Bariatric surgery (BS) is an increasingly used therapeutic option for severe obesity which allows patients to achieve sustained weight loss over time and resolution or improvement in most associated pathological conditions. Major mid- and long-term complications of BS include iron deficiency and iron-deficient anemia, which may occur in up to 50% of cases and significantly impair patient quality of life. These changes may be present before surgery. The aim of this review was to prepare schemes for diagnosis and treatment of iron deficiency and iron-deficient anemia before and after bariatric surgery.
La cirugía bariátrica es una modalidad terapéutica para la obesidad grave que se utiliza cada vez con más frecuencia, y permite que el paciente consiga una pérdida de peso mantenida en el tiempo y una resolución o mejoría de la mayor parte de las enfermedades asociadas. Una de las principales complicaciones a medio y a largo plazo es el déficit de hierro y la anemia ferropénica, que puede afectar hasta al 50% de los casos, y deteriora de manera importante la calidad de vida del paciente. Estas alteraciones pueden estar presentes desde el preoperatorio. El objetivo de la presente revisión es elaborar unos esquemas de diagnóstico y tratamiento del déficit de hierro y la anemia ferropénica en el pre y postoperatorio de la cirugía bariátrica.
Morbid obesity is a chronic disease characterized by excess body weight and fat, and is associated with an increased risk of mortality and chronic diseases such as type 2 diabetes, high blood pressure, dyslipidemia, cardiovascular disease, steatohepatitis, or respiratory disease, amongst others, in addition to a significant impairment in the quality of life.1–4 Bariatric surgery (BS)5 is a treatment which has been shown to be effective in the long term for achieving weight loss and the resolution of or an improvement in patient comorbidity. BS is indicated for patients with severe obesity (body mass index [BMI]>40kg/m2 or BMI>35kg/m2 with an associated disease),5,6 although in recent years various scientific societies have proposed its use in specific patients with lower degrees of obesity.7,8
However, BS is not free from risk, particularly from nutritional complications. The most common of these are iron deficiency and iron deficiency anemia. In fact, these are already detected in a high proportion of obese patients before BS, and may affect up to 50% of patients during mid and long term follow-up after surgery.9–11
The different BS procedures currently performed, usually through a laparoscopic approach, involve a reduction of gastric capacity, whether or not associated with small bowel bypass, which results in a variable degree of micronutrient malabsorption.12 The number of BS procedures increases every year. Gastric bypass continues to be the most commonly used procedure, but other techniques, such as vertical gastrectomy, have been increasingly used in recent years.13 Although BS is usually associated with less risk of bleeding as compared to other major surgeries of the gastrointestinal tract,14 it increases the risk of developing anemia and iron deficiency after surgery. Moreover, anemia or iron deficiency are commonly found in patient candidates for BS, and are associated with an increased risk of perioperative complications and with the occurrence of anemia in the mid and long term after surgery.10,15–17
The most significant causes of iron deficiency and iron deficiency anemia after BS include: decreased intake due to food (especially red meat) intolerance; decreased gastric proteases and acidity (hypochlorhydria), which causes the incapacity to release iron from food and to reduce ferric to ferrous iron for subsequent absorption; and decreased absorptive surface (especially in the duodenum and proximal jejunum), together with blood losses caused by perioperative bleeding, menses, etc.6,11–13 Folic acid and vitamin B12 deficiency may also exist, associated with various factors such as impaired intake, malabsorptive procedures, or decreased intrinsic factor production.11,17,18
The pathogenesis of iron deficiency anemia also depends on the procedure used for BS. When an adjustable gastric band is used, anemia mainly results from a decreased intake of iron-rich foods, in vertical gastrectomy it is due to the malabsorption component (decreased Fe3+ to Fe2+ conversion) due to gastric resection, and in procedures bypassing the duodenum and the first jejunal loops (gastric bypass and biliopancreatic diversion) it is caused by the prevention of iron absorption at that level.11
Various studies have estimated that iron deficiency and iron deficiency anemia occur after BS in 30–50% and 20–30% of patients respectively.9,11,15,16 This prevalence increases over time after surgery, and is more common in women of childbearing age, in patients with iron deficiency before surgery, and after procedures with a malabsorptive component.15,16 The long-term monitoring of anemia in patients undergoing BS is therefore indispensable, because the treatment of anemia has been shown to decrease complications and to prevent an impairment in patient quality of life.9,15,19,20 A multidisciplinary approach is therefore required to assess and treat anemia and/or iron deficiency, in order to optimize the available resources, provide the greatest benefit to the patient, and minimize the associated complications.20,21
The treatment of iron deficiency anemia consists of the administration of iron, for which different preparations and administration schemes and routes may be used. The oral route is the most commonly used for iron administration. In BS, however, because of the different factors associated with oral iron therapy, such as the high rate of gastrointestinal side effects, poor treatment adherence, or difficult absorption, the intravenous route is becoming increasingly used.11,18,22–24
The purpose of this review, conducted by a multidisciplinary group of physicians (surgery, endocrinology and nutrition, hematology, and internal medicine), was to prepare flow charts for the diagnosis and treatment of iron deficiency and iron deficiency anemia in patients who undergo BS, both before surgery and in short and long term postoperative follow-up, based on the evidence available in the literature on the prevention and treatment of iron deficiency and iron deficiency anemia both before and after BS.
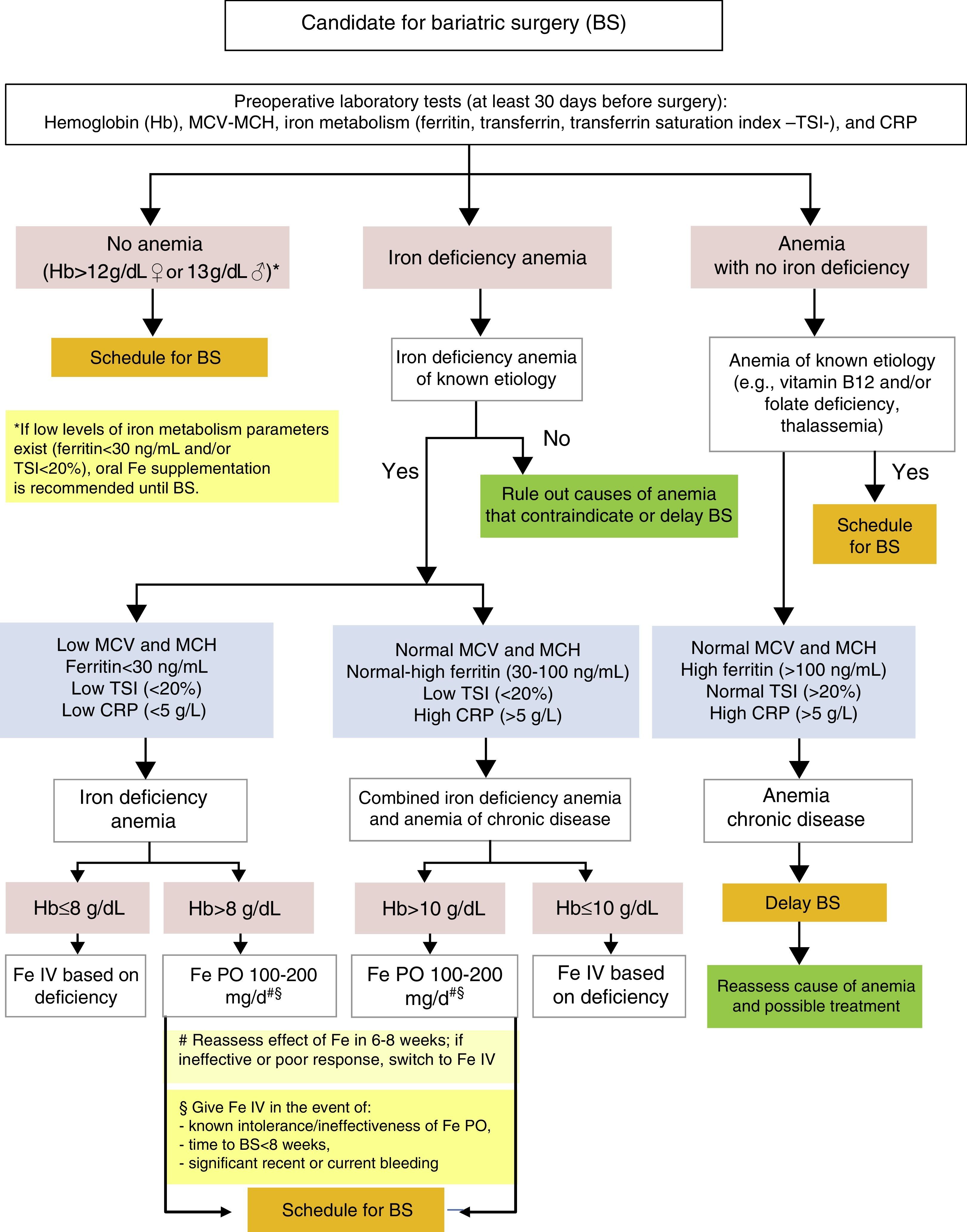
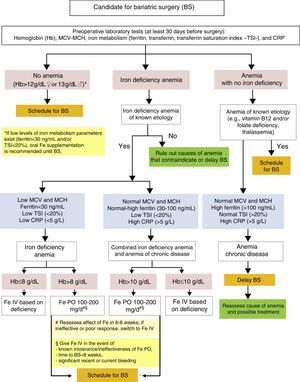
Diagnosis and treatment of iron deficiency anemia before bariatric surgery (Fig. 1)The evaluation of candidates for BS includes laboratory tests such as a complete blood count, coagulation tests, blood chemistry (kidney and liver function, lipid profile, carbohydrate metabolism), and the measurement of mineral and micronutrient levels (iron, folic acid, vitamin B12, and vitamin D),11,25 in addition to other measurements considered to be indicated based on the patient's clinical condition and on the type of surgery to be performed. This evaluation should be conducted at least 30 days before surgery.26 Various studies have reported prevalence rates of anemia before BS ranging from 6 to 22%.9,11
BS is associated with nutritional risk, and prior nutritional deficiencies, such as iron or vitamin D deficiency, should therefore be treated. Although folate and/or vitamin B12 deficiencies are not uncommon in candidates for BS, iron deficiency is the most common cause of anemia in these patients, and may be found in up 60% of cases.27
The evaluation of preoperative iron deficiency anemia should include the following measurements: hemoglobin (Hb), mean corpuscular volume (MCV), mean corpuscular Hb (MCH), iron metabolism (ferritin, transferrin, and the transferrin saturation index [TSI]), and C-reactive protein (CRP) as an inflammatory activity marker.9
In patients with no anemia based on the criteria of the World Health Organization (WHO) (Hb levels less than 12g/dL in females or 13g/dL in males), surgery may be normally scheduled. However, if iron deficiency is found (ferritin <30ng/mL and/or TSIS <20%), oral iron supplementation is recommended before surgery.26
The detection of preoperative iron deficiency anemia at the initial clinical evaluation or the preoperative visit requires a careful clinical and laboratory assessment, especially if anemia is significant (Hb ≤10g/dL) or is not due to a known prior etiology such as gynecological losses in premenopausal women. These patients should be referred for assessment by hematology or internal medicine to identify the cause of anemia and its potential correction. The correction of anemia before surgery, if possible, is advised.26 That is to say, either elective BS should be delayed until anemia has been corrected, or the indication for surgery should be reconsidered.
Once the cause of iron deficiency anemia is determined, it should be classified as iron deficiency anemia or combined iron deficiency-inflammatory anemia.28
Iron deficiency anemia is characterized by microcytosis and hypochromia (low MCV and MCH), low iron deposits and circulating levels (ferritin <30ng/mL, TSI <10%), and usually normal inflammatory parameters (C-reactive protein [CRP] levels less than 5mg/L). In this isolated iron deficiency anemia, treatment is recommended with oral iron (100–200mg of elemental iron daily). Ferrous salts are preferred because they are better absorbed, and ferrous sulphate is especially recommended because of its acceptable tolerability, efficacy, and low cost.29 Re-evaluation with laboratory tests is advised after 6–8 weeks of treatment, and the patient should be switched to intravenous (IV) iron supplementation if no improvement is seen in Hb. In the event of previously known oral iron intolerance or inefficacy, recent or current significant bleeding (e.g. gynecological losses), a very low initial Hb level (≤8g/dL), or moderate to severe anemia (Hb 8–10g/dL) in patients who are symptomatic or in whom surgery is scheduled in less than 8 weeks, IV iron supplementation is initially recommended after the total dose to be administered in each individual patient has been calculated. The traditional Ganzoni formula30 may be used to estimate deficiency, although this calculation may underestimate iron requirements. As an alternative, a simplified, easy to use dosage scheme validated for ferric carboxymaltose IV in a clinical trial conducted in patients with intestinal bowel disease, which takes patient weight and Hb level as a reference, is available.31Fig. 2 shows the calculation of the total IV dose of iron to be administered based on the Ganzoni formula, usually as iron sucrose, or on the simplified table validated for ferric carboxymaltose.11
In patients in whom work-up suggests combined iron deficiency anemia and anemia of chronic disease (ACD), the latter usually shows red blood cells (RBC) with normal corpuscular volume and Hb (MCV and MCH), normal or slightly increased ferritin levels (30–100ng/mL) associated with functional iron deficiency (TSI <20%), and CRP increase (>5g/L), although CRP levels are usually lower than those seen in isolated ACD.32–34 In these patients, once other concomitant diseases have been ruled out, BS recommendation is maintained and the consideration of preoperative treatment with IV iron is advised, especially if anemia is moderate to severe (Hb lower than 10g/dL), because it has been shown to be more effective than oral iron therapy in these cases.18,22–24 Oral iron supplementation may be considered in patients with moderate preoperative anemia (Hb >10g/dL) with no prior intolerance to or prior ineffective reaction to oral iron therapy and without significant or active blood losses. As in isolated iron deficiency anemia, the efficacy of oral supplementation in these patients should be reassessed at 6–8 weeks, and preoperative treatment should be completed with IV iron if required.
In patients in whom anemia with no iron deficiency is detected, the presence of known causes that may justify moderate anemia, such as the patient being a carrier of thalassemia minor, which would not contraindicate surgery, must be assessed. Vitamin B12 and/or folate deficiency should also be ruled out. After supplementation with these vitamins and additional studies, if required, BS may also be scheduled. All other patients may be classified as isolated ACD, characterized by normocytosis and normochromia (normal MCV and MCH), normal or elevated ferritin (>100ng/mol) with normal transferrin saturation (TSI >20%), and increased inflammatory markers (CRP >5g/L). It is recommended that BS be delayed until the chronic disease with which the anemia is associated (e.g. connective disease or kidney failure) has been re-evaluated and treated if appropriate. In practice, in such cases surgery is usually canceled.
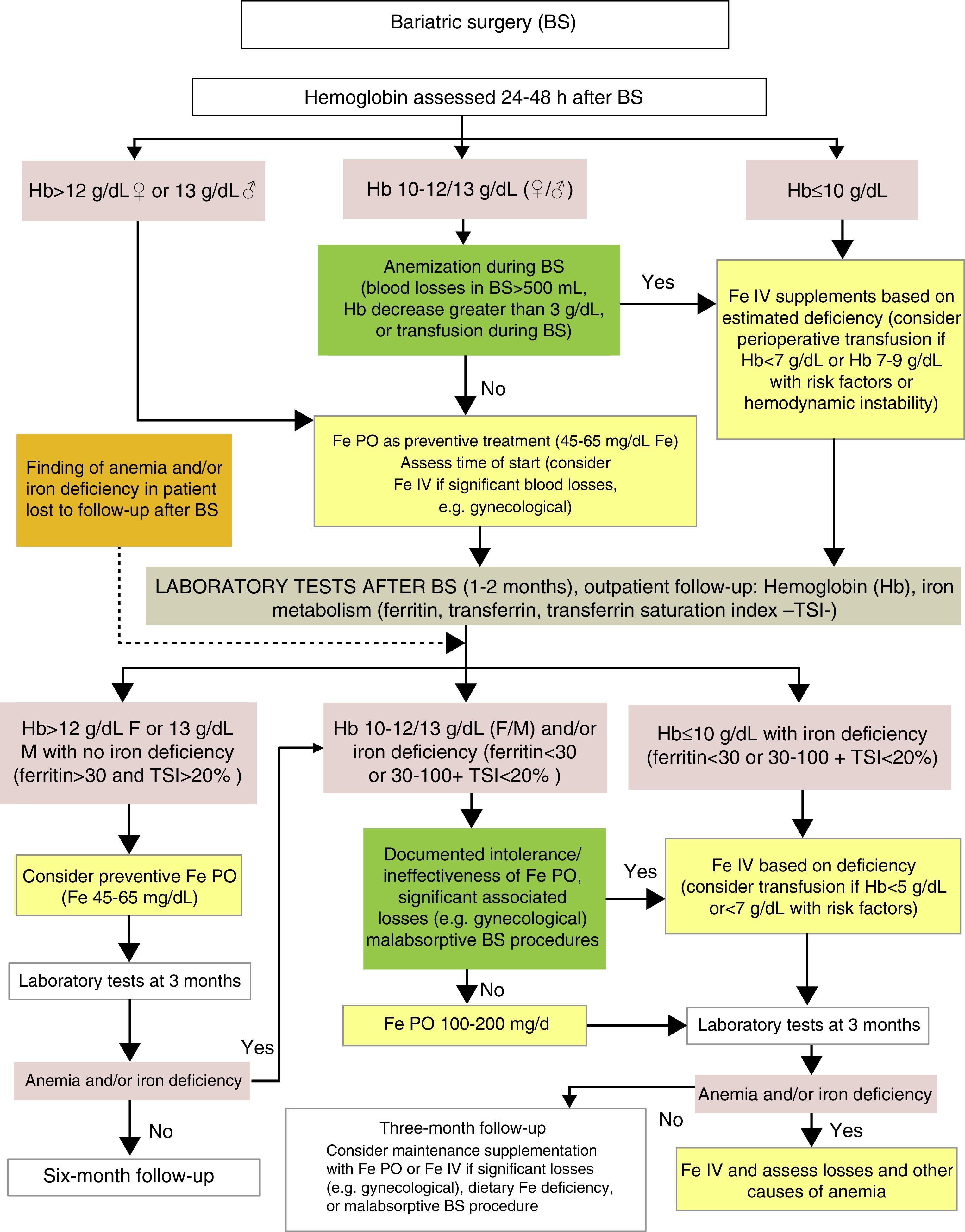
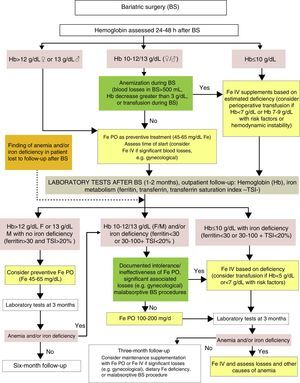
Diagnosis and treatment of iron deficiency anemia after BS (Fig. 3)Although BS procedures do not usually involve significant peroperative bleeding, an assessment of the Hb level is recommended 24–48h after surgery.
Clinical practice guidelines advise the prescription of long-term supplementation with micronutrients to all patients undergoing BS, the dosage being adapted to the characteristics and risk of each surgical procedure. The most common deficiencies are those of vitamin B12, calcium, vitamin D, and iron. The usually recommended dose of iron is 45–60mg of elemental iron, included in a multivitamin preparation.25 It should be borne in mind that oral iron tolerability may be compromised in the first weeks after surgery due to a greater risk of gastrointestinal side effects. In any case, both the time of the start and the dose will depend on such factors as prior iron deposits, perioperative blood losses, the characteristics of the surgery (higher requirements in procedures bypassing the duodenum and the first jejunal loops), diet tolerance (with foods providing iron), and the presence of blood losses (e.g. from menses).11,21 If iron therapy is required in the first few weeks, the use of a fluid preparation or sachets is advised, to avoid the intake of big tablets.
If no anemia exists (Hb >12g/dL in females or >13g/dL in males) in the early postoperative period, the start of supplementation with 40–65mg of elemental iron, included in or associated with the multivitamin complex usually prescribed at discharge, may be considered depending on the gastrointestinal tolerance of the patient.25
In patients with moderate postoperative anemia (Hb 10–12g/dL in females or 10–13g/dL in males), IV iron therapy based on the estimated deficiency should be considered if significant intraoperative or perioperative anemia occurs (quantified blood losses greater than 500mL, Hb decrease >3g/dL from preoperative levels or the need for intraoperative RBC transfusion). In all other patients with Hb >10g/dL, oral iron therapy should be prescribed at doses of 100–200mg daily, following an assessment of individual treatment tolerance and expected treatment compliance.
In patients with significant postoperative anemia (Hb <10g/dL), the presence of transfusion criteria should be assessed based on current recommendations, which advise a restrictive transfusional strategy (Hb <7g/dL or Hb 7–9g/dL with associated risk factors, or hemodynamic instability).26 In addition to potential transfusion support in the described conditions, all these patients may benefit from the rapid replacement of iron deposits with IV iron therapy depending on the calculated iron deficiency.21,26,35
In addition to recommendations for preventive supplementation, the specific monitoring of iron deficiency and iron deficiency anemia, which allows for the early detection of changes, is advised after BS. One to two months after surgery, a clinical and laboratory assessment, including a complete blood count and iron metabolism, is recommended.36 In patients who have undergone BS months or years before and have been lost to follow-up, this should be restarted if anemia and/or iron deficiency is found. It is essential, particularly in patients lost to follow-up, to assess the cause of anemia and rule out diseases with a specific treatment such as vitamin B12 or folate deficiency, or other less common deficiencies such as copper deficiency.11
If this evaluation done months (or years in the event of follow-up loss) after BS detects absolute (ferritin <30ng/mL) or functional iron deficiency (ferritin 30–100 and/or TSI <20%), with or without anemia, treatment with oral iron 100–200mg/d is recommended, with a re-evaluation of its efficacy after 8–12 weeks of treatment. If the Hb level is <10g/dL in patients with a prior intolerance to oral iron or in whom it is ineffective, with significant concomitant blood losses (e.g. gynecological), or who have undergone malabsorptive BS procedures, treatment with IV iron is advised until the estimated iron deficiency has been replaced, especially if the anemia is symptomatic. In these patients, severe iron deficiency (Hb <8g/dL) may be detected. This usually becomes chronic in weeks or months and is generally well tolerated due to the development of compensatory mechanisms that ensure adequate tissue oxygenation under normal conditions, so that almost no symptoms occur even with very low Hb levels (5g/dL). The maintenance of a restrictive transfusion strategy is therefore recommended, provided a specific treatment alternative to allogeneic blood transfusion is available and the few clinical symptoms allow for waiting until the specific treatment has taken effect.37 In patients with severe iron deficiency anemia after BS, IV iron therapy is especially recommended to minimize the need for a blood transfusion.
In patients with no anemia or iron deficiency in postoperative control (ferritin >30ng/mL and TSI >20%), or in those administered treatment for recovery, follow-up is advised with laboratory tests every three months, and routine supplementation with micronutrients, including iron, should be continued. Special care should be taken with conditions associated with a risk of iron deficiency (women of childbearing age, diet very poor in iron, or BS procedures with a malabsorptive component) or the prior ineffectiveness of oral iron. In such cases, regular treatment with IV iron may be necessary to maintain iron deposits.18 It should also be noted that iron deficiency may cause villous atrophy, which contributes to the ineffectiveness of oral iron by additionally impairing intestinal iron absorption.38 The correction of iron deficiency may improve this situation.33
In the event of anemia and/or iron deficiency relapse, treatment with oral or IV iron should be restarted, and it is recommended that blood losses or other causes of anemia (e.g. vitamin B12 or folic acid deficiency) should be ruled out once more.11
In patients with no evidence of new or relapsing anemia and/or iron deficiency one year after surgery, the time between measurements may be increased to six months or even one year.36
DiscussionThe prevalence of iron deficiency anemia is greater among candidates for BS than in the general population, and is even greater after the surgical procedure.9,11,16,17,20,23 The evaluation and treatment of iron deficiency is therefore recommended both before and after surgery,9,11,15,19,27 although clinical practice guidelines do not include specific recommendations about the most adequate treatment and prevention measures for iron deficiency with or without anemia before and after BS.5,25,36
Anemia is detected before surgery in 10–15% of candidates for BS, in which iron deficiency is the main etiological factor.9,11,27 Iron deficiency alone occurs in up to 50% of these patients, and especially in females, because of the high prevalence among candidates for BS of women of childbearing age, in whom menstrual losses are the most common cause of anemia and/or iron deficiency.16,27 It is also recommended that other causes of iron deficiency with or without anemia which are more frequent in patients with morbid obesity than in the general population, such as gastritis or peptic esophagitis should be ruled out.39
While obese patients often have an associated metabolic, respiratory, or bone and joint disease, they do not usually have other causes of anemia such as chronic diseases (kidney failure, inflammatory bowel disease). A greater prevalence of tumors has however been reported in obese patients as compared to the general population.40 Tumors have also been found to be associated with anemia, but obviously the finding of a malignancy in the preoperative evaluation of a patient with morbid obesity would contraindicate BS, at least in the short term.
In addition, obesity itself is often associated with a low-grade inflammatory state that may impair iron metabolism, resulting in a slight increase in iron deposits (hyperferritinemia) but with a functional restriction of the iron required for erythropoiesis (which can be estimated by transferrin saturation).28,33 This inflammatory state, which may be inferred from moderately elevated C-reactive protein (CRP) levels in the absence of another concomitant inflammatory disease, is often associated with a slight reduction in hemoglobin levels among candidates for BS.27
The prevalence of bleeding before BS and in the early postoperative period is low (2–4%), and the transfusion of allogeneic blood is required in 2% of cases.14
It is well known, however, that even mild anemia increases morbidity and mortality after any surgery.41 Preoperative anemia evaluation and correction, if possible, is therefore recommended to try and improve postoperative prognosis and decrease the administration of allogeneic blood transfusions and their complications.42,43 If anemia and/or iron deficiency is detected before surgery, the start of iron deficiency replacement is recommended along with etiological work-up in an attempt at correction before surgery.26 Oral iron is usually given, but treatment with IV iron is recommended if oral iron is found to be ineffective on reassessment after 6–8 weeks of treatment. IV iron should be directly started in patients with known iron intolerance or ineffectiveness, significant recent or current bleeding, severe iron deficiency anemia (Hb <8g/dL), or moderate mixed anemia (iron deficiency-inflammatory anemia) (Hb <10g/dL).9,11,21,44–46
A recent document on the treatment of iron deficiency anemia47 has proposed a treatment algorithm including the administration of erythropoiesis-stimulating agents (ESAs) for anemia of chronic disease with no iron deficiency or kidney failure. A review of anemia and BS by Munoz et al. also included ESA administration before surgery for anemia of chronic disease with no iron deficiency or anemia from an unknown cause.9 This paper includes no such preoperative measure because of the little evidence available, in contrast to what occurs in other elective major surgeries such as elective orthopedic major surgery.26,48,49
The clinical practice guidelines of the American Society for Metabolic and Bariatric Surgery advise the start of daily supplementation with oral iron (45–60mg of elemental iron plus vitamin C) early after surgery to prevent iron deficiency, especially in patients undergoing malabsorptive surgery and in women of childbearing age, while the European guidelines do not include specific recommendations in this regard.25,36 This review recommends the individualization of preventive oral iron supplementation. Dosage depends on several factors such as the presence of anemia and/or iron deficiency, the type of surgery, or the persistence of menstrual losses in women of childbearing age. IV administration of iron may also be considered necessary, because oral iron tolerance may be compromised during the first few weeks after surgery.
Preventive iron supplementation should be maintained during mid and long-term follow-up in patients undergoing BS, although oral iron is frequently insufficient to maintain oral deposits in these patients, especially if malabsorptive procedures are used.24,50,51 The most recent update of the European practice guidelines for BS recommends regular laboratory tests including complete blood count and iron levels from one month after surgery,36 treatment being started with oral iron (100–200mg/day) in the event of isolated iron deficiency or moderate iron deficiency anemia (Hb >10g/dL), with a reassessment of its efficacy at 8–12 weeks of treatment. IV iron therapy is considered as the initial treatment option if severe iron deficiency anemia is detected after BS, or if the above mentioned factors causing the ineffectiveness of oral iron treatment are present,11,16,36 because IV iron therapy rapidly increases iron deposits, facilitates the rapid recovery of hemoglobin levels, and decreases the transfusion rate.21–23,26,52–54 Post-BS anemia is considered to be among the emergent causes for the use of IV iron.55
Two studies of IV iron treatment in patients undergoing BS reported series of patients treated with iron sucrose,22,53 a molecule with good clinical tolerance that provides a rapid availability of iron transfer to the body, but requires the supplementation as infusions not exceeding 200mg of the drug (with a maximum of 600mg weekly) administered over 30min to avoid toxicity.56 Other molecules such as low molecular weight iron dextran, ferric carboxymaltose, or iron isomaltoside allow for IV administration of higher iron doses, so that the complete iron deficiency may be replaced by a single infusion. The administration of low molecular weight iron dextran as a single infusion corrected anemia and iron deficiency for longer than one year in 23 patients undergoing gastric bypass surgery with no response to prior treatment with oral iron.23 No significant adverse reactions were seen in the study, but a preventive hypersensitization treatment was given, and IV iron was administered over 6h. It should be kept in mind that there is a risk of severe anaphylactic reactions associated with this iron molecule, and that high-dose iron supplementation should be performed as infusions administered over at least 4h.57 The Malone et al. study reported data on the efficacy and safety of ferric carboxymaltose as compared to other oral iron preparations in 123 patients undergoing BS (gastric bypass in most cases) which showed a similar or superior efficacy for the correction of iron deposits and an increase in hemoglobin levels as compared to all other treatments with no significant adverse events.54 These findings agree with the efficacy and safety data available for ferric carboxymaltose in a large number of patients with multiple diseases who showed iron deficiency with or without anemia.58–60 In addition, the possibility of administering a dose up to 1,000mg of ferric carboxymaltose IV over 15min61 confers on this drug the possibility of rapidly replacing iron deposits and correcting anemia in patients undergoing BS who are refractory to oral iron, thus minimizing the number of hospital visits.18,54 This measure has been shown to be cost-effective.62,63 In any case, when IV iron supplementation with any preparation is planned in these patients, the recent recommendations of the Spanish Agency of Medicinal Products and Medical Devices should be taken into account. The recommendations include adverse effect monitoring for 30min after treatment, and the non-indication of IV iron therapy during the first term of pregnancy.64
Finally, once the deficiency has been corrected, iron levels should be monitored in patients undergoing BS and preventive iron supplementation should be continued as recommended by the clinical practice guidelines to avoid the mid or long-term recurrence of iron deficiency.16,17 This maintenance supplementation may consist of oral iron, particularly in patients in whom this route has previously been shown to be effective or if chronic blood losses stop (e.g. menopause). By contrast, in patients with persisting intolerance to oral iron therapy or permanently impaired duodenal iron absorption (malabsorptive BS procedures), maintenance treatment with IV iron may be required.11,24 The introduction of new oral ferric iron preparations such as liposomal iron or ferric maltol, which have apparently better tolerability and bioavailability than other orally absorbed iron salts, could represent an alternative to IV iron therapy in this maintenance phase. However, these preparations are still in the development phase, and the few results reported for them in the treatment of iron deficiency do not include patients with anemia after BS.65,66
In conclusion, iron deficiency with or without anemia is a common complication of BS. The prevalence of iron deficiency significantly increases after surgery. It may occur in the short or long-term during postoperative follow-up and significantly impair quality of life.
The identification and treatment of preoperative anemia may contribute to decreased morbidity and mortality and the need for perioperative transfusion. Postoperative evaluation and an early start to specific supplementation with oral iron is recommended in patients undergoing BS, particularly those at a greater risk of iron deficiency such as women of childbearing age or those who have undergone surgical procedures with a malabsorptive component. The efficacy of oral iron treatment may be limited by the occurrence of adverse effects, poor adherence, or impaired absorption caused by some BS procedures. In such cases, IV iron therapy is a convenient and effective alternative, and sometimes should be administered in the long term. In addition, in patients with severe iron deficiency anemia after BS, IV iron therapy is especially recommended in order to minimize the possibility of receiving an allogeneic blood transfusion. In any case, additional studies are needed to determine the most adequate dosage scheme for the prevention and treatment of iron deficiency, with or without anemia, in these patients.
FundingThis review article was supported by a grant from Vifor Pharma España SL.
Conflicts of interestThe authors state that they have no conflicts of interest with regard to the text submitted, and that they have received fees as consultants from Vifor Pharma España SL.
Please cite this article as: Jericó C, Bretón I, García Ruiz de Gordejuela A, de Oliveira AC, Rubio MÁ, Tinahones FJ, et al. Diagnóstico y tratamiento del déficit de hierro, con o sin anemia, pre y poscirugía bariátrica. Endocrinol Nutr. 2016;63:32–42.