Amiodarone is a widely used, effective antiarrhythmic drug. Its high iodine contents and its direct toxic effect on the thyroid gland cause thyroid function changes in up to 50% of patients, which may occur even after drug discontinuation because of its long half-life.
There are two main forms of amiodarone-induced thyrotoxicosis (AIT). Type 1 AIT is characterized by increased thyroid hormone synthesis (due to iodine overload in patients with underlying thyroid disease), while type 2 AIT triggers the release of preformed hormones by causing destructive thyroiditis (due to direct drug toxicity). It is important to differentiate between the two types in order to optimize treatment, which consists of thionamides for type 1 AIT and glucocorticoids for type 2 AIT.
We report a case illustrating the complex management that may be required in some cases of AIT and the potential treatment alternatives when there is resistance to conventional treatment.
This was a 51-year-old male patient with a history of obstructive hypertrophic cardiomyopathy and an episode of atrial fibrillation four years before who had been treated with amiodarone (200mg/24h) since then. In the setting of a new episode of atrial fibrillation, primary hyperthyroidism was detected (TSH, 0.008mIU/mL [0.27–4.2mIU/mL]; T4, 7.5ng/dL [0.93–1.7ng/dL]; and T3, 10.99pg/mL [2.57–4.43pg/mL]). The patient reported nervousness, palpitations, and loss of 4kg in the previous month. Physical examination disclosed a grade II/VI systolic murmur in the left sternal margin with arrhythmic heart rate of 86bpm and fine distal tremor. No goiter was palpated, and no proptosis or changes in extraocular motility were found.
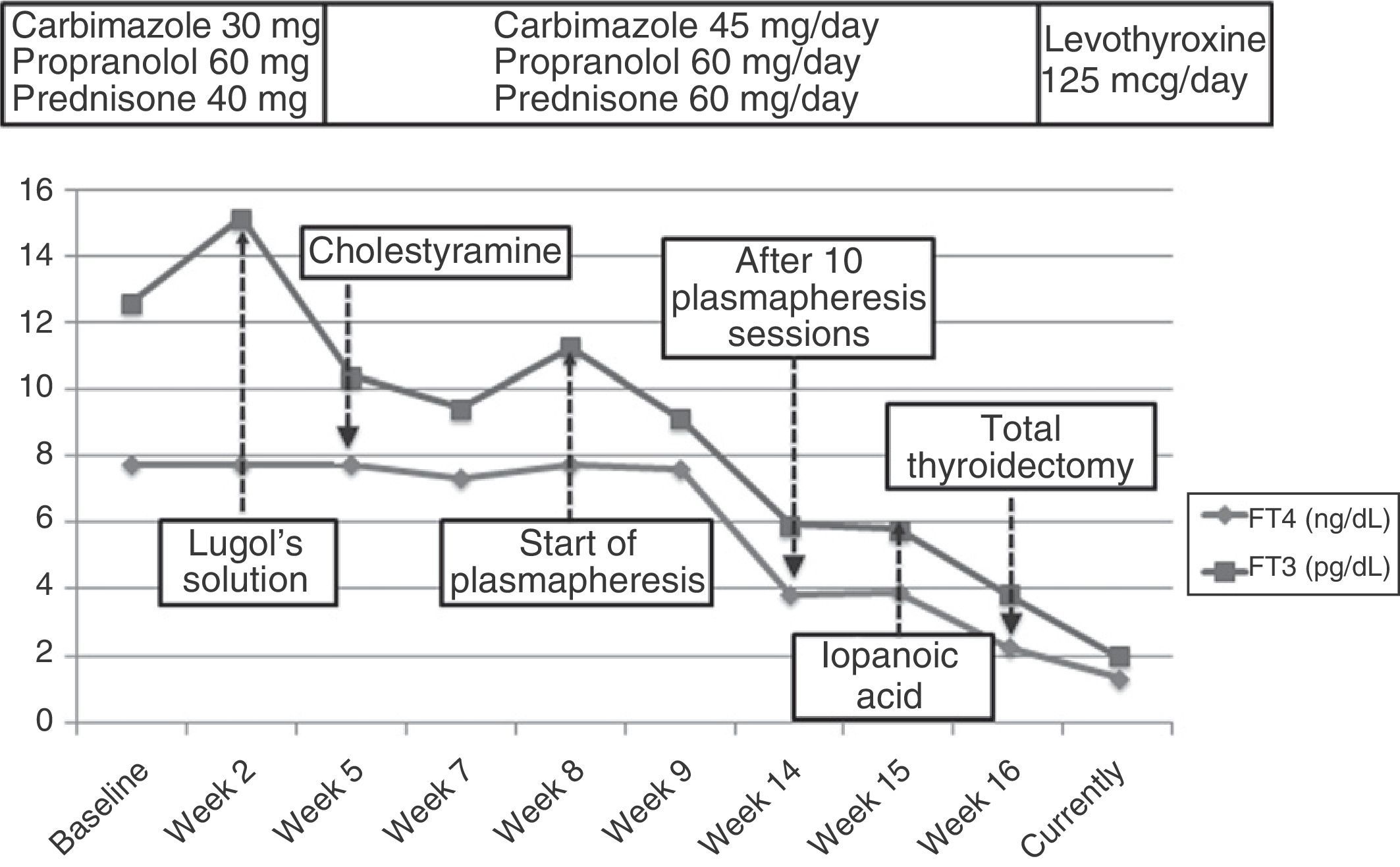
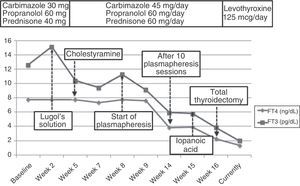
There was no thyroid autoimmunity. Thyroid ultrasound examination showed preserved morphology with no nodular lesions and generalized hypovascularization, while scintigraphy revealed an almost complete thyroid gland blockade. Type 2 AIT was diagnosed based on these results. Amiodarone was discontinued and, because of the high risk of atrial fibrillation and decompensated heart failure, mixed treatment was started with high doses of thionamides (carbimazole 45mg/d), glucocorticoids (prednisone 60mg/d), and beta-blockers (propranolol 60mg/d). Fifteen days later, hyperthyroidism worsened (TSH, 0.008mIU/mL; T4 higher than 7.7ng/dL; and T3, 15.14pg/mL). A rapid blockade of thyroid hormone release was attempted with Lugol's solution (5 drops/12h) for one week. No improvement occurred, and cholestyramine (12g/d) was therefore added.
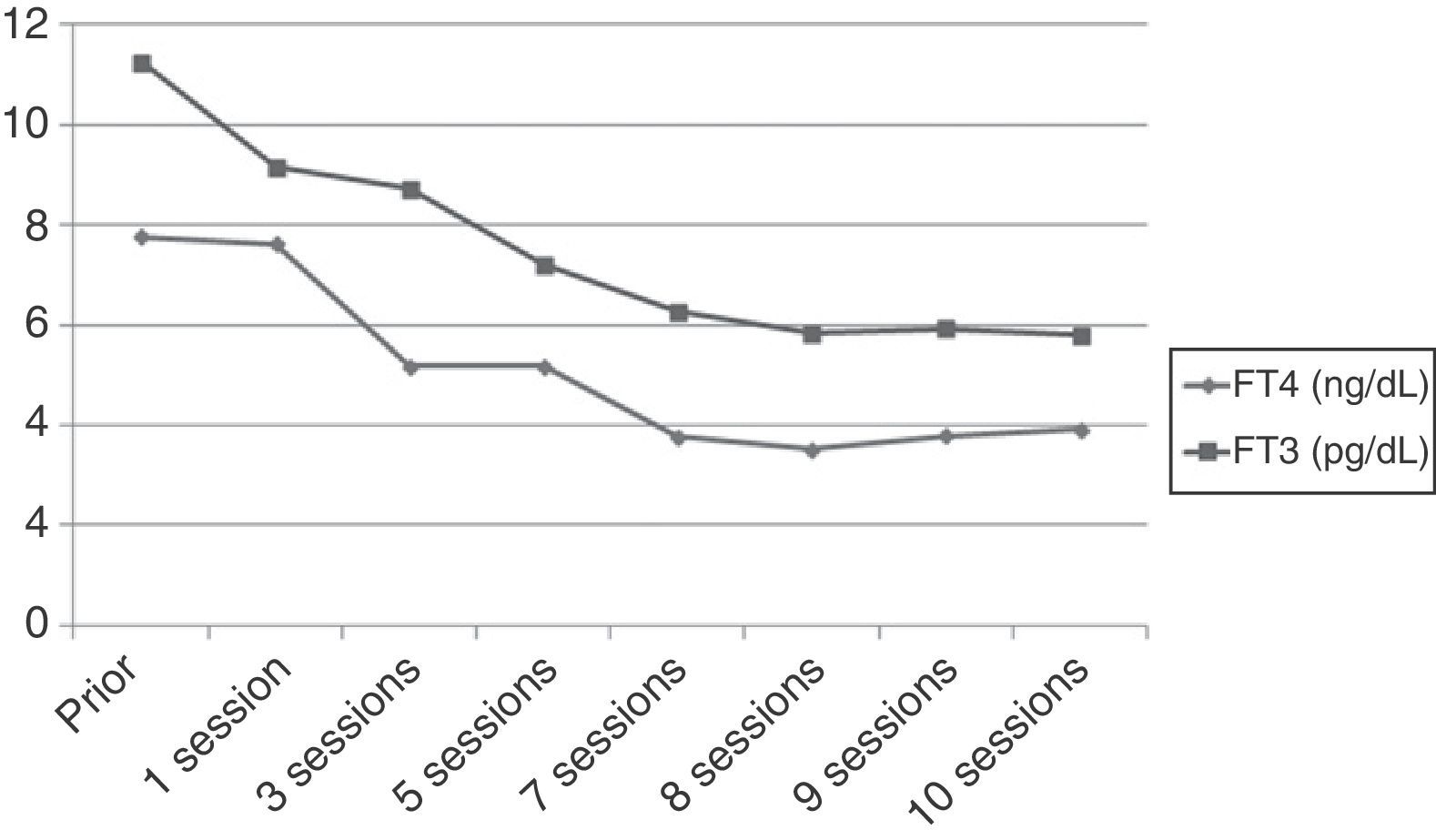
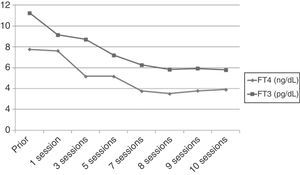
However, after 25 days of treatment the patient experienced a difficult to control atrial flutter which required hospitalization. Laboratory tests upon admission showed the following levels: TSH 0.005mIU/mL, FT4 higher than 7.77ng/dL, and FT3 11.25pg/mL. Because of refractoriness to medical treatment and high surgical risk, treatment with plasmapheresis was started to rapidly decrease thyroid hormone levels. Apheresis of 2L of plasma was decided upon. Plasmapheresis was initially performed on alternate days, and with longer intervals subsequently. An early benefit (Fig. 1) (TSH, 0.005mIU/mL; FT4, 3.84ng/dL; FT3, 5.93pg/mL) was achieved, with the recovery of sinus rhythm, but thyroid hormone levels subsequently stabilized, and discontinuation of plasmapheresis was therefore decided upon after 10 sessions.
With a view to surgical treatment, thyroid blockade was again attempted with iopanoic acid (500mg/12h for one week), and acceptable hormone levels (Fig. 2) were achieved (FT4, 2.26ng/dL; FT3, 3.84pg/mL). Total thyroidectomy was finally performed, and a pathological examination found a normal sized thyroid gland with no nodules, involution of thyroid follicles, and degenerative changes. These findings were consistent with type 2 AIT. After 12 months of follow-up on treatment with levothyroxine 125mcg/d, the patient has experienced no new episodes of tachyarrhythmia.
When decompensation of an underlying heart disease or atrial fibrillation occurs in a patient treated with amiodarone, AIT should be ruled out. There is no agreement regarding the recommendation to discontinue amiodarone. Because of the long half-life of the drug, treatment discontinuation does not result in an immediate benefit. It also causes a blockade of both T4 to T3 conversion and beta-adrenergic receptors, so that drug discontinuation may initially worsen the symptoms of hyperthyroidism.1
Bile acid sequestrants are another therapeutic option for the control of thyrotoxicosis. Cholestyramine and colestipol are better known for their indication as lipid lowering drugs, but also inhibit the enterohepatic circulation of thyroid hormones, and various studies have shown that, when combined with antithyroid drugs, a more rapid normalization of thyroid profile is achieved.2
On the other hand, iodine-saturated solutions or cholecystographic agents (such as iopanoic acid or Lugol's solutions) mainly act by blocking T4 to T3 conversion and have been shown to be helpful in the preoperative control of hyperthyroidism in various studies.3 In our patient, however, the effective control of thyrotoxicosis was not achieved with any of these therapeutic options.
Plasmapheresis is a process consisting of blood filtration to remove some plasma components, including thyroid hormones. In addition to decreasing circulating hormone and iodine levels, it may remove TSI (thyroid-stimulating immunoglobulin) antibodies and some drugs that bind to plasma proteins. Unlike hemodialysis, plasmapheresis allows for a transient decrease in plasma amiodarone levels. The use of plasmapheresis should therefore be considered in patients with severe thyrotoxicosis refractory to treatment. It is a relatively safe procedure with a mortality rate of approximately 0.03–0.05%, and complications rarely occur.4 Ezer et al. reported a marked decrease in preoperative FT3 and FT4 levels with plasmapheresis in a series of 11 patients with thyrotoxicosis from various causes refractory to conventional antithyroid treatment or for which this treatment was contraindicated.4 The role of plasmapheresis in patients with AIT is controversial, according to the literature reports. Diamond et al. reported a series of three patients with AIT treated with plasmapheresis. While treatment had to be stopped in one patient due to complications, the other two patients experienced a marked decrease in thyroid hormone levels after only two sessions (from 77.2pmol/L to 54.3pmol/L and from 77pmol/L to 47.1pmol/L respectively).5 These favorable results agree with those reported for other patients with AIT.6–8
However, the effect of plasmapheresis may be transient.9 The mechanism underlying recurrence is complex. Decreased plasma amiodarone levels imply the release of the drug from adipose tissue to plasma, with the resultant risk of prolonging thyroid dysfunction over time.
When patients with underlying heart disease do not respond to conventional treatment, thyroidectomy is sometimes required.10 However, a high surgical risk exists because of the severity of thyrotoxicosis, and prior optimization of thyroid function is therefore needed.
To sum up, the reported case demonstrates the complexity of management of some cases of AIT. Arrhythmia in acutely decompensated heart failure may sometimes require a rapid decrease in circulating thyroid hormone levels. If the response to conventional treatment is unsatisfactory, plasmapheresis may represent a transient therapeutic alternative before definitive surgery.
Please cite this article as: Mateo Gavira I, Vilchez López F, Larrán Escandón L, Roldán Caballero P, Aguilar Diosdado M. Manejo de la tirotoxicosis grave por amiodarona cuando fracasa el tratamiento médico convencional. Endocrinol Nutr. 2013;60:e43–e45.