The self-determination of blood glucose is relevant for diabetes mellitus (DM) insulin-treated patients. The use of glucometers with advanced features and measuring glycated hemoglobin (HbA1c) may help improve metabolic control. The main objective of this study was to determine the percentage of insulin treated patients who reduced HbA1c by at least 0.4% after 6 months of using Contour and A1CNow+.
Materials and methodsObservational, prospective, multicentre study in adult DM insulin treated patients, with HbA1c >8%.
ResultsOf the 454 recruited patients analyzed, a total of 333 were evaluable. After 6 months the HbA1c decreased (p<.05) in both groups [−0.89 (95% CI −1.01 to −0.76) and −0.98 (95% CI: −1.21 to −0.76), in type 1 and 2 DM, respectively]. An HbA1c reduction of 0.4% was observed in 73% of patients after 6 months of device use. A decrease in the number of patients with HbA1c >8% was observed, with this reaching: 41% for all, 45% in type 1 DM, and 25% in type 2 DM. In the glycaemic profile, a reduction (p<.05) was observed in pre- and post-prandial glycaemia in both groups (−20.7±36.4 and −37.1±47.1mg/dL, respectively), with 23% pre-prandial glucose <130mg/dL and post-prandial <180mg/dL
ConclusionThe use of glucometers with advanced features, and measuring glycated hemoglobin (HbA1c) may help improve metabolic control and to monitor insulin treated DM patients more closely.
El autoanálisis glucémico es relevante en el autocontrol de pacientes diabéticos (DM) insulinizados. Usar glucómetros con funciones avanzadas y determinar la hemoglobina glucosilada (HbA1c) puede contribuir a mejorar el control metabólico. El objetivo principal fue conocer el porcentaje de pacientes que consigue una disminución como mínimo del 0,4% de la HbA1c a los 6 meses de usar los dispositivos Contour USB y A1CNow+.
Material y métodosEstudio observacional, prospectivo, multicéntrico en pacientes adultos con DM tratados con insulina, con HbA1c ≥ 8%.
ResultadosSe analizó a 333 pacientes con DM valorables de 454 reclutados. A los 6 meses la HbA1c disminuyó (p < 0,05) en ambos grupos (−0,89 [IC 95%: −1,01; −0,76] y −0,98 [IC 95%: −1,21; −0,76], DM tipo 1 y tipo 2, respectivamente]. Un 73% de los pacientes consiguió una disminución ≥0,4% de HbA1c tras 6 meses de utilizar los dispositivos. Esto supuso una reducción del porcentaje de pacientes con HbA1c >8%, que llega al 41% en global, al 45% en diabéticos tipo 1 y al 25% en los tipo 2. En el perfil glucémico, se observó una reducción (p < 0,05) de la glucemia pre- y posprandial en ambos grupos (−20,7±36,4 y −37,1±47,1mg/dL, respectivamente). En el 23% de los pacientes la glucemia preprandial fue <130mg/dL y la posprandial <180mg/dL.
ConclusiónLa utilización de un glucómetro con funciones avanzadas y la determinación de HbA1c en sangre capilar podrían contribuir a mejorar el control metabólico y a monitorizarlo de manera más detallada en pacientes con DM tratados con insulina.
Diabetes mellitus (DM) has an 8% prevalence rate in the adult population worldwide.1 The cross-sectional study Di@bet.es, conducted in Spain on 5072 subjects, showed a 13.8% prevalence of type 2 diabetes mellitus (T2DM). Half of the patients did not know they had the disease.2 On the other hand, the prevalence of type 1 diabetes mellitus (T1DM) ranges from 0.08% to 0.2%.3 A recent study conducted in the autonomous community of Navarre between 2009 and 2011 showed an incidence of T1DM of 8.7 cases per 100,000 people, with higher rates in the population under 15 years similar to those seen in other studies conducted in Spain.4
The complications associated with this disease increase morbidity and mortality, impair the quality of life of patients, and have a considerable social and economic impact. According to a study reported in 2013, the cost of DM for the Spanish national health system amounts to 5809 million euros, which represents 8.2% of total healthcare costs. Drug treatment and hospital costs account for 38% and 33% of the total cost, with an annual cost per patient with DM of €1770.5
Blood glucose control is an essential part of DM management. Glycosylated hemoglobin (HbA1c) provides information on blood glucose during the previous 2–3 months and is used in assessing the degree of control and in deciding on the most adequate treatment. The American Diabetes Association (ADA) recommends the maintenance of HbA1c levels under 7%,6 but goal individualization is a key point in the approach to patients with DM. The results of interventions in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study showed that patients with T1DM on intensive blood glucose control therapy (mean HbA1c: 7.2%) had a 35–76% reduction in microvascular complications as compared to the group on standard therapy (mean HbA1c: 9.1%).7 In the UKPDS study, a 1% decrease in la HbA1c in patients with TD2M was found to be associated with a 14% decrease in the risk of myocardial infarction and a 37% decrease in microvascular complications.8
The Spanish Society of Diabetes (SED) recommends a different frequency of self-monitoring of capillary blood glucose (SMCBG) depending on the type of treatment and on blood glucose stability9: the group of patients using insulin should perform SMCBG with the greatest frequency. This recommendation is particularly necessary, useful, and effective in patients with multiple insulin doses, and especially in those with T1DM. The value of SMCBG is more controversial in patients not treated with insulin, although Bosi et al. (the PRISMA study) recently found decreases in HbA1c ranging from −0.39 to −0.45% in patients with T2DM not treated with insulin using highly structured and intensive SMCBG.10
Contour USB and A1CNow+ (Bayer Hispania S.L., Sant Joan Despí, Spain) are two devices that may be helpful for blood glucose monitoring in patients with DM. Contour USB is a meter for SMCBG with an Autolog® function that requires patients to flag their results as: preprandial, postprandial, or SMCBG at any other time of the day. Contour USB has in-built software that allows for access to all blood glucose data stored both by patients and healthcare professionals. A1CNow+ is a portable device that allows for the rapid (in 5min) quantitative measurement of the percentage of HbA1c in capillary blood.
The primary objective of this study was to ascertain the proportion of insulin-treated patients who achieve at least a 0.4% decrease in HbA1c after using the Contour USB and A1CNow+ devices for six months. Secondary objectives included the assessment of the proportion of patients with improvements in preprandial and postprandial blood glucose levels after six months of use of the above devices.
Subjects and methodsStudy design and subjectsThis was a multicenter, observational, prospective study conducted at endocrinology departments and primary care offices throughout Spain in 2011 and 2012. The inclusion criteria were as follows: patients over 18 years of age diagnosed with DM and treated with insulin for at least 12 months or for 12 months before study entry with HbA1c levels ≥8% at baseline, who had never used the study devices, had basic computer skills, and performed SMCBG at least three times daily after study entry. The exclusion criteria were as follows: patients with DM not treated with insulin, patients with DM and total cholesterol levels >500 mg/dL or triglyceride levels >3000 mg/dL, or any relevant disease advising against participation in the study in the investigator's judgment. Written informed consent was obtained from all participants before study entry. The study was approved by the evaluation, support, and prevention unit of Hospital Clínic in Barcelona and was conducted in accordance to the principles of the Declaration of Helsinki and Good Clinical Practice standards.
The patients enrolled into the study were trained in the use of the Contour USB system and its advanced features. Contour USB is a system for testing capillary blood glucose. Its Autolog® function requires patients to flag results as preprandial or postprandial. The device also has built-in software for data management that may be accessed at any time and at any place for data visualization. Healthcare professionals responsible for the HbA1c of patients were trained in taking measurements using the A1cNow+ system. This is a multi-test system for the quantitative measurement of HbA1c levels in capillary blood taken from a small prick in the finger; the reading time is 5min.
Background and biochemical dataData were collected for the previous 12 months, and also at the time of the study visit. Sociodemographic (age, race, and sex), anthropometric (weight, height, and abdominal circumference), and clinical data (time since DM onset, cardiovascular risk factors and target organ lesions) were collected. Data collected at the different visits also included preprandial, 2-hour postprandial, and mean daily (the mean of measurements taken during the 14 days prior to each visit) SMCBG values, hypoglycemic episodes in the previous year, and hygienic and dietary habits. Finally, the last HbA1c value available was collected, and HbA1c was measured using the A1cNow+ at baseline and 3 and 6 months after study entry. Suggestions for changes in treatment after the evaluation of blood glucose profiles were left to the discretion of each investigator.
An ad hoc questionnaire specifically developed for this study was used to assess patient satisfaction with the device (Contour USB+) and physician satisfaction with Contour USB and A1cNow+.
Statistical analysisThe sample size was estimated in order that a 0.4% decrease in HbA1c after using the Contour USB and A1cNow+ for six months could be seen. Assuming 5% of invalid participants and a 95% confidence interval, the number of participants to be recruited was 520.
Results are given as frequencies and percentages for qualitative variables, and as mean and standard deviation (SD) for quantitative variables. Quantitative variables were compared using a non-parametric Mann–Whitney U test, while qualitative variables were compared using a Chi-squared test for independent samples or a McNemar test for related samples. A Wilcoxon test was used to test changes over time.
Statistical analysis was performed using SAS statistical software (version 9.3). The significance level used in all statistical tests was 0.05 for two-sided tests.
ResultsA total of 454 patients were recruited, of whom 333 (73.3%) were evaluable. The reasons for exclusion were as follows: an HbA1c level less than 8% at study entry (n=19), not performing SMCBG three times daily after study start (n=87), not being on insulin therapy (n=23), the unavailability of HbA1c levels for the previous 6 months (n=32), or non-attendance at the 3 and 6-month visits (n=30). There could have been more than one reason for a patient's exclusion.
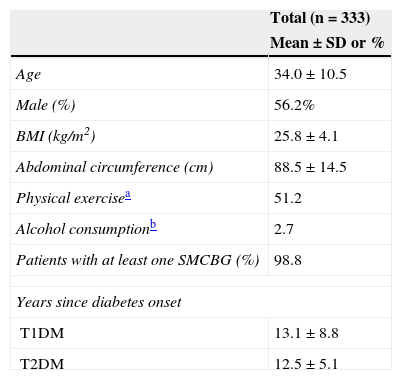
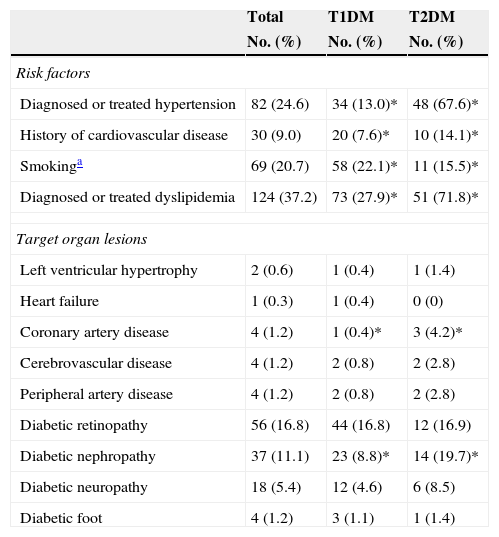
Of all the participants, 78.7% had T1DM and 21.3%, T2DM. Table 1 shows their anthropometric characteristics and hygienic and dietary habits. Of all the patients who performed SMCBG, 39.3% did three tests daily, and 36.9% more than three tests daily. Table 2 shows cardiovascular risk factors and target organ lesions in all the patients by DM type. Significant differences were seen between patients with T1DM and T2DM in all risk factors. Twelve percent of patients with both types of DM had experienced some hypoglycemia requiring medical care over the previous year.
Anthropometric characteristics and hygienic and dietary habits of the study population.
| Total (n=333) | |
|---|---|
| Mean±SD or % | |
| Age | 34.0±10.5 |
| Male (%) | 56.2% |
| BMI (kg/m2) | 25.8±4.1 |
| Abdominal circumference (cm) | 88.5±14.5 |
| Physical exercisea | 51.2 |
| Alcohol consumptionb | 2.7 |
| Patients with at least one SMCBG (%) | 98.8 |
| Years since diabetes onset | |
| T1DM | 13.1±8.8 |
| T2DM | 12.5±5.1 |
SMCBG: self-monitoring of capillary blood glucose; BMI: body mass index.
Cardiovascular risk factors and target organ lesions by type of DM.
| Total | T1DM | T2DM | |
|---|---|---|---|
| No. (%) | No. (%) | No. (%) | |
| Risk factors | |||
| Diagnosed or treated hypertension | 82 (24.6) | 34 (13.0)* | 48 (67.6)* |
| History of cardiovascular disease | 30 (9.0) | 20 (7.6)* | 10 (14.1)* |
| Smokinga | 69 (20.7) | 58 (22.1)* | 11 (15.5)* |
| Diagnosed or treated dyslipidemia | 124 (37.2) | 73 (27.9)* | 51 (71.8)* |
| Target organ lesions | |||
| Left ventricular hypertrophy | 2 (0.6) | 1 (0.4) | 1 (1.4) |
| Heart failure | 1 (0.3) | 1 (0.4) | 0 (0) |
| Coronary artery disease | 4 (1.2) | 1 (0.4)* | 3 (4.2)* |
| Cerebrovascular disease | 4 (1.2) | 2 (0.8) | 2 (2.8) |
| Peripheral artery disease | 4 (1.2) | 2 (0.8) | 2 (2.8) |
| Diabetic retinopathy | 56 (16.8) | 44 (16.8) | 12 (16.9) |
| Diabetic nephropathy | 37 (11.1) | 23 (8.8)* | 14 (19.7)* |
| Diabetic neuropathy | 18 (5.4) | 12 (4.6) | 6 (8.5) |
| Diabetic foot | 4 (1.2) | 3 (1.1) | 1 (1.4) |
T1DM: type 1 diabetes mellitus; T2DM: type 2 diabetes mellitus.
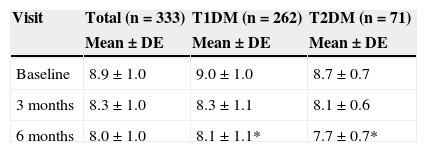
The HbA1c value at baseline was compared to the value found in the last prior visit available. Very similar values were seen in both patients with T1DM and TD2M (9.0% vs 9.1% and 8.7% vs 8.8% respectively). Table 3 shows HbA1c levels at baseline and during follow-up, overall and by type of DM. After 6 months of follow-up, HbA1c levels were statistically lower (p<0.05) in patients with T2DM as compared to patients with T1DM.
HbA1c levels at baseline and 3 and 6 months by type of DM.
| Visit | Total (n=333) | T1DM (n=262) | T2DM (n=71) |
|---|---|---|---|
| Mean±DE | Mean±DE | Mean±DE | |
| Baseline | 8.9±1.0 | 9.0±1.0 | 8.7±0.7 |
| 3 months | 8.3±1.0 | 8.3±1.1 | 8.1±0.6 |
| 6 months | 8.0±1.0 | 8.1±1.1* | 7.7±0.7* |
T1DM: type 1 diabetes mellitus; T2DM: type 2 diabetes mellitus.
Mann–Whitney U test between DM types: *p<0.01.
Change in HbA1c was assessed by comparing the baseline value to the levels found at 3 and 6 months. At 3 months, a mean −0.66 decrease (95% CI: −0.77; −0.56) in HbA1c was seen in patients with T1DM, as compared to a −0.55 decrease (95% CI: −0.72; −0.38) in those with T2DM; the differences between the DM types were not significant. Fifty-nine percent of patients achieved a ≥0.4% decrease in HbA1c at 3 months as compared to baseline. However, more than half the patients (56%) continued to have HbA1c values >8%. At 6 months, mean HbA1c reduction was −0.89 (95% CI: −1.01; −0.76) in patients with T1DM and −0.98 (95% CI: −1.21; −0.76) in patients with T2DM; the differences between the DM types were not significant. Seventy-three percent of all patients achieved ≥0.4% decreases in HbA1c after using the Contour USB and A1CNow+ devices for 6 months. This led to a decrease in the proportion of patients with HbA1c levels >8%, which achieved 41% for the combined groups, by 45% in T1DM and by 25% in T2DM.
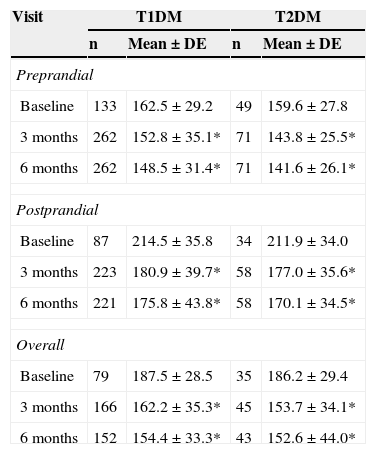
Capillary blood glucoseDaily SMCBG tests increased from 3.1±1.3 at study start to 3.5±1.3 at study completion (p<0.05). Daily tests increased from 3.3±1.3 to 3.6±1.3 (p<0.05) in patients with T1DM and from 2.4±1.1 to 3.0±1.1 (p<0.05) in patients with T2DM. Ninety percent of all patients achieved an improvement in SMCBG results at 6 months. The mean decrease in preprandial capillary blood glucose was 20.7mg/dl, and an improvement was seen in 75% of patients. Postprandial blood glucose decreased by 37.1mg/dl, with an improvement being seen in 83% of patients. Table 4 shows the changes in total, preprandial, and postprandial capillary blood glucose from baseline to 6 months by type of DM. Preprandial, postprandial, and total glucose levels significantly improved in both patients with T1DM and T2DM at 3 and 6 months.
Overall, preprandial, and postprandial capillary blood glucose levels (mg/dL) at baseline and 3 and 6 months by type of DM.
| Visit | T1DM | T2DM | ||
|---|---|---|---|---|
| n | Mean±DE | n | Mean±DE | |
| Preprandial | ||||
| Baseline | 133 | 162.5±29.2 | 49 | 159.6±27.8 |
| 3 months | 262 | 152.8±35.1* | 71 | 143.8±25.5* |
| 6 months | 262 | 148.5±31.4* | 71 | 141.6±26.1* |
| Postprandial | ||||
| Baseline | 87 | 214.5±35.8 | 34 | 211.9±34.0 |
| 3 months | 223 | 180.9±39.7* | 58 | 177.0±35.6* |
| 6 months | 221 | 175.8±43.8* | 58 | 170.1±34.5* |
| Overall | ||||
| Baseline | 79 | 187.5±28.5 | 35 | 186.2±29.4 |
| 3 months | 166 | 162.2±35.3* | 45 | 153.7±34.1* |
| 6 months | 152 | 154.4±33.3* | 43 | 152.6±44.0* |
T1DM: type 1 diabetes mellitus; T2DM: type 2 diabetes mellitus.
Wilcoxon test compared to baseline values: *p<0.05.
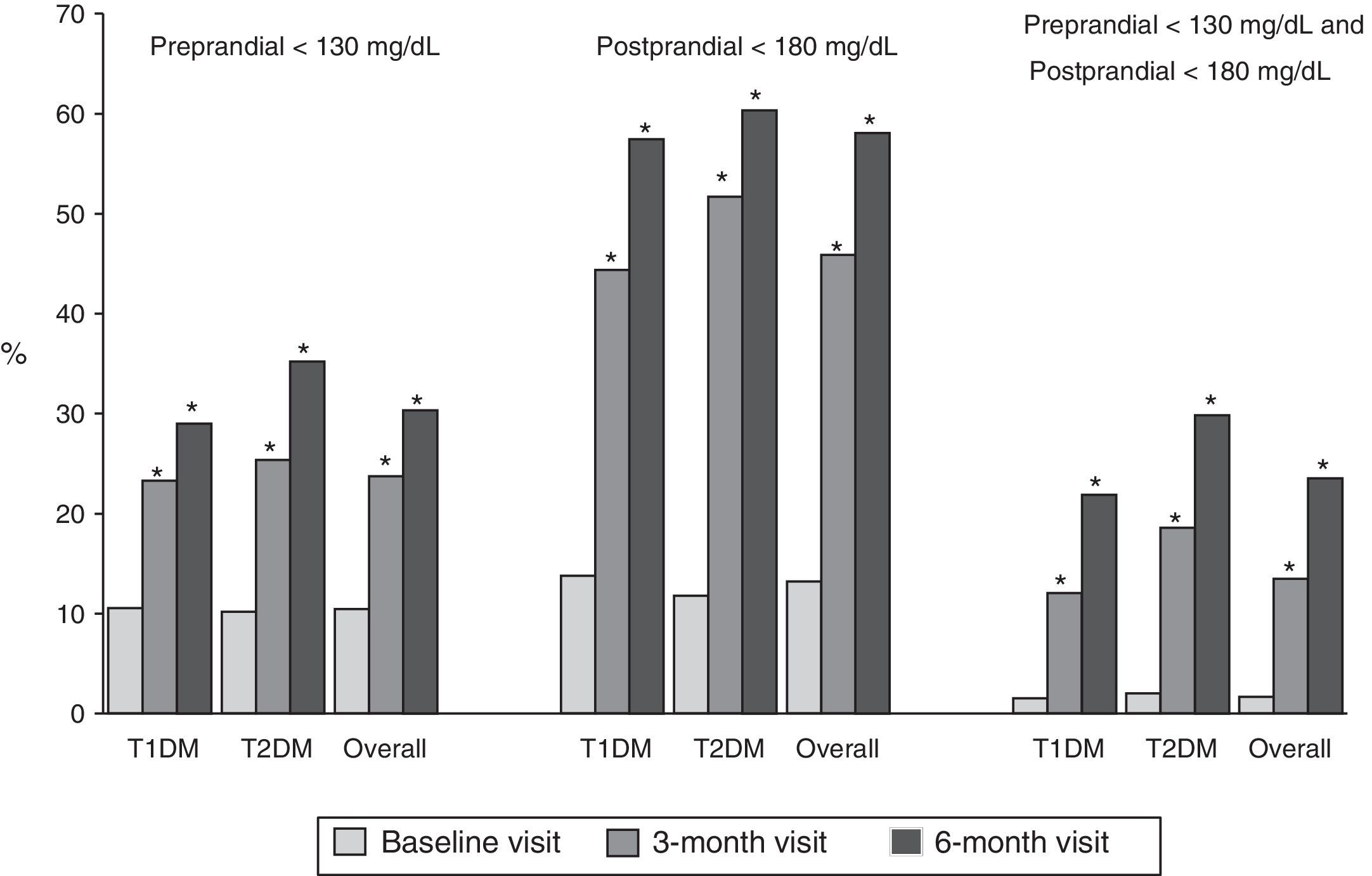
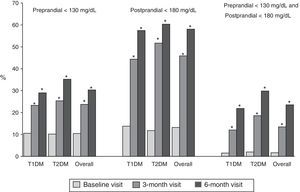
Fig. 1 shows the proportion of patients who had at baseline and at 3 and 6 months a preprandial glucose level <130mg/dl, those who had a postprandial glucose level <180mg/dl and those who had both, overall and by type of DM. Thirty percent of patients had preprandial glucose levels <130mg/dl at 6 months, as compared to 10% at the baseline visit. Fifty-eight percent of patients had postprandial glucose levels <180mg/dl, while only 13% of patients had such levels at baseline, with significant differences in both cases. Finally, 24% of patients met both objectives, as compared to 2% at baseline.
Proportion of patients with preprandial capillary blood glucose <130mg/dL and postprandial capillary blood glucose <180mg/dL at baseline and at 3 and 6 months, overall and by type of DM. T1DM: type 1 diabetes mellitus; T2DM: type 2 diabetes mellitus; McNemar test compared to baseline: *p<0.05.
At study end, more than 97% of patients were satisfied with their use of the Contour USB glucometer. As regards the physicians participating in the study, more than 95% were satisfied with the use of both devices, and a similar proportion thought that they had a positive impact on patient management or monitoring.
DiscussionThis observational, prospective study showed that the use of a glucometer with advanced features (Contour USB) and a device to measure HbA1c levels (A1CNow+) may contribute toward improving blood glucose control and monitoring in insulin-treated patients with DM.
SMCBG is an indispensable component for the self-control of diabetes, which is in turn a critical part of the integral management of the disease. Its use has been shown to have beneficial effects on metabolic control in DM, as demonstrated by HbA1c, especially in patients treated with insulin. It is also able to predict hypoglycemia and to contribute to patient awareness of the disease. Both the SED and some European consensuses have recently published guidelines and recommendations on the most effective use of SMCBG in different settings and patients with DM.9,11 Specifically, different recommendations are made for insulin-treated patients on the frequency of SMCBG depending on the insulin therapy regimen prescribed and on the stability of blood glucose control.9,11 In patients with T1DM, the SED recommends 3–4 SMCBG measurements daily and a 7-point weekly profile (measurements before and after meals and at bedtime) in those with stable metabolic control and increased frequency in those with unstable control. SMCBG frequency in our study patients was within these recommendations (specifically, 3.6 daily in the group of patients with T1DM) and significantly increased during the study, both in patients with T1DM and in patients with T2DM.
There is increasing evidence that postprandial blood glucose control is critical for controlling HbA1c levels, and that a decrease in these levels may help to prevent the occurrence of complications. It has recently been shown that the frequency of postprandial glucose measurements is related to HbA1c decrease in insulin-treated patients with DM, and that measurement is promoted by the use of glucometers with advanced features that allow for flagging blood glucose as preprandial or postprandial.12 In our study, the use of such features made it possible to confirm blood glucose improvement both before and after meals. Throughout our study, an improvement in the blood glucose profile resulted in a progressive decrease in HbA1c, which was 0.9% on average at study end. In almost two thirds of patients a reduction greater than expected was achieved (≥0.4%). This figure can be better appreciated if it is borne in mind that in 24-week clinical trials with drugs HbA1c decreases of this magnitude are considered to be clinically significant.13,14
The main strengths of our study are the high number of patients recruited, the high proportion who completed follow-up (75%), and its conduct in the setting of standard clinical practice. It has however some limitations: it was not a controlled or randomized study, and the different interventions to achieve the predefined objectives were not specified. Consequently, we have been unable to establish the contributions of the different interventions used to the improvement in blood glucose profile and HbA1c, nor can we rule out a Hawthorne effect (i.e. that the results could have been affected by patient awareness of being studied). It should be noted, however, that the information provided by randomized, controlled clinical trials and that derived from studies in standard clinical practice are being increasingly considered as complementary.
In conclusion, the use of a glucometer with advanced features and the measurement of HbA1c in capillary blood may contribute to both the improving and the closer monitoring of metabolic control in insulin-treated patients with DM.
Conflicts of interestThe study was sponsored by Bayer Hispania S.L., Sant Joan Despí, Spain. IV and IC were members of the advisory committee for study design and had independent access to the data and their analysis. They received fees for their participation. IV and IC state that they have no conflicts of interest in relation to the results of this study.
PA and NG are employees of Bayer Hispania S.L. GR is an employee of Adknoma Health Research S.L.
The authors thank the invaluable collaboration of all investigators and participants in the COMET study.
Please cite this article as: Vinagre I, Álvarez P, García N, Roura G, Conget I, en representación de los investigadores del estudio Comet. Evaluación del control metabólico en pacientes con diabetes tratados con insulina mediante la utilización de los dispositivos Contour USB y A1cNow+ (Estudio COMET). Endocrinol Nutr. 2015;62:384–390.