The most common functional ovarian tumors are strumal and sexual cord tumors. Depending on the histological variant, these may secrete various hormones, including estrogens, androgens, human chorionic gonadotropin (hCG), and thyroid hormones. The ovary has a complex embryological development, and tumors of a very diverse histology may therefore arise in it.1 Mature cystic teratoma of the ovary or benign dermoid cyst is the most common benign ovarian tumor and derives from germ cells.2 It may consist of a wide variety of mature tissues, and from the hormonal viewpoint it may behave as a carcinoid tumor or as a neoplasm secreting thyroid hormones, the so-called struma ovarii. Struma ovarii accounts for 3% of all benign teratomas and may lead to a true thyrotoxicosis in up to 8% of cases.3 It is usually benign in nature. Struma ovarii occurs most commonly between the fifth and sixth decades of life, and is rare before puberty. A unilateral adnexal tumor mass corresponding to the ovarian tumor may sometimes be palpated.4
The case of a patient in whom struma ovarii was incidentally found during follow-up for a follicular thyroid carcinoma is reported below. This was a 32-year-old female who attended the endocrinology clinic for goiter. The patient reported a mass in the anterior side of the neck appearing two months before, but with no local or general clinical signs or symptoms suggesting thyroid dysfunction. She reported no toxic habits or routine use of medication. She also had no family history of goiter. At a physical examination, a left thyroid nodule approximately 3cm in diameter and of medium consistency which rose upon swallowing was palpated, as well as a slightly enlarged right thyroid lobe. No adenopathies were palpated.
Laboratory tests showed no changes in complete blood count and chemistry tests, thyrotropin (TSH) 1.3mIU/mL (normal range (NR), 0.2–3.5), free thyroxine (fT4) 14ng/dL (NR, 0.8–2.1), and negative anti-thyroid and anti-thyroglobulin antibodies (Ab). A thyroid scan revealed a normally located, enlarged thyroid gland with a low global uptake of irregular distribution, showing a low uptake area in the left lobe coinciding with the palpable nodule. A neck ultrasound showed a left thyroid lobe 25mm×32mm×58mm in size with preserved echogenicity, while the middle and lower thirds were occupied by a single hypoechoic nodule 24mm×26mm×30mm in size and with mixed vascularization. The isthmus had an anteroposterior diameter of 22mm and showed no parenchymal lesions. The right thyroid lobe measured 24mm×28mm×49mm and had a preserved echo structure. No pathological adenopathies were seen in the neck. Based on these findings, fine needle aspiration of the nodule was requested. The cytological diagnosis was follicular tumor.
Left hemithyroidectomy and isthmectomy were performed. The pathological laboratory reported a 3cm follicular carcinoma with focal oncocytary features in the base of the left lobe. Right thyroidectomy was therefore performed at repeat surgery. No pathological changes were reported in the right side of the thyroid gland.
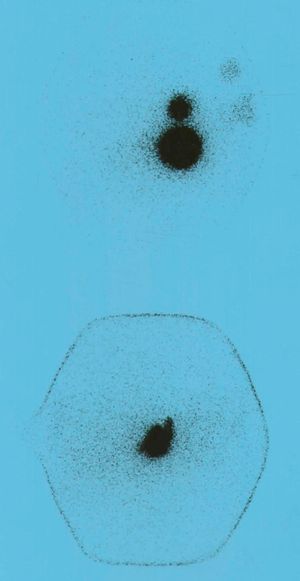
A pT2N0 Mx follicular thyroid carcinoma was diagnosed, and the patient was subsequently administered an ablation dose of 100 millicuries (mCi) of 131I as supplemental treatment. The results of laboratory test performed before 131I administration included TSH 58mIU/mL, fT4 0.1ng/dL, thyroglobulin (Tg) 46ng/mL (NR, 2–16), and negative anti-thyroglobulin Ab. A whole body scan showed uptake at neck level and another active area in the lesser pelvis, slightly displaced to the right, suggesting ovarian uptake (Fig. 1).
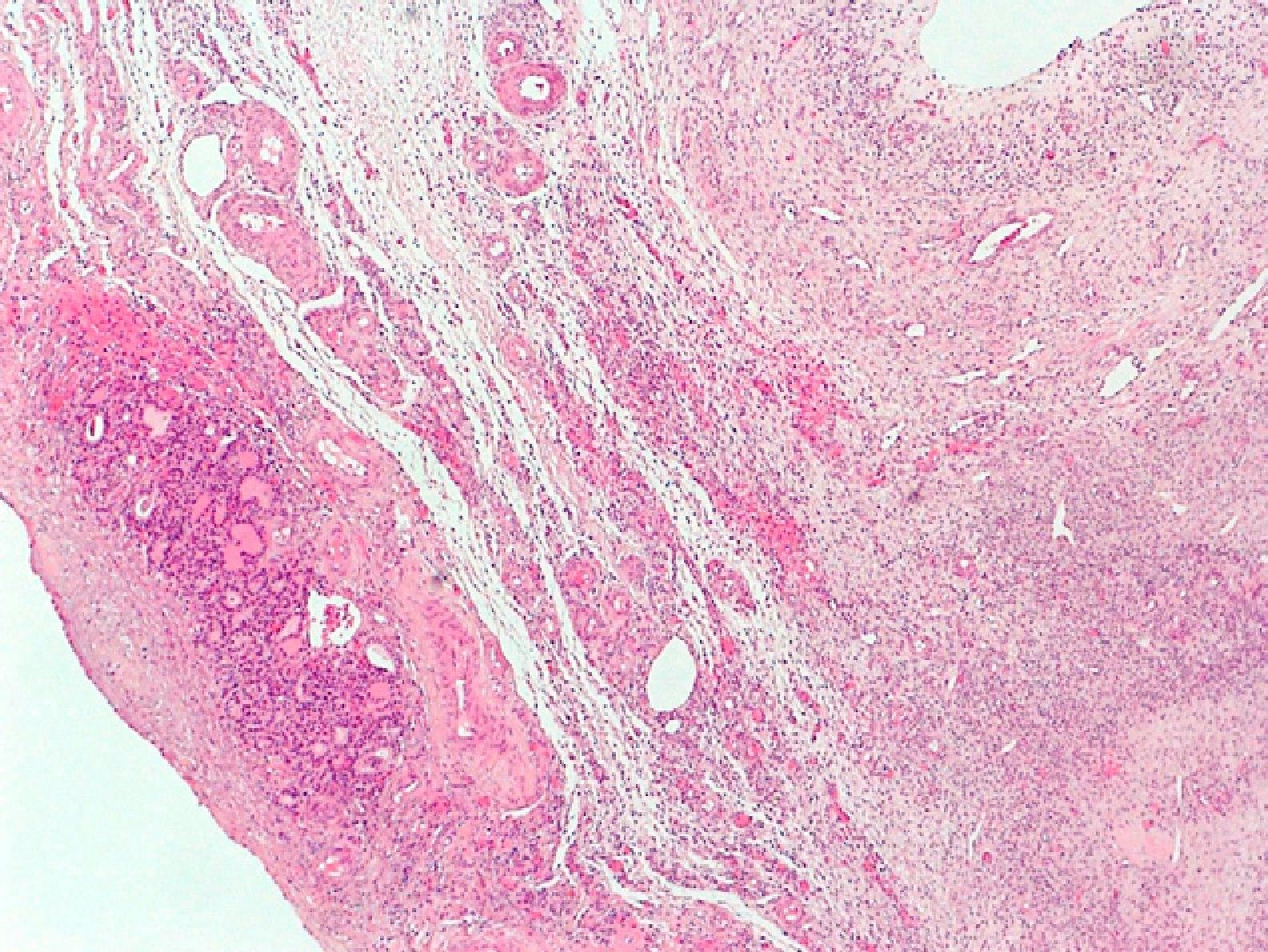
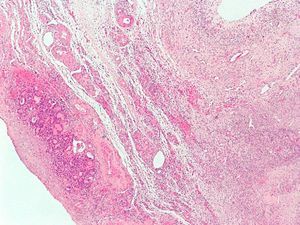
The patient was referred to the gynecology department for evaluation. A gynecological ultrasound revealed a uterus of normal size with a 25-mm myoma in the posterior and lateral aspect, a right ovary with scant stroma and abundant follicles (polycystic), and a left ovary with predominant follicular tissue. The patient underwent right laparoscopic oophorectomy. The surgical specimen, 3cm in size, consisted of the ovary, with two small gaps. The pathological diagnosis was an ovary with very abundant follicles and a fragment of thyroid tissue (2mm) with no remarkable changes (struma ovarii) (Fig. 2).
The results of laboratory tests performed four months after the 131I ablation dose were as follows: TSH 0.22mIU/mL, fT4 1.34ng/dL, Tg<0.2ng/mL, and negative anti-thyroglobulin Ab.
Six months later, a whole body scan performed after the discontinuation of levothyroxine treatment was negative, and laboratory test results included: TSH 38mIU/mL, fT4 0.1ng/dL, Tg<0.2ng/mL, and negative anti-thyroglobulin Ab. The patient had uncomplicated pregnancies two and four years after this whole body scan. At the next follow-up visits, data continued to suggest complete remission, with Tg<0.2ng/mL, negative anti-thyroglobulin Ab, and normal neck ultrasound examinations.
The ability to concentrate radioiodine depends on the expression of the NIS iodine symporter in the cell membrane. Such expression may be detected in extrathyroid tissues such as the ovary. When ovarian uptake of 131I is seen, a differential diagnosis should be made between struma ovarii, ovarian metastases of thyroid carcinoma, and thyroid carcinoma arising in struma ovarii.5 Ovarian uptake of 131I has rarely been reported in the literature, probably because a whole body scan is reserved for thyroid carcinoma and for hyperthyroidism with prior total thyroidectomy.
A literature review found only a few reported cases where struma ovarii was incidentally found during follow-up for differentiated thyroid carcinoma.6–10 In four of these cases, similarly to what occurred in our patient, struma ovarii was detected in a whole body scan following the 131I ablation dose for a differentiated thyroid carcinoma.6,8–10
In the reported case, struma ovarii was an incidental finding during the follow-up of follicular carcinoma. Ovarian metastasis should always be considered in differential diagnosis, especially when no other teratoma elements are identified in the ovarian surgical specimen.10 Metastases account for less than 10% of malignant ovarian tumors. If metastasis occurs, a primary thyroid tumor is rare. The pathological study will establish whether the condition is benign or malignant. In the reported case, metastasis from a follicular carcinoma was ruled out. After oophorectomy was performed, basal Tg remained undetectable at all times, and subsequent scans were negative.
Please cite this article as: Rojo Álvaro J, et al. Hallazgo incidental de estruma ovárico. Endocrinol Nutr. 2013;60:268–70.