High weight is a protective factor against osteoporosis and risk of fracture. In obesity, however, where overweight is associated to excess fat, this relationship does not appear to be so clear, excess weight has sometimes been associated to decreased bone mass. Obesity interferes with bone metabolism through mechanical, hormonal, and inflammatory factors. These factors are closely related to weight, body composition, and dietary patterns of these patients. The net beneficial or harmful effect on bone mass or risk of fracture of the different components of this condition is not well known. We need to recognize patients at a greater risk of bone disease related to obesity to start an adequate intervention.
El peso elevado es un factor protector de la osteoporosis y el riesgo de fractura. Aunque en el caso de la obesidad, donde el sobrepeso se encuentra asociado a un acúmulo excesivo de grasa, esta relación no parece estar tan clara y, en ocasiones, se ha relacionado la misma con un descenso de la masa ósea. La obesidad interfiere con el metabolismo óseo a través de factores mecánicos, hormonales e inflamatorios. Estos factores se encuentran íntimamente relacionados con el peso, composición corporal y patrón dietético de estos pacientes. El efecto perjudicial o beneficioso neto sobre la masa ósea o sobre el riesgo de fractura de los distintos componentes de esta enfermedad todavía no es bien conocido. Es necesario reconocer a aquellos pacientes con mayor riesgo de padecer enfermedad ósea relacionada con la obesidad para la realización de una intervención adecuada.
Bone is an active organ on which a great number of factors act. Osteoporosis and increased risk of fracture are conditions which have gained relevance in recent years because of gradual aging of the population and their effects on quality of life and financial impact for society as a whole. In 2010, prevalence rates of osteoporosis in population older than 50 years in the European Union were 6.6% in males and 22.1% in females, and 5.5% in the overall population. In the most populated countries (Germany, France, Italy, Spain, and United Kingdom), approximately 21% of females aged 50 to 84 years meet the criteria for osteoporosis.1,2
Obesity is the most prevalent metabolic disease in developed countries and one of the main causes of morbidity and mortality. In 2008, the WHO estimated that 1400million adults over 20 years were overweight, more than 200million males and approximately 300million females were obese. To sum up, one out of every 10 people in the worldwide adult population were obese.3 According to the ENRICA study, conducted from 2008 to 2010 in a Spanish population, prevalence of obesity in Spain was 22.9% (24.4% in males and 21.4% in females).4
The relationship between overweight and bone metabolism is controversial. Increased body weight has been considered a factor that increases bone mass and decreases risk of fracture.5 On the other hand, increasing evidence has been found in recent years showing that overweight, particularly accumulation of fat mass, may have a negative impact on risk of fracture, particularly when adjusting for bone mass of the patient.6
The available evidence shows that postmenopausal women with obesity have an increased risk of humeral fractures and osteoporotic fractures of the ankle and lower limb, and a decreased risk of hip, pelvis, and wrist fractures. Fewer studies are available in males, but they have a pattern similar to females.7 It has been postulated that increased risk of fracture may be related to difficult mobility and increased falls in patients with obesity.8 In any case, there is no clear explanation yet for the effect of obesity on bone and its consequences.
Prevalence of obesity is greatly increasing in recent years, and population is gradually aging. These two circumstances are intrinsically associated to bone health, and this review is therefore intended to:
- -
Ascertain the mechanisms that relate obesity to changes in bone metabolism.
- -
Detect the factors associated to obesity with a negative impact on bone mineral density and risk of fracture.
- -
Determine the intervention required to prevent or improve the impact on bone of these factors related to obesity.
A narrative review was carried out. An online search was conducted in the medical databases PUBMED and EMBASE for the following terms: “Obesity” [MESH] AND “Bone metabolism” [MESH]; “Body Composition” [MESH] AND “Bone Metabolism” [MESH]; “Obesity” [MESH] AND “Bone” [MESH]; “Obesity” [MESH] and “Vitamin D” [MESH] or “PTH” [MESH]. Original articles, meta-analyses, systematic reviews, and narrative reviews were considered. The search was done on February 1, 2016, and no limits were established for the publication date.
Priority was given to articles with methodology of higher scientific evidence (clinical guideline, meta-analysis, randomized clinical trial, systematic review, original article, and narrative review) published in the last five years.
Body compositionWhen the concept of obesity is considered, it is very important to assess the contribution of the different components of weight (lean mass, fat mass, and water). Lean mass and fat mass are independent determinants of bone mass, and each of them will therefore have a different influence and will depend on several factors.9
Lean mass and boneIncreases in lean or fat-free mass are associated to increased bone mass. The reason for this may be an increased mechanical load on bone related to weight and muscle hypertrophy. In this regard, a meta-analysis by Ho-Pham et al. showed that correlation between lean mass and bone mineral density in the femoral neck was greater than that of fat mass (r=0.39 [0.34–0.43] vs r=0.28 [0.22–0.33]). The effect of lean mass was greater in men (r=0.43) and postmenopausal women (r=0.45).10
The positive effect of increased lean mass is attributed to factors related to lifestyle such as exercise and diet, estrogen sufficiency, genetic influences, or a combination of these factors. On the other hand, an increase in muscle mass has an independent effect on risk of fracture by decreasing fragility and falls related to osteoporotic fracture.11
Although obesity is characterized by a predominant increase in fat mass, there is also an increase in lean mass that may partly account for the beneficial effect of this on bone mineral density. This situation was studied by Neubecker et al., who compared bone mineral density, bone fragility, and risk of fracture in obese patients and patients with anorexia nervosa and found that female obese patients (with greater lean mass) had greater bone mineral density and less bone fragility, which suggests a lower fracture risk.12
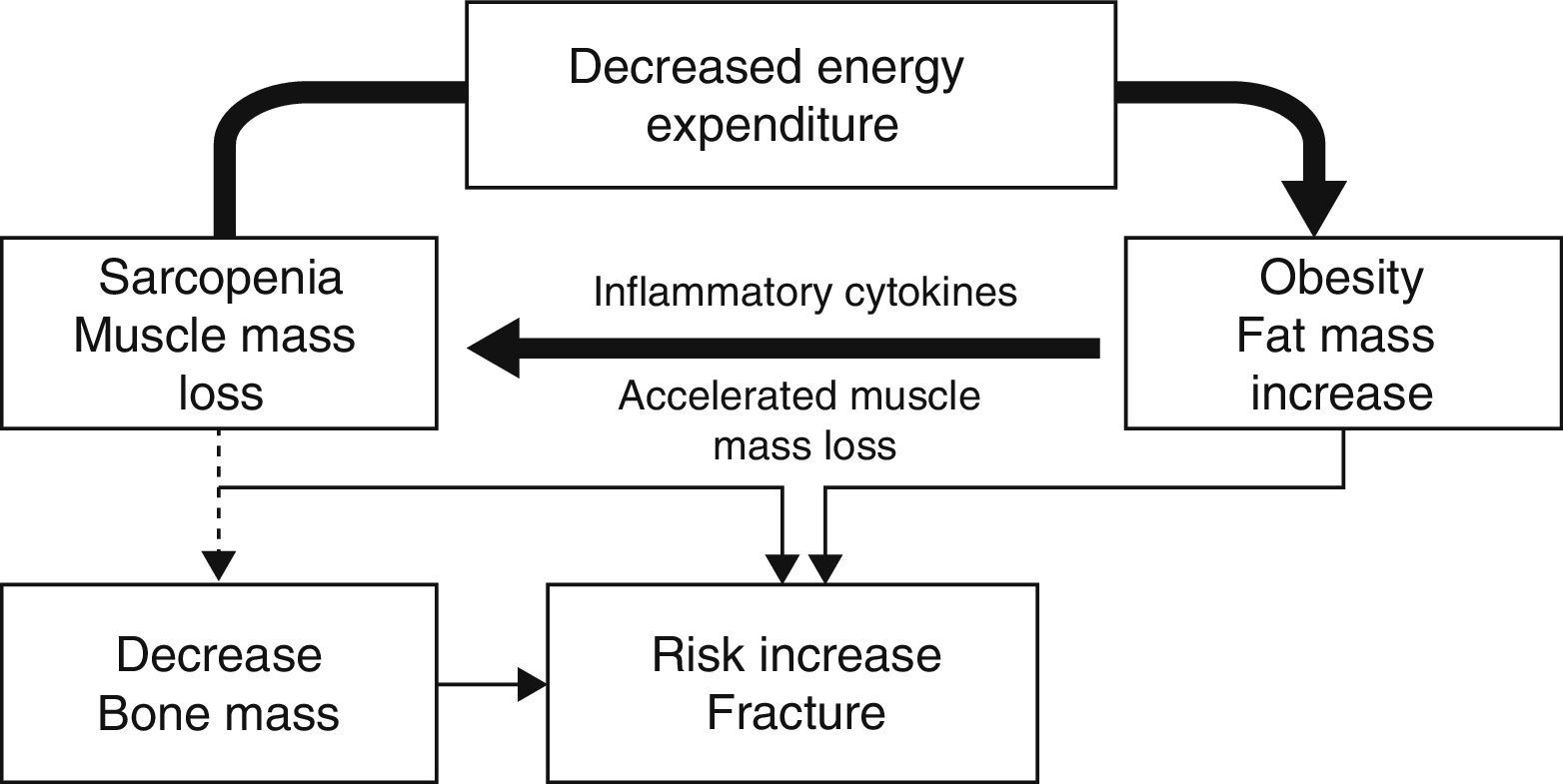
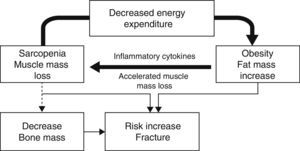
In elderly patients or obese patients with associated severe disease, the so-called sarcopenic obesity is acquiring special interest. In sarcopenic obesity, weight increase is related to a relative decrease in muscle mass.13 The genesis of this disease is related to muscle damage caused by inflammatory mediators in the setting of a vicious cycle of progressive physical inactivity that increases adipose tissue and diseases related to obesity, which increase inactivity in turn. Sarcopenia is enhanced by other factors such as loss of sensitivity to alpha motor neurons, changes in anabolic hormones, and malnutrition.
Several circumstances occur in this pro-inflammatory condition: (1) preferential mobilization of muscle instead of fat for energy consumption; (2) a high inflammation level that causes a progressive decrease in muscle mass; and (3) an impairment in muscle quality caused by fat deposition in muscle due to increased insulin resistance.14
This sarcopenia leads to a lower positive influence of muscle mass on bone, together with decreased mobility and an increase in the number of falls. A recent study by Scott et al. showed that patients with non-sarcopenic obesity had greater bone mineral density in all sites studied (lumbar spine and hip). Patients with sarcopenic obesity also had higher rates of fractures, which were non-vertebral in men (OR: 3.0 [1.7–5.5]) and in all sites in women (OR: 2.8 [1.4–56]), although no significance was found when the latter were adjusted based on bone mineral density in the hip.11 This situation of decreased mobility and increased fragility is associated to a relative increase in fat mass, which leads to a pro-inflammatory state associated to this condition that may have a negative influence on bone15 and increase risk of fracture (Fig. 1).
Fat mass and boneWeight increase in obesity mainly results from fat mass. Fat mass has an impact on bone metabolism mainly through several mechanisms:
- -
From the quantitative viewpoint, an increase in total body mass increases mechanical load, with the already discussed effects. This does not appear to be the main effect, however, because in some cases percent fat mass is not the best predictor for fracture risk reduction, as noted by Moayyeri et al., who found a relationship between fat mass and risk of hip fracture in women, but not in men.16
- -
From the qualitative viewpoint, increased fat mass is associated to an increase in adipose tissue (adipocytes), which has been shown to have several functions: (a) secretes hormones such as leptin, adiponectin, and sex hormones; (b) promotes a pro-inflammatory state; (c) has a common mesenchymal precursor for osteoblasts and adipocytes. These characteristics have an influence on bone mineral density.17
- -
On the other hand, fat mass distribution (visceral or subcutaneous) may be related to bone metabolism.18
The systemic influence of adipose tissue through mediators is widely recognized. Action of these mediators (adipokines and inflammatory factors) may have a significant role in bone mass development:
AdipocytokinesAdipocytokines are hormonal mediators produced by adipose tissue. The two main adipocytokines are leptin and adiponectin, having multiple effects on satiety and insulin resistance, among other functions, but their role in bone metabolism is controversial.
Leptin has a two-way effect on bone. On the one hand, leptin has an in vitro effect on bone marrow, promoting differentiation to osteoblasts and inhibiting differentiation to adipocytes of pluripotent stem cells.19 On the other hand, leptin may act through the sympathetic nervous system to inhibit bone formation by decreasing insulin resistance and reducing the anabolic effect of insulin on bone.20 Leptin has been seen to have a directly proportional action on bone formation markers and an inverse action on resorption markers in postmenopausal women and young adults.21 Because of this, high leptin levels are associated to greater bone mineral density (r=0.18–0.33), which results in fracture risk reduction, as shown by the systematic review performed by Biver et al.22 Despite this beneficial effect, bone metabolism profile has been seen to be better in thin as compared to obese females, even though the latter have higher leptin levels. This last circumstance has two possible explanations: (a) first of all, resistance to leptin action is seen in high degrees of obesity; (b) elevated leptin levels cause a pro-inflammatory state that may lead to poorer bone and cartilage health.23
Based on this data, it was hypothesized that leptin injection could enhance bone formation and inhibit bone resorption, but when the hormone was injected to a group of patients on a weight loss diet, no effect was seen on bone metabolism.24
Adiponectin is another hormone produced by adipose tissue that increases insulin sensitivity. Osteoblasts express adiponectin and its receptors, increasing their differentiation in response to the hormone, which also induces inhibition of osteoclastogenesis and osteoclast activity. However, adiponectin also increases production of the receptor activator for nuclear factor κB ligand (RANKL) and decreases osteoprotegerin levels.25 Analysis of the in vivo effect of adiponectin showed that lower levels were associated to greater bone mass.23 Modification of obesity with the increase in adiponectin associated to weight reduction does not appear to be related to improvement in bone mineral density.26 Adiponectin levels are decreased in obesity and type 2 diabetes mellitus, which could have an effect in patients with these conditions, but no adequate evidence exists to support such effects or use it as a therapeutic target.
Resistin is another adipokine that increases proportionally to obesity and is related to insulin resistance. Effects of resistin on bone metabolism are also bidirectional because, on the one hand, a modest increase has been seen in osteoblast proliferation and, on the other hand, resistin increases osteoclast formation in bone marrow cultures. Otrowska et al. noted that in women with anorexia nervosa, changes in resistin could serve as independent predictors of elevation of crosslaps and RANKL. These changes may negatively affect the osteoprotegerin/RANKL balance and decompensate the balance state of bone remodeling.27
GhrelinGhrelin is an orexigenic peptide secreted by the stomach which, among other functions, stimulates differentiation of preadipocytes into adipocytes and antagonizes lipolysis. Levels of this hormone are inversely related to body mass index and insulin resistance, and are therefore decreased in metabolic syndrome.28 Ghrelin may have a protective effect on bone metabolism by inhibiting precursors of osteoblasts and osteoclastogenic cytokines (tumor necrosis factor-α [TNFα], interleukin [IL]-1, IL-6), and modulating osteoblast differentiation through the GH-IGF-1 axis, as shown by different in vitro studies.29 However, most studies have found no data relating ghrelin levels to bone mineral density,22 with the exception of studies conducted by Makovey et al., who related lower bone mass in the hip of young women to increased ghrelin levels,30 and by Misra et al., who found a positive relationship of bone mineral density in the lumbar spine and hip to ghrelin levels in healthy female adolescents.31 Additional studies are needed of the relationship of this hormone to bone metabolism and its presence in obesity.
Endocrine effect of adipose tissueObesity is related to low-grade chronic inflammation. Adipose tissue is infiltrated by macrophages that induce chronic production of pro-inflammatory cytokines, increases in acute phase reactants, and activation of inflammatory pathways. These cytokines cause an increase in osteoclast differentiation and, thus, in bone resorption. Long-term maintenance of this situation results in osteopenia and osteoporosis.32 The cytokines having greater effects on bone are IL-6 and TNF-α.33
IL-6 is increased in obesity and in patients with increased insulin resistance. This molecule causes an increase in osteoclastogenesis, increasing bone resorption.34 On the other hand, however, stimulation of osteoblast proliferation in situations of high bone turnover has also been seen.35
TNF-α has an effect similar to IL-6. In addition, TNF-α enhances the negative effect on bone by modulating osteoclastogenesis through the RANK receptor (receptor activator for nuclear factor κB), stimulating RANKL, decreasing osteoprotegerin production, and overexpressing the RANK receptor.36 The combination of these factors has a synergistic effect that promotes bone resorption and deteriorates bone.
EstrogensRelationship between estrogens and bone metabolism is clear, and is the main reason for age-related bone mass decrease in postmenopausal women. In postmenopausal obese women, circulating estrogens are partially maintained due to peripheral aromatization (related to adipose tissue) of increased androgens in relation to insulin resistance. Increased circulating estrogen levels also stimulate osteoblast production in bone marrow. This situation may lead to a partial increase in bone mass and some protective role against osteoporosis.17 Few studies designed to identify this functionality are however available, and in the studies conducted, such as the one by Corina et al., the estrogenic action of adipose tissue has not been seen to have a significant effect on bone, particularly in postmenopausal patients.37
Fat mass distributionDistribution of fat mass in the body has been characterized in different compartments in relation to cardiovascular disease, diabetes mellitus, and mortality. It also appears that it could have an effect on bone mineral density. Two types of adipose tissue and obesity are distinguished:
- -
Visceral adipose tissue, related to central obesity: When indirectly measured, abdominal circumference has shown a variable, modest association to bone mass and risk of fracture.18 In the few studies measuring fat mass with advanced techniques (CT and MIR), visceral fat was negatively associated to bone mineral density, content, structure, and strength. In a study conducted by Cenci et al., visceral fat measured in postmenopausal women by CT was seen to be associated to lower bone quality (lower trabecular bone volume and stiffness, and high cortical porosity).38 The Choi et al. study analyzed the relationship between presence of visceral adiposity and bone mineral density and found an inversely proportional relationship after adjusting for confounding metabolic factors.39 This negative relationship may be due to greater secretion of pro-inflammatory cytokines produced in this type of tissue and having the effect on bone previously discussed.
- -
Subcutaneous adipose tissue, related to peripheral obesity: Less evidence is available for subcutaneous adipose tissue, and the few studies conducted have provided conflicting information, ranging from no relationship to a positive association to bone mass. A study by Gilsanz et al. analyzed the relationship between fat distribution and bone strength, and bone strength was found to be positively associated to subcutaneous fat and inversely associated to visceral fat.40
While no sufficient studies comparing the effect of both types of obesity in the same context are available, it may be concluded that the main factor influencing bone metabolism is the amount of adipose tissue, rather than its distribution.19
In this regard, in different diseases such as obesity, anorexia nervosa, and type 2 diabetes mellitus, an inverse relationship has been noted between bone marrow fat and bone mineral density. Hypotheses postulated for this increase include presence of a same mesenchymal precursor for osteoblasts and adipocytes. In obese patients, there would be a greater differentiation to adipose tissue with a reduction of bone formation potential. This data is currently difficult to measure, and no adequate evidence to ascertain whether there may be an increased risk of fracture is available yet.41,42
To sum up, increased adiposity, associated to a decrease in fat-free mass, has been related to decreased bone mineral density and increased fragility.43 However, the effect of this excess fat mass and inflammation on this bone metabolism is not very well known yet.
Insulin resistanceObesity is intrinsically related to the concept of insulin resistance and metabolic syndrome. It has been reported that this insulin resistance and its metabolic consequences (increased insulin, amyline, and preptin levels) may be associated to bone metabolism.
Insulin and related peptidesInsulinElevated insulin levels are positively associated to bone mass increase. This situation may be due to decreased osteoclast activity and increased osteoblast function related to increased estrogen levels as the result of aromatization and an increase in free estrogen fractions due to decreases in sex hormone binding proteins.44 Among patients with insulin resistance, those with type 2 diabetes mellitus have been shown to have a decreased bone mass, which may be related to the relative decrease in insulin secretion.44 As regards risk of fracture, the meta-analysis of Vestergaard et al. demonstrated that patients with both type 1 and type 2 diabetes mellitus had an increased risk of hip fracture, although bone mineral density was lower in patients with type 2 diabetes.45 The increase in risk of fracture and changes seen in bone mineral density in diabetic patients may be due to multiple factors, including a negative calcium balance, hypoinsulinemia, kidney function impairment, advanced glycation products, and increased inflammatory cytokine levels. Further studies are needed to assess the weight of each of these factors on risk of fracture and be able to act on them.
AmylineAmyline is a peptide co-secreted with insulin, a member of the calcitonin family, which is therefore increased in patients with insulin resistance. It appears that this hormone may have effects similar to calcitonin, inducing osteoblast proliferation in vitro and decreased osteoclast production.46
PreptinThis peptide is secreted in the same way as the previous ones and has also shown in vitro an anabolic effect on bone, but has no effect on osteoblasts.47
According to these findings, insulin resistance, at least in its early phases, may be related to increased bone formation with decreased bone resorption and, thus, to an increase in bone mass.44 This condition is gradually lost as insulin secretion decreases, which may cause in diabetic patients decreases in bone mineral density and an increased risk of fracture.
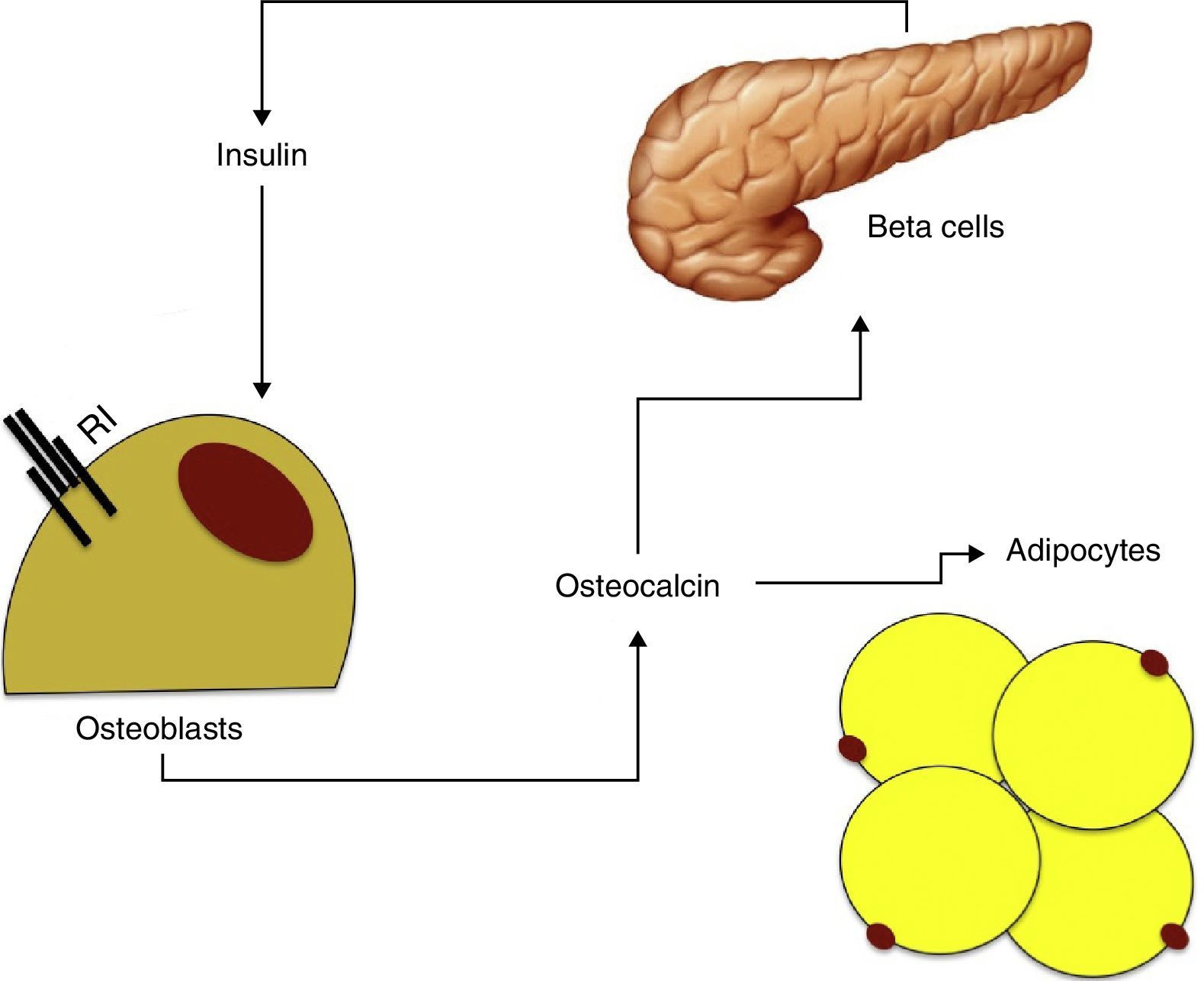
Insulin resistance, osteocalcin, and metabolic syndromeOsteocalcin is a 49-amino acid peptide synthesized by osteoblasts only and stored in the bone mineral matrix as hydroxyapatite crystals. Because of its characteristics, osteocalcin is used as bone formation marker.
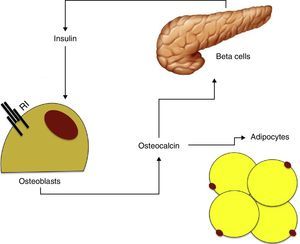
Osteocalcin has been seen to stimulate proliferation of beta cells and insulin secretion in vitro, and also increases adiponectin production, thus improving insulin sensitivity.48 As already noted, insulin has in turn an effect on the production and differentiation of osteoblasts and adiponectin. These findings suggest the existence of a feedback mechanism between pancreatic beta cells, adipose tissue, and bone (Fig. 2).
Based on the available data, it may be hypothesized that osteocalcin could have a central role in the relationship between bone metabolism and insulin resistance. Hyperinsulinism would increase osteoblast and osteocalcin production, which would in turn stimulate insulin production and improve insulin sensitivity through adiponectin.49
In obesity, characterized by high insulin resistance and a relative decrease in insulin secretion, decreased osteocalcin production may be seen according to different studies. The study recently conducted by Bador et al. found a negative correlation of osteocalcin to glucose and insulin resistance as measured using HOMA-R. In addition, diabetic patients were seen to have lower osteocalcin levels.50 This situation may lead to decreased bone formation with a potential negative effect on bone mineral density in these patients.
Secondary hyperparathyroidismVitamin DEffects of vitamin D on bone metabolism are well known: (a) it has an influence on calcium and phosphorus levels, promoting the mineralization process; (b) controls function and differentiation of osteoblasts and osteoclasts, promoting bone formation through inhibition of parathormone (PTH) function.51
Vitamin D deficiency is highly prevalent in the general population. In a Spanish population (including healthy, middle-aged subjects only), baseline vitamin D deficiency was found, with 27.58% of subjects showing levels less than 20ng/mL and 56.03% values ranging from 20 and 30ng/mL.52 On the other hand, the effect of age is important, because vitamin D levels are low in 79.6% of European postmenopausal women. In Spain, however, slightly higher levels are found as compared to the rest of Europe.53 This situation may be enhanced by increased adiposity in the older age group (>65 years).54
Vitamin D levels are decreased in obese population, particularly in those with high-grade obesity.55 This decrease in 25OH vitamin D levels in obese patients are probably related to: (a) decreased bioavailability due to sequestration of this vitamin by adipose tissue,56 (b) decreased 25-hydroxylation of vitamin D in the liver, particularly in patients with non-alcoholic steatohepatitis,57 and (c) decreased sun exposure due to clothes and less time spent outdoors.58 This hypovitaminosis is usually related to high PTH levels.59 These two changes may be associated to an adverse effect on cortical bone. A study conducted on women aged 25–71 years showed that those with severe obesity (BMI>35kg/m2) had greater bone mineral density, but trabecular bone mineral density was decreased in patients with higher PTH levels.60
Vitamin D deficiency may be associated to impaired bone metabolism and muscle function and increased insulin resistance. Maintenance of adequate vitamin D levels is especially important, but we do not know yet the levels to be reached or the adequate supplementation amount or source.
Plasma levels of 25OH vitamin D required to prevent changes secondary to deficiency are above 30ng/mL, but optimum concentrations range from 36 to 40ng/mL.
The recommended amounts of vitamin D in the diet are 15μg/day (600IU) until 71 years of age and 20μg/day (800IU) thereafter.61,62 However, these amounts are often insufficient to maintain adequate vitamin D levels in blood, and amounts higher than 1000IU/day have to be recommended.63 Obese patients usually need greater amounts of this vitamin to achieve adequate plasma levels, requiring 2.5IU/kg to increase plasma level by 1ng/mL.64
Calcium and phosphorus intakeThe different guidelines recommend consumption of 1000–1500mg/day of calcium depending on age and physiological status of the subject.65 Calcium absorption through the bowel is influenced by vitamin D levels, circulating estrogens, and calcium intake. Thus, obese patients appear to have an increased calcium absorption due to increased circulating estrogen levels, greater calcium consumption, and larger absorption surface in the bowel.66 Because of the influence of dietary calcium on bone mass increase, this may mean that overweight and obesity provide some protection of bone metabolism.
Phosphorus consumption in the general Spanish population is very high as compared to dietary recommendations, being similar in both sexes and in different age groups.67 Exact characterization of phosphorus requirements in people with obesity is however difficult due to underestimation of phosphorus-rich products such as soft drinks and some food additives.
High phosphorus intake, particularly when associated to low calcium intake, may be related to secondary hyperparathyroidism leading to increased bone resorption, decreased peak bone mass, and increased bone fragility. Similarly, increased serum phosphorus levels may increase acid load which, although partially buffered by the associated increase in protein intake, may also be related to bone damage.68 Most these relationships are hypothetical, and further research is needed to confirm them.
ConclusionsObesity is a pathological condition that changes the body, influencing bone metabolism through mechanical, hormonal, and inflammatory factors.
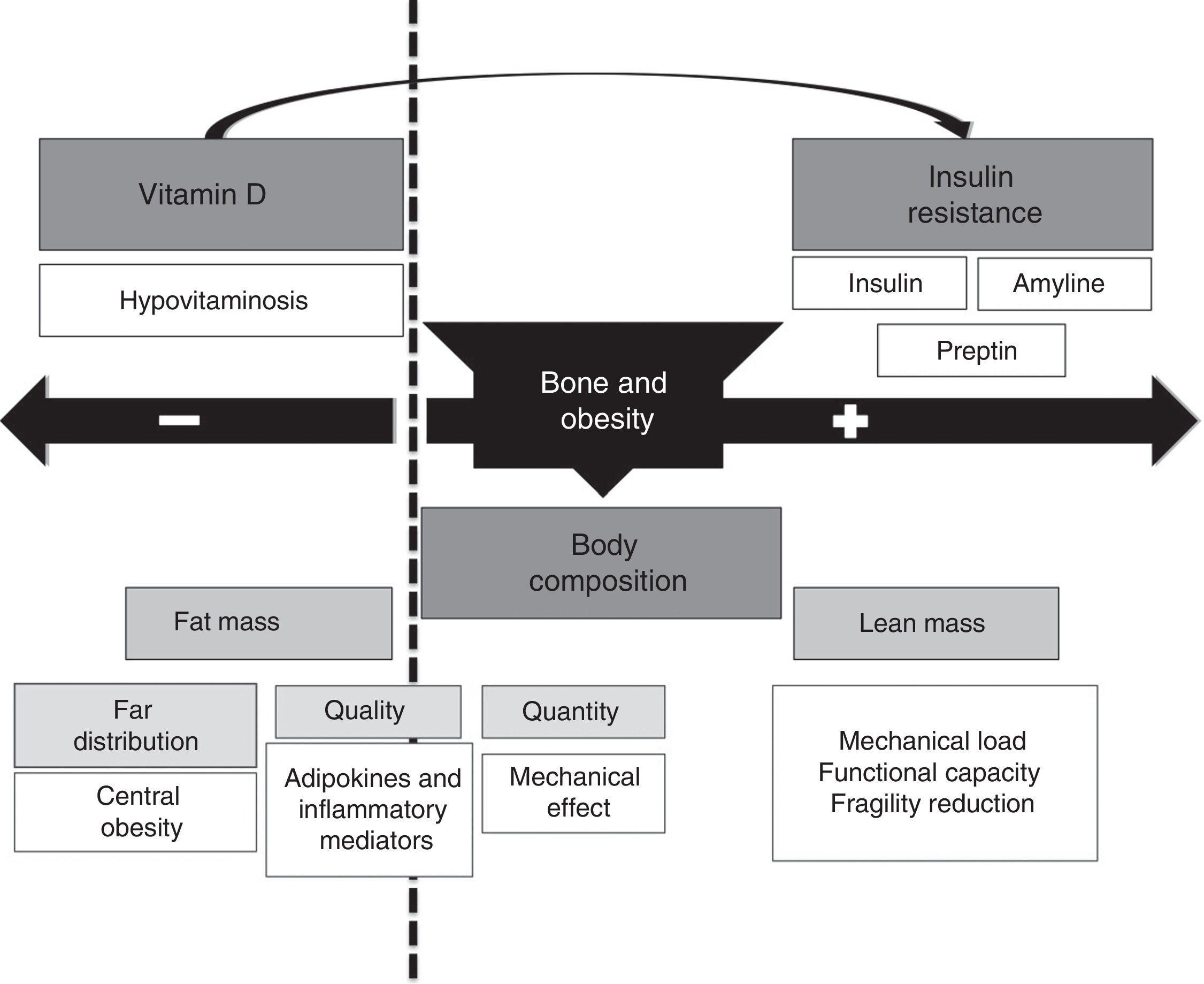
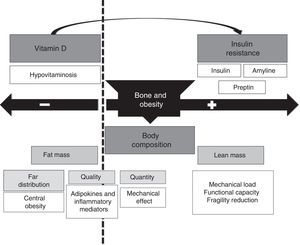
The relationship between obesity and bone mass may be bidirectional (Fig. 3), including a bone mass increase based on: (1) a greater body mass associated to an increase in mechanical load that stimulates bone mass increase as a response; (2) the increase in fat mass is associated to increased androgen to estrogen conversion, which represents a positive stimulus for bone metabolism; (3) increased insulin and amyline secretion secondary to insulin resistance by beta cells.
On the other hand, there is a harmful effect on bone metabolism resulting from the following: (1) obesity is a pro-inflammatory state associated to secretion of a number of cytokines (IL-6, TNF-α) and adipocytokines (adiponectin, leptin etc.). While cytokines have been shown to have a negative influence on bone, the role of adipocytokines is in humans is partly unknown yet; (2) obese patients have decreased circulating levels of 25OH vitamin D, mostly due to vitamin sequestration in adipose tissue. This may lead to impaired bone formation, with altered bone quantity and quality (architecture); (3) decrease vitamin D levels, combined with higher dietary phosphorus levels, may be associated to increased PTH levels that may independently influence bone metabolism.
The net harmful or beneficial effect of obesity on bone mass or of these factors on risk of fracture is not well known yet, and additional observational and interventional studies are needed in this area of knowledge.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: López-Gómez JJ, Pérez Castrillón JL, de Luis Román DA. Influencia de la obesidad sobre el metabolismo óseo. Endocrinol Nutr. 2016;63:551–559.