adenopathy (5.36%).
Clinical characteristics, types of treatment, postoperative complications, tumor staging*, and risk categories** of DTC. Data are given as percentage or median and their interquartile range.
| Baseline characteristics | |
| n | 150 |
| Female | 79 |
| Age (years) | 49 (35.5–57.7) |
| Incidental presentation | 52.5 |
| Tumor size (cm) | 1.4 (0.6–2.5) |
| Microcarcinoma | 44 |
| Multifocality | 29.3 |
| Total thyroidectomy | 72.7 |
| Lymph node surgery | 37 |
| Treatment with131I | 82 |
| Laryngeal nerve palsy | 5.8 |
| Permanente hypoparathyroidism | 17.3 |
| Extrathyroid extension | 13.4 |
| Nodal metastases | 20.5 |
| Distant metastases | 2.1 |
| TNM stage (6th edition, 2002)10* | |
| • I | 75.9 |
| • II | 9 |
| • III | 6.2 |
| • IVa, b and c | 8.9 |
| Risk categories (ETA 2006)11** | |
| • Very low risk | 29.5 |
| • Low risk | 38.9 |
| • High risk | 31.5 |
DTC: differentiated thyroid carcinoma; ETA: European Thyroid Association; TNM: tumor, node, metastasis.
In 36% of patients no FNA was performed or the FNA sample was taken from a nodule different from the one affected by TC. FNA results were diagnostic in 30.7%, benign in 18.7%, indeterminate in 12%, and inadequate in 2.7%.
Fig. 1 shows the histological TC subtypes. Eighty-six percent of patients in our series had papillary carcinoma (PC), of which 56.7% were classical papillary carcinoma (CPC) or encapsulated carcinoma.
Table 1 shows the different treatments, postoperative complications, tumor staging, and risk categories for DTC. Among DTC patients, total thyroidectomy was performed in 99 patients (72.5%), subtotal thyroidectomy in one (0.72%), lobectomy plus isthmectomy in seven (5.1%), and lobectomy in 30 patients (21.16%).
Some lymph node surgery (central compartment, ipsilateral laterocervical, contralateral laterocervical, and supraclavicular) was performed in 37% of patients with TC. In the DTC subgroup, lymph node surgery was performed in 18% of low risk patients, 75.6% of high-risk patients, and non very low risk patients.
Two types of surgery (thyroid and/or lymph node) were performed in 17.5% of patients with very low risk DTC, while 29% of low and high risk patients underwent three types of surgery. After excluding patients with medullary, poorly differentiated, and anaplastic carcinoma, rates of permanent hypoparathyroidism and recurrent nerve palsy were 14.7% and 4%, respectively.
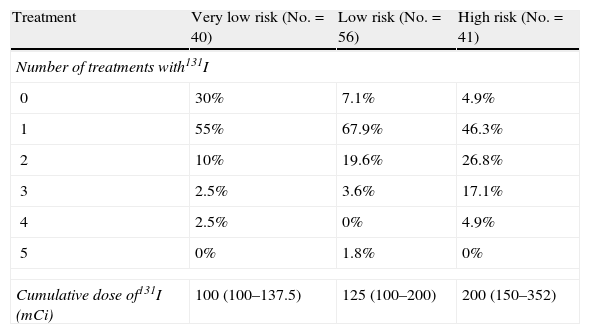
Eighty-two percent of patients with TC were treated with 131I. Table 2 shows the number of doses and the cumulative dose of 131I. Two or more doses of 131I were administered to 70% of very low risk patients, 93% of low risk patients, and 95% of high risk patients. Six patients (4%) received external radiotherapy, with a total cumulative dose of 5300cGy (2262.5–7000).
Number of doses and cumulative dose of 131I in patients with DTC.
| Treatment | Very low risk (No.=40) | Low risk (No.=56) | High risk (No.=41) |
| Number of treatments with131I | |||
| 0 | 30% | 7.1% | 4.9% |
| 1 | 55% | 67.9% | 46.3% |
| 2 | 10% | 19.6% | 26.8% |
| 3 | 2.5% | 3.6% | 17.1% |
| 4 | 2.5% | 0% | 4.9% |
| 5 | 0% | 1.8% | 0% |
| Cumulative dose of131I (mCi) | 100 (100–137.5) | 125 (100–200) | 200 (150–352) |
DTC: differentiated thyroid carcinoma.
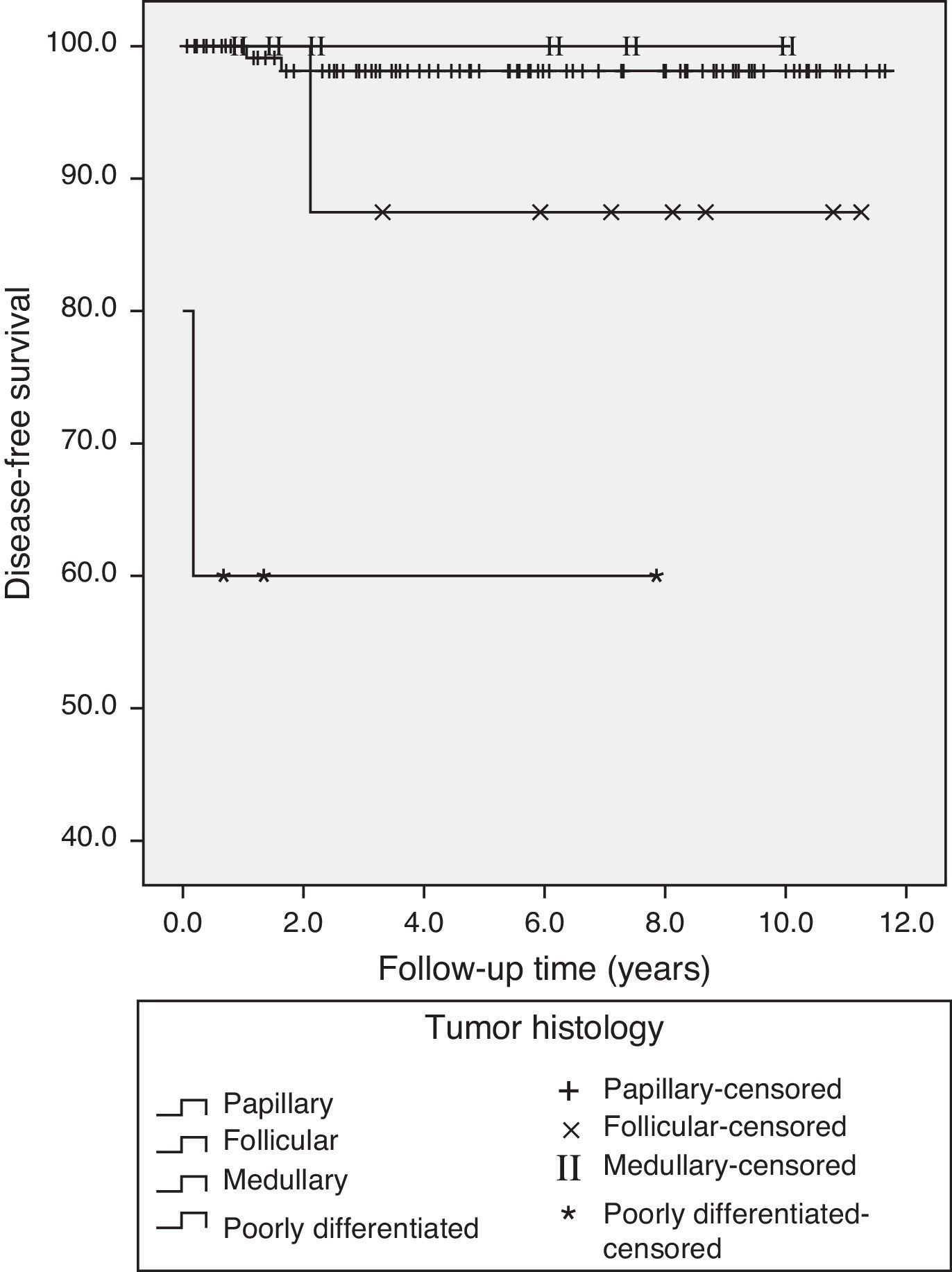
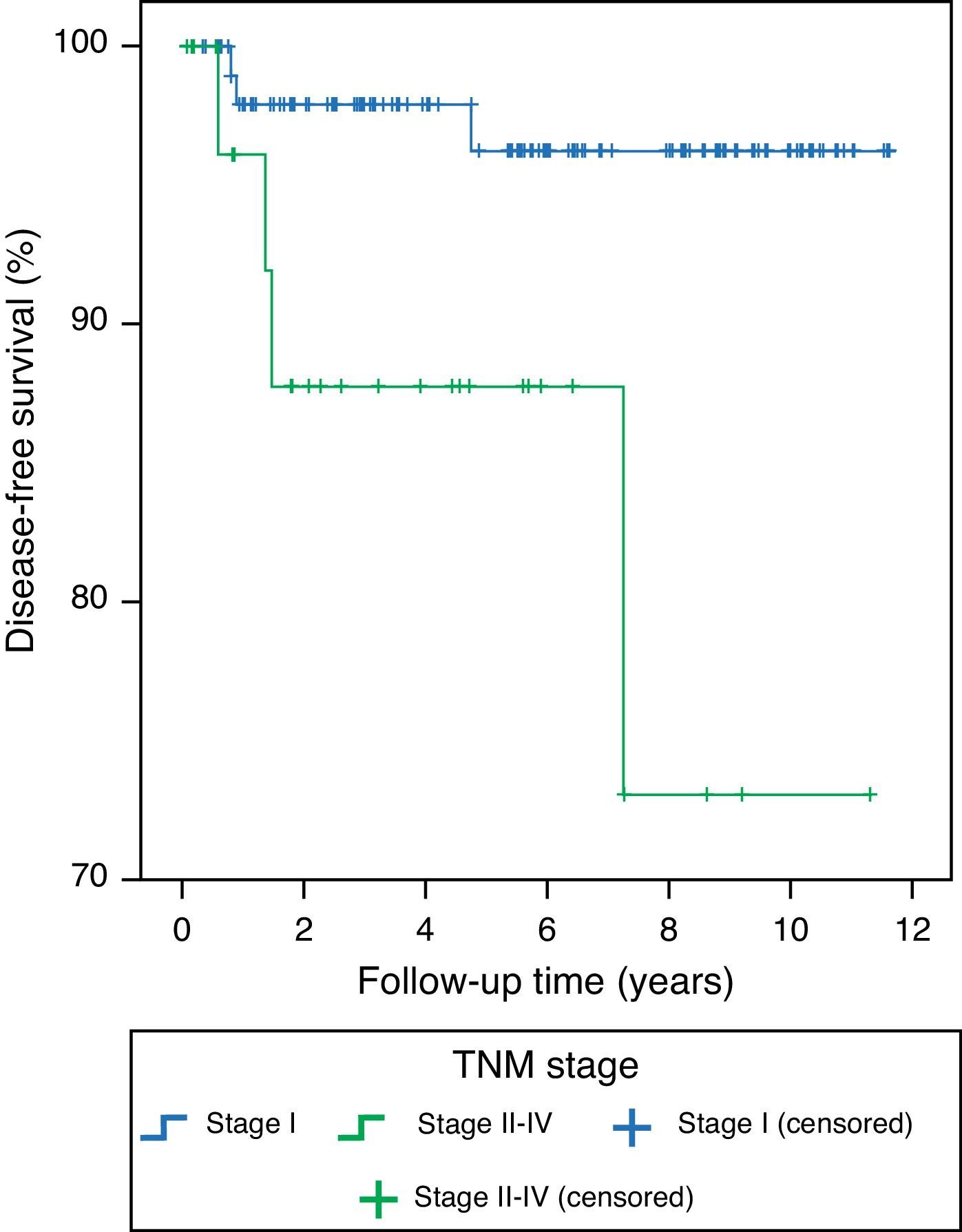
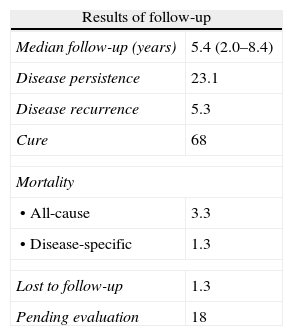
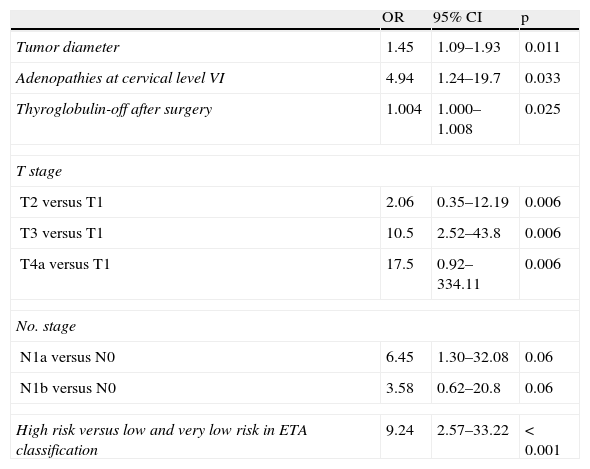
Table 3 shows the results of follow-up at the end of the study. Sixty-eight percent of patients met cure criteria, and specific TC-related mortality was 1.3% (one patient each with anaplastic and poorly differentiated carcinoma). In the univariate analysis of the DTC subgroup, variables associated with persistent disease or death included (Table 4): tumor diameter (OR 1.45 [95% CI 1.09–1.93], p=0.011), adenopathies at cervical level VI (OR 4.94 [95% CI 1.24–19.7], p=0.033), Tg-off after surgery (OR 1.004 [95% CI 1.000–1.008], p=0.025), T category (T2 versus T1, OR 2.06 [95% CI 0.35–12.19]; T3 versus T1, OR 10.5 [95% CI 2.52–43.8], T4a versus T1, OR 17.5 [95% CI 0.92–334.11], p=0.006), N category (N1a versus N0, OR 6.45 [95% CI 1.30–32.08], N1b versus N0, OR 3.58 [95% CI 0.62–20.8] p=0.06), and ETA risk classification11 (high risk versus low and very low risk), OR 9.24 (2.57–33.22) p<0.001. Variables associated with recurrent disease were the TNM stage (II–IV versus I). HR 5.91 (95% CI 1.31–26.59), p 0.023 and ETA risk classification11 (high versus low/very low risk), HR 3.91 (95% CI 0.87–17.61), p=0.077. No other individual variable showed a significant association with recurrence. Figs. 2 and 3 show the Kaplan–Meier curves for disease-free survival as a function of histological type and TNM classification in patients with TC. Patients with microcarcinoma (T less than 1cm) had no less chance of recurrence, persistent disease, or death than those with macrocarcinoma.
Results of follow-up of patients with thyroid carcinoma, given as percentage or median and interquartile range.
| Results of follow-up | |
| Median follow-up (years) | 5.4 (2.0–8.4) |
| Disease persistence | 23.1 |
| Disease recurrence | 5.3 |
| Cure | 68 |
| Mortality | |
| • All-cause | 3.3 |
| • Disease-specific | 1.3 |
| Lost to follow-up | 1.3 |
| Pending evaluation | 18 |
Variables of the DTC subgroup associated with persistent disease or death.
| OR | 95% CI | p | |
| Tumor diameter | 1.45 | 1.09–1.93 | 0.011 |
| Adenopathies at cervical level VI | 4.94 | 1.24–19.7 | 0.033 |
| Thyroglobulin-off after surgery | 1.004 | 1.000–1.008 | 0.025 |
| T stage | |||
| T2 versus T1 | 2.06 | 0.35–12.19 | 0.006 |
| T3 versus T1 | 10.5 | 2.52–43.8 | 0.006 |
| T4a versus T1 | 17.5 | 0.92–334.11 | 0.006 |
| No. stage | |||
| N1a versus N0 | 6.45 | 1.30–32.08 | 0.06 |
| N1b versus N0 | 3.58 | 0.62–20.8 | 0.06 |
| High risk versus low and very low risk in ETA classification | 9.24 | 2.57–33.22 | < 0.001 |
ETA: European Thyroid Association; DTC: differentiated thyroid carcinoma; 95% CI: 95% confidence level; OR: odds ratio.
The best prognostic model of DTC recurrence was TNM staging (II–IV versus I) HR 5.91 (95% CI 1.31–26.59) p=0.023, and the best predictive model of disease persistence or death was the risk categorization of the European consensus11: high versus low or very low risk, OR 9.24 (95% CI 2.6–33.2), p<0.001. Eight percent of stage I patients experienced recurrence, as compared to 23.8% of patients with disease stages II, III, and IV.
DiscussionThe results of our study showed a clear predominance of the histological variant of PC (found in more than 80% of cases), which agrees with the reports from TC and DTC series, both in Spain and the US, including higher numbers of patients and longer follow-up times.5–7,12,13
The follicular carcinoma (FC) subtype accounted for 6.6% of TCs in our patients (3.3% follicular and 3.3% Hürthle cell carcinomas), a proportion similar to that reported in the DTC series from the Móstoles12 and Toledo6 hospitals and lower than those reported in the series of Hospital Príncipe de Asturias in Alcalá de Henares7 (11.3%), Hospital de Basurto11 (11%), and the joint series of two Catalan hospitals5 (12%). In this regard, several studies2,14–16 show a progressive reduction of the FC variant in favor of PC in recent decades. Among other factors, improved nutritional iodine status,17 associated with an increase in BRAF mutations, more common in PC,18 a better histological definition of the follicular variant of PC,19 and an increased detection of smaller tumors,15 most of them of the PC variant, have been implicated in this trend.
In more than half the patients in our series, the form of presentation of TC was considered as incidental. Similarly to our study, in the Italian series reported by Elisei et al.,2 who defined incidental finding as the detection of a nodule during diagnostic tests requested for reasons other than thyroid disease, the incidental presentation of DTC doubled from 1990, accounting for 20% of patients diagnosed during the 1990–2004 period. These data support the notion that increased use of the best imaging diagnostic procedures has contributed to the higher prevalence of DTC seen in recent decades. In our opinion, the inclusion and analysis of this variable, incidental presentation, not considered in most current publications, could contribute to a greater understanding of the epidemiological changes in TC in future studies.
The high proportion of patients with microcarcinoma in our series, close to 45%, is similar to that reported in the series from Hospital de Móstoles13 and clearly higher than those reported in all other Spanish series,5–7,12 ranging from 15% to 20%. These differences may be due to the different observation periods used in some of the above studies. Thus, in the Hospital de Basurto series12 the rate of microcarcinomas increased from 12% during the 1996–2000 period to 29% during 2001–2005. In the Elisei et al. study,2 one of the European series including a greater number of patients with DTC treated at a single hospital using standardized treatment and follow-up protocols, the proportion of patients with microcarcinoma increased from 7.9% during the period 1969–1989 to 28.7% during the period 1990–2004. This increase in the proportion of patients with microcarcinoma reflects the increased use of imaging methods more sensitive for the diagnosis of thyroid diseases and other non-thyroid diseases in recent decades. This has resulted in the detection of smaller tumors, allowing for the diagnosis of early stage or small TCs which otherwise would not have become clinically apparent.2 However, and similarly to other series,2,13 the probability of disease persistence or death was not lower in patients with microcarcinoma in our study as compared to patients with macrocarcinoma. In this regard, some of the authors2,16 have emphasized that papillary microcarcinomas are frequently located near the thyroid capsule and have a limited microscopic extrathyroid extension. Thus, although these tumors have a good prognosis, they meet the definition of tumor with local extension, thus increasing the proportion of these tumors in stage T3. On the other hand, the increase in intrathyroid tumors and the reduction in nodal and distant metastatic involvement seen in the last decades have not been associated with a corresponding decrease in locally advanced DTCs, as reported in some studies.2,21,22 Complete molecular typing of DTCs with different biological behavior will very likely allow for a better understanding of these clinical observations in the future.23
In agreement with the small tumor size and the low rate of extrathyroid or metastatic involvement in patients in our series, more than 65% had early stage disease (75.9% were in stage I from the TNM scale, and 68.4% in the very low or low risk categories of the European consensus11). This proportion is higher than reported in all other Spanish series,5,6,12 where stage I disease was found in 35.5–69% of patients. Multifocal tumors were found in approximately 30% of cases, a proportion similar to that reported in all other Spanish series. In this regard, the progressive increase in this histological parameter seen in some series comparing different observation periods2,12 may very likely reflect a better histological definition of PC during recent decades,19,21 rather than a change in the biological behavior of the tumor.
As regards types of treatment, total or almost total thyroidectomy was performed in 72.7% of our patients, a lower proportion as compared to those reported in all other Spanish series,5–7,12 ranging from 86% to 92%. This difference is probably due to the high proportion of patients with microcarcinoma and incidental presentation in our series. Thus, almost one-third of our patients were diagnosed TC based on histological study of the specimen from thyroidectomy performed for thyroid disease not suspected to be malignant, with 76% of patients in stage I, which would explain the lower proportion of total thyroidectomies performed at our hospital. Similarly, the low lymph node surgery rate at our hospital, lower than those reported in all other Spanish series,5–7,12 could also be related to these circumstances. The indication of lymph node microdissection only for cases where malignancy is suspected before surgery or where lymph node metastases are found during surgery and the questionable benefits of prophylactic dissection of the central compartment with no improvement of recurrence and mortality indices, as stated in the European consensus,11 the protocol in our country since 2007, would have led to a decreased performance of lymph node surgery. In any case, a review of lymph node surgery rates as a function of risk categories of the European consensus11 showed that lymph node surgery was performed in 75% of high risk patients in our series.
More than 80% of our patients received 131I ablation treatment after surgery, which is similar to the proportion reported in DTC series in Spain. Ablation of remnant tissue with 131I as an effective alternative to repeat surgery to complete thyroidectomy in patients at low risk of recurrence11 would explain the greater percentage of patients in our series undergoing treatment with 131I as compared to total thyroidectomy. The high proportion of very low risk patients treated with 131I mainly occurred during the first half of the study (1998–2004), a period where our hospital had not still implemented any standardized treatment and follow-up protocol for TC.
Surgical complications included permanent hypoparathyroidism in 17.3% and permanent recurrent nerve palsy in 5.8% of our patients. Although most Spanish studies of TC do not report these data, our surgical complication rate was higher than reported in the pooled series from Catalan hospitals5 and the series from Hospital Príncipe de Asturias,7 which found permanent hypothyroidism in 14% and 9% and recurrent nerve palsy in 3.7% and 3.3% of patients, respectively. The repeat surgery rate for complete thyroidectomy, close to 25% in our series, as well as the inclusion of histological subtypes of TC other than DTC, requiring more aggressive initial surgery, would have contributed, amongst other factors, to the greater surgical morbidity seen in our study. We should note in this regard that almost one-third of patients from the group with low and high risk DTC underwent up to three surgical procedures, which would partly explain the high postoperative morbidity seen in our series. On the other hand, when patients with medullary, poorly differentiated, and anaplastic carcinoma were excluded, the rates of permanent hypoparathyroidism and recurrent nerve palsy decreased to 14.7% and 4%, respectively. Finally, the possibility of a greater postoperative morbidity in the first few years after our hospital started to operate (1998), related to an initial lack of specialization in thyroid surgery, should not be ruled out.
DTC-specific mortality in our study was 1.3%, similar to the specific mortality rates of 1.8% and 1.9% reported in the pooled series from Catalan hospitals5 and the Hospital de Basurto series,12 respectively, and markedly lower than the rates of 3.1%, 3.9%, 4.9%, and 7.9% reported by hospitals in the surrounding areas of Móstoles,12 Leganés,20 Toledo,6 and Alcalá de Henares,7 respectively. It is also lower than the mortality rates of approximately 4% reported by other European2,21,24 and American25,26 groups. In this regard, the nationwide Spanish study conducted by Lope et al.27 of more than 2500 deaths from TC during the 1989–1998 decade showed differences in TC mortality rates and risk even between different towns in the same autonomous community. The different mortality rates seen may be due to the different follow-up times and surgical procedures for TC patients, a circumstance which was noted in some,26 but not all,2 studies. The different clinical practice standards and various as yet unknown etiopathogenetic factors very likely modify the form of presentation of, and the treatment approach to TC in each area,11 thus conditioning the different mortality rates seen.
In our series, univariate analysis in the patient subgroup with DTC showed that tumor size, the presence of cervical adenopathies, and the different T and N categories were associated with persistent disease or death. Such prognostic factors are similar to those reported in many publications.28 Patient age,6 male sex,13 histological type,6 extrathyroid extension,6,13 and the presence of nodal13 and distant metastases6 are some of the independent predictors of survival and persistent disease reported in other Spanish series. As it is well-known, some of the variables associated with DTC recurrence or mortality in the univariate analysis are not identified as independent predictors in the multivariate analysis.28 In addition, the risk factors for mortality and recurrence in DTC vary, depending on the study.28 Moreover, some of the recent studies have suggested that both variables predicting for mortality and for recurrence, which are not always the same, should be identified.29 In this regard, the multivariate analysis showed that in patients with DTC in our series, the best prognostic model for recurrence was the staging system of the TNM scale. The risk rate multiplied almost six times in patients in stages II, III, and IV as compared to stage I patients. By contrast, the risk categories of the European consensus provided the best model for predicting persistent disease or death.11
The different staging systems currently proposed include different prognostic factors and allow for differentiating between patients with low and high risk of death related to TC and disease recurrence. Among the more than 15 systems proposed,29 the TNM system has been shown to be helpful in predicting mortality, and is therefore recommended for all patients with DTC. Based on this scale and on information derived from WBS after surgery, the European consensus11 defined three risk categories for the subsequent management and treatment of patients. Factors predicting for persistent disease or death in our series support the value of these risk categories of the European consensus. In any case, the choice of a staging system, over and above individual experience and taking into account that prior studies have shown no significant differences between several systems,30 should be guided by recommendations in guidelines and expert consensuses, which appear to be accepted by a sizeable number of Spanish endocrinologists.4
The retrospective nature of our review and the short time elapsed from the implementation of the TC management guidelines at our unit (which occurred in 2004 and 2007 for the British Thyroid Association guidelines8 and European consensus,11 respectively) to study completion are the most important limitations of our study. It should be noted that there was no standardized protocol for TC treatment and follow-up in force at our hospital during the first half of the study. This undoubtedly decreased the extent of compliance with such guidelines in a significant number of our patients with TC, as it had previously been shown to occur in other Spanish hospitals.4
We conclude that patients in our series/cohort had low rates of C-related specific mortality and persistent disease. Stratification of DTC patients into the risk categories of the European consensus11 was shown to be a good model for predicting persistent disease or death.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Donnay Candil S, et al. Estudio de cohorte retrospectivo de pacientes diagnosticados de cáncer de tiroides del área suroeste de Madrid. Factores pronósticos en el cáncer diferenciado de tiroides. Endocrinol Nutr. 2013;60:60–8.