To study the frequency of non-alcoholic fatty liver disease (NAFLD), its relationship to clinical and biochemical variables, and the effect 12-month's lifestyle intervention in obese children and adolescents.
MethodsThirty-six obese patients aged 7–18 years, 42% female and 58% male, 72.2% prepubertal and 27.8% pubertal, were selected. Anthropometric measurements and glucose, insulin (baseline and after a glucose load), lipid profile, C-reactive protein, and aminotransferase tests were performed before and 12 months after dietary and physical activity intervention. Liver ultrasound was performed to determine the presence of NAFLD.
ResultsNAFLD was found in 66.7% (n=24), and was mild in 30.6%, moderate in 27.8%, and severe in 8.3%. Subjects with NAFLD had higher body mass index (BMI, p=0.007), waist (p=0.005), fat area (p=0.002), basal insulin (p=0.01), and HOMA-IR (p=0.008) values and lower QUICKI (p=0.02) values than those with no NAFLD. After intervention, physical activity increased (p=0.0001) and calorie intake remained unchanged. NAFLD disappeared in 9 patients (37.5%, p=0.02) and disease severity decreased in 3 patients (12.5%). In addition, BMI Z-score (p=0.005), fat area (p=0.0001), basal insulin (p<0.05), insulin resistance (p<0.005), lipid profile (p<0.03), and transaminases decreased. Weight loss was the main variable accounting for NAFLD improvement.
ConclusionThis group of obese children and adolescents showed a high frequency of NAFLD. The lifestyle intervention with weight reduction is effective for the treatment of NAFLD.
Estudiar la frecuencia de hígado graso no alcohólico (HGNA), su relación con variables clínicas y bioquímicas, y el efecto de la intervención durante 12 meses en el estilo de vida en niños y adolescentes obesos.
MétodosSe seleccionaron 36 pacientes obesos entre 7 y 18 años, 42% femeninos y 58% masculinos, 72,2% prepuberales y 27,8% puberales. Antes y 12 meses después de intervención sobre dieta y actividad física, se tomaron medidas antropométricas y se cuantificaron glucosa e insulina (basal y poscarga de glucosa), perfil lipídico, proteína C reactiva y aminotransferasas. Se realizó ecografía hepática para determinar presencia de HGNA.
ResultadosEl 66,7% (n=24) presentó HGNA, 30,6% de grado leve, 27,8% moderado y 8,3% grave. Aquellos con HGNA tenían índice de masa corporal (IMC; p=0,007), circunferencia abdominal (p=0,005), área grasa (p=0,002), insulina basal (p=0,01) y HOMA-IR (p=0,008) más altos, y QUICKI (p=0,02) más bajo, que aquellos sin HGNA. Con la intervención, la actividad física aumentó (p=0,0001) y la ingesta calórica se mantuvo igual; el HGNA desapareció en 9 pacientes (37,5%; p=0,02) y en 3 mejoró el grado de alteración (12,5%). Además, el Z-Score del IMC (p=0,005), el área grasa (p=0,0001), la insulina basal (p<0,05), la resistencia a la insulina (p<0,005), el perfil lipídico (p<0,03) y las transaminasas disminuyeron. La disminución de peso fue la principal variable explicativa de la mejoría del HGNA.
ConclusiónEn este grupo de niños y adolescentes obesos se observó una alta frecuencia de HGNA. La intervención en estilo de vida con reducción de peso es efectiva en el tratamiento del HGNA.
Non-alcoholic fatty liver disease (NAFLD) is abnormal fat accumulation in hepatocytes, mainly as triglycerides.1,2 The natural history of NAFLD ranges from simple steatosis, a slowly progressing disorder, to non-alcoholic steatohepatitis (NASH), fibrosis, and even cirrhosis.3,4 Most studies suggest that NAFLD may develop very early in life, and has a direct relationship with the degree of obesity.5 Indeed, while the overall frequency of NAFLD in children and adolescents ranges from 2.6% to 9.6%, it may occur in 20–80% of those with obesity.6,7 A prior study conducted in Merida, Venezuela, reported a 45% frequency of NAFLD in obese children, with no sex differences.2
Liver histology (biopsy) is the gold standard for the diagnosis of NAFLD.3,8 However, ultrasonography is the most commonly used imaging method because it is non-invasive, inexpensive, safe, and widely available.9 In addition, ultrasonography has shown 70% sensitivity and 100% specificity as compared to liver biopsy,10 and an excellent correlation with the histological grade of hepatic steatosis in children.11 The presence, degree, and pattern of aminotransferase elevation (AST, aspartate aminotransferase; ALT, alanine aminotransferase) is non-specific, and up to 70% of patients with NAFLD have no abnormal aminotransferase levels, and their increase may lead to suspected NASH.12
The treatment of pediatric NAFLD may reverse the process and prevent its progression to fibrosis, portal hypertension, and cirrhosis. Most patients with NAFLD are obese and resistant to insulin. Thus, based on the understanding of its pathophysiology, the first-line treatment is weight loss through a low-calorie diet and the practice of aerobic exercise.13 In obese children with NAFLD, Nobili et al.14 and Reinehr et al.15 reported NAFLD improvement in 50–60% of patients after lifestyle intervention.
Because of the increased frequency of obesity in children and adolescents and its complications,16 it was decided to assess a group of obese children and adolescents in order to determine the presence of NAFLD, its association with clinical and biochemical variables, and the effects of lifestyle changes during a year of follow-up.
Materials and methodsSubjectsA non-randomized clinical trial where each subject acted as his/her own control was conducted. Thirty-six obese patients aged 7–18 years with BMI>97th percentile (P97) for age and sex, according to the Fundacredesa tables,17 taken from the outpatient clinics of Instituto Autónomo Hospital Universitario de Los Andes (IAHULA) and educational institutions of the city of Merida, evaluated in a prior study,16 were enrolled into the study. Subjects with underlying endocrine disease, acute or chronic liver disease, genetic syndromes associated with obesity, drug treatments interfering with hepatic or immune function and carbohydrate and lipid metabolism, any acute or chronic disease, and any physical condition restricting exercise were excluded from the study. The study was conducted in compliance with the ethical recommendations of the Declaration of Helsinki, and legal representatives signed their informed consent.
ProtocolThe information collected included personal particulars and any family history of cardiovascular and metabolic disease. Hours per week of physical exercise and sedentary activities such as watching TV and using computers were recorded. The 24-h recall procedure was used for examining dietary habits. Participants, in underwear, were weighed using a standard scale. Height was measured as the mean of three measurements using a Harpenden stadiometer. BMI (weight/height2) and Z-score of BMI were calculated. Measurements were made of the tricipital skin fold with a specific caliper, left arm circumference, abdominal circumference (AC) and hip circumference as indicated in the 2000 National Health and Nutrition Examination Survey. Waist/hip ratio (WHR), fatty area, and muscular area were calculated.17 Abdominal obesity was defined as AC>P90 of the multiethnic population.18 Blood pressure was measured and categorized according to the Fundacredesa tables17 as high blood pressure (HBP) when>P97 and as normal-high BO (pre-HBP) when it ranged from P90 to P97 for age and sex.
A fasting blood sample was drawn for measuring blood glucose, insulin, a lipid profile including triglycerides (TG), total cholesterol (TC), HDL-C and LDL-C, C-reactive protein (CRP), AST, and ALT. A glucose load of 1.75g/kg of weight (maximum 75g) was administered, and blood glucose and insulin levels were measured at 2h. Glucose, TC, TG, and HDL-C were tested using colorimetric enzymatic methods with biosystem reagents in an OLYMPUS AU-640 analyzer. LDL-C was calculated using the Friedewald formula: LDL-C=TC−(TG/5+HDL-C). Insulin and highly sensitive CRP were measured by chemiluminescence (Siemens Medical Solutions Diagnostics), with inter- and intra-assay coefficients of variation of 6.5% and 5.4% and 7.5% and 4.1%, respectively. Aminotransferases were tested using the method of Reitman S. and Frankel S. The upper normal limits were 40U/mL for AST and 38U/mL for ALT. Tests were performed at the hormone laboratory of IAHULA. HOMA-IR (Homeostasis Model Assessment-Insulin Resistance) was calculated using the formula fasting insulin (μU/mL)×fasting glucose (mmol/L)/22.5, and insulin sensitivity index QUICKI (quantitative insulin-sensitivity check index) by the formula 1/[log(insulin 0min)+log(glucose 0min)]. Fasting hyperglycemia or diabetes mellitus (DM) was diagnosed based on ADA criteria.19 Insulin resistance was defined as HOMA-IR>2.5, and dyslipidemia using the 1991 National Institute of Health criteria.20 The diagnosis of metabolic syndrome (MS) was based on the 2003 Cook et al. criteria,21 except for fasting blood glucose, for which a cut-off point of 100mg/dL instead of 110mg/dL was used. The presence of three or more criteria established the diagnosis.
Liver ultrasonography was performed by a pediatric gastroenterologist using Esaote Medical equipment with a 5MHz transducer and software version 4.7. At examination after intervention, the gastroenterologist was blinded to prior results. Three grades of NAFLD were distinguished.8,22
Type I NAFLD. Mild: characterized by minimally increased liver echogenicity with preserved visualization of diaphragm and margins of intrahepatic vessels.
Type II NAFLD. Moderate: moderately increased liver echogenicity and a slight difficulty in assessing diaphragm and intrahepatic vessels.
Type III NAFLD. Severe: markedly increased echogenicity, poor or no visualization of the walls of intrahepatic vessels, the diaphragm, and the posterior part of the right lobe of the liver due to poor ultrasound penetration.
Dietary and physical exercise interventionParticipants and their representatives were provided a general program including dietary and physical activity recommendations, with tables for the weekly recording of weight and physical activity. Recommendations included the intake of lower amounts of food and the decreased intake of refined sugar, carbonated beverages, carbohydrates, and fat in general, and an increased fiber consumption in the form of fruit and vegetables. Moderate physical exercise like walking or jogging, or any preferred physical activity, was recommended for 30min daily or at least three times a week, together with a decrease in time spent watching TV or playing video games. Information was given about the expected changes, and the active and responsible participation of parents and representatives was encouraged. Daily steps were counted in 18 subjects using a pedometer (Omron model HJ-112INT). Lifestyle intervention lasted 12 months and was monitored every month. The complete assessment previously performed was repeated after intervention. Benefit was defined as partial or total NAFLD improvement after intervention.
Statistical analysisContinuous variables are given as mean and standard deviation, and categorical variables as numbers and percentages. A Chi-square test was used for associations between categorical variables. A McNemar test was used for differences between categorical variables before and after intervention. Continuous variables were compared using a Student's t test for dependent or independent samples as appropriate. Logistic regression was used to determine which of the variables had the greatest influence on NAFLD improvement. A value of p<0.05 was considered statistically significant.
ResultsThe mean age of the 36 children and adolescents was 10.59±2.96 years. Of all participants, 15 (42%) were female and 21 (58%) male, and 26 (72.2%) prepubertal (Tanner stages I and II) and 10 (27.8%) pubertal (Tanner stages III, IV, and V). They were all obese.
Before intervention, 14 participants (38.9%) had hypercholesterolemia; 8 (22.2%) high LDL-C levels; 19 (52.8%) elevated TG; 27 (75%) low HDL-C levels; 10 (27.8%) elevated blood pressure, including six with pre-HBP (P90–P97) and 4 with HBP (>P97); 5 (13.9%) insulin resistance; 24 (66.7%) abdominal obesity; and 13 (38.9%) MS. NAFLD was diagnosed in 24 subjects (66.7%), while no ultrasonographic signs suggesting NAFLD were seen in the remaining 12 subjects (33.3%). No cases of DM, fasting hyperglycemia, or glucose intolerance were found.
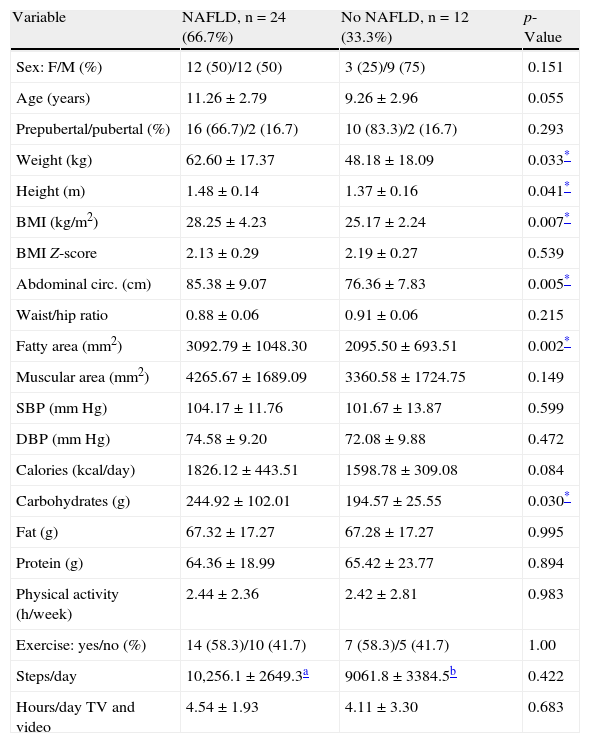
No differences were seen in age, sex, pubertal stage, blood pressure, total calorie intake, and physical activity between subjects with or without NAFLD. Subjects with NAFLD had higher mean values of weight (p=0.03), height (p=0.04), BMII (p=0.007), abdominal circumference (p=0.005), and fatty area (p=0.002). Carbohydrate consumption was greater in the NAFLD group (p=0.03) (Table 1).
Clinical and dietary characteristics and physical activity before intervention of children and adolescents with and without NAFLD.
| Variable | NAFLD, n=24 (66.7%) | No NAFLD, n=12 (33.3%) | p-Value |
| Sex: F/M (%) | 12 (50)/12 (50) | 3 (25)/9 (75) | 0.151 |
| Age (years) | 11.26±2.79 | 9.26±2.96 | 0.055 |
| Prepubertal/pubertal (%) | 16 (66.7)/2 (16.7) | 10 (83.3)/2 (16.7) | 0.293 |
| Weight (kg) | 62.60±17.37 | 48.18±18.09 | 0.033* |
| Height (m) | 1.48±0.14 | 1.37±0.16 | 0.041* |
| BMI (kg/m2) | 28.25±4.23 | 25.17±2.24 | 0.007* |
| BMI Z-score | 2.13±0.29 | 2.19±0.27 | 0.539 |
| Abdominal circ. (cm) | 85.38±9.07 | 76.36±7.83 | 0.005* |
| Waist/hip ratio | 0.88±0.06 | 0.91±0.06 | 0.215 |
| Fatty area (mm2) | 3092.79±1048.30 | 2095.50±693.51 | 0.002* |
| Muscular area (mm2) | 4265.67±1689.09 | 3360.58±1724.75 | 0.149 |
| SBP (mmHg) | 104.17±11.76 | 101.67±13.87 | 0.599 |
| DBP (mmHg) | 74.58±9.20 | 72.08±9.88 | 0.472 |
| Calories (kcal/day) | 1826.12±443.51 | 1598.78±309.08 | 0.084 |
| Carbohydrates (g) | 244.92±102.01 | 194.57±25.55 | 0.030* |
| Fat (g) | 67.32±17.27 | 67.28±17.27 | 0.995 |
| Protein (g) | 64.36±18.99 | 65.42±23.77 | 0.894 |
| Physical activity (h/week) | 2.44±2.36 | 2.42±2.81 | 0.983 |
| Exercise: yes/no (%) | 14 (58.3)/10 (41.7) | 7 (58.3)/5 (41.7) | 1.00 |
| Steps/day | 10,256.1±2649.3a | 9061.8±3384.5b | 0.422 |
| Hours/day TV and video | 4.54±1.93 | 4.11±3.30 | 0.683 |
Data are given as X±SD and No. (%). BMI: body mass index; DBP: diastolic blood pressure; SBP: systolic blood pressure.
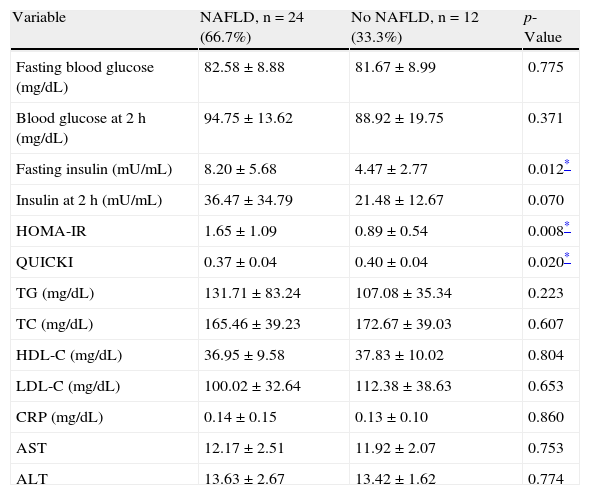
Before intervention, the NAFLD group had higher fasting insulin (p=0.01) and HOMA-IR (p=0.008) values and lower QUICKI values (p=0.02). Insulin levels at 2h and TG tended to be higher in the NAFLD group, without reaching statistical significance, while there was no difference in blood glucose, TC, LDL-C, HDL-C, CRP, and transaminase levels between the groups. No patients had transaminase levels higher than the reference range (Table 2).
Levels of metabolic variables, C-reactive protein, and aminotransferases before intervention in children and adolescents with and without NAFLD.
| Variable | NAFLD, n=24 (66.7%) | No NAFLD, n=12 (33.3%) | p-Value |
| Fasting blood glucose (mg/dL) | 82.58±8.88 | 81.67±8.99 | 0.775 |
| Blood glucose at 2h (mg/dL) | 94.75±13.62 | 88.92±19.75 | 0.371 |
| Fasting insulin (mU/mL) | 8.20±5.68 | 4.47±2.77 | 0.012* |
| Insulin at 2h (mU/mL) | 36.47±34.79 | 21.48±12.67 | 0.070 |
| HOMA-IR | 1.65±1.09 | 0.89±0.54 | 0.008* |
| QUICKI | 0.37±0.04 | 0.40±0.04 | 0.020* |
| TG (mg/dL) | 131.71±83.24 | 107.08±35.34 | 0.223 |
| TC (mg/dL) | 165.46±39.23 | 172.67±39.03 | 0.607 |
| HDL-C (mg/dL) | 36.95±9.58 | 37.83±10.02 | 0.804 |
| LDL-C (mg/dL) | 100.02±32.64 | 112.38±38.63 | 0.653 |
| CRP (mg/dL) | 0.14±0.15 | 0.13±0.10 | 0.860 |
| AST | 12.17±2.51 | 11.92±2.07 | 0.753 |
| ALT | 13.63±2.67 | 13.42±1.62 | 0.774 |
Data are given as X±SD. AST: aspartate aminotransferase; ALT: alanine aminotransferase; CRP: C-reactive protein.
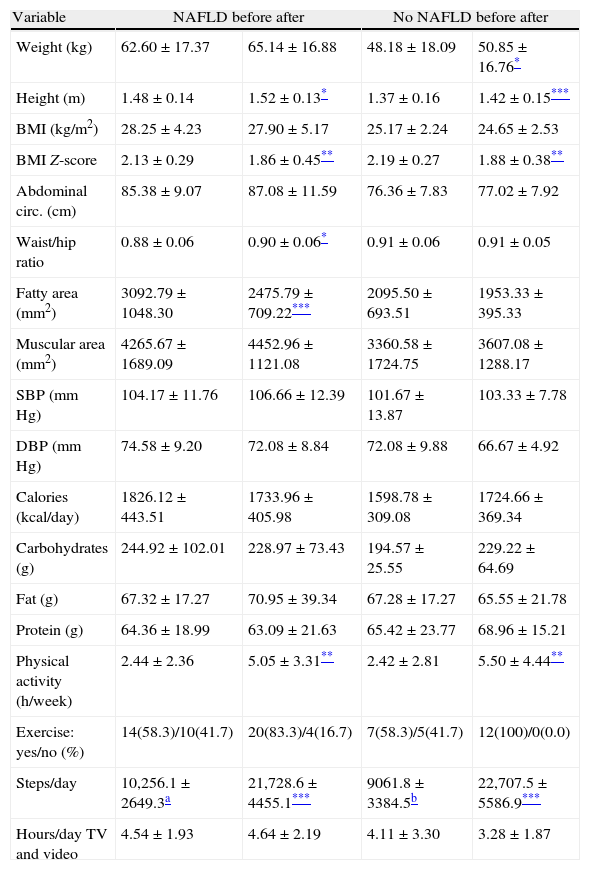
Table 3 shows the comparative clinical, dietary, and physical activity data for obese children and adolescents at study start and after 12 months of intervention, categorized by the presence or absence of NAFLD. The BMI Z-score decreased in both groups (p<0.005 for both), with no changes in calorie intake. An increase was seen in physical activity (p<0.005 in h/week and p<0.0001 in steps/day), with no change in the hours of sedentary life. Patients with NAFLD showed a decreased fatty area (p<0.0001), a change not seen in patients without NAFLD. No significant changes were found in any of the other variables tested.
Clinical and dietary characteristics and physical activity before and after intervention of children and adolescents with and without NAFLD.
| Variable | NAFLD before after | No NAFLD before after | ||
| Weight (kg) | 62.60±17.37 | 65.14±16.88 | 48.18±18.09 | 50.85±16.76* |
| Height (m) | 1.48±0.14 | 1.52±0.13* | 1.37±0.16 | 1.42±0.15*** |
| BMI (kg/m2) | 28.25±4.23 | 27.90±5.17 | 25.17±2.24 | 24.65±2.53 |
| BMI Z-score | 2.13±0.29 | 1.86±0.45** | 2.19±0.27 | 1.88±0.38** |
| Abdominal circ. (cm) | 85.38±9.07 | 87.08±11.59 | 76.36±7.83 | 77.02±7.92 |
| Waist/hip ratio | 0.88±0.06 | 0.90±0.06* | 0.91±0.06 | 0.91±0.05 |
| Fatty area (mm2) | 3092.79±1048.30 | 2475.79±709.22*** | 2095.50±693.51 | 1953.33±395.33 |
| Muscular area (mm2) | 4265.67±1689.09 | 4452.96±1121.08 | 3360.58±1724.75 | 3607.08±1288.17 |
| SBP (mmHg) | 104.17±11.76 | 106.66±12.39 | 101.67±13.87 | 103.33±7.78 |
| DBP (mmHg) | 74.58±9.20 | 72.08±8.84 | 72.08±9.88 | 66.67±4.92 |
| Calories (kcal/day) | 1826.12±443.51 | 1733.96±405.98 | 1598.78±309.08 | 1724.66±369.34 |
| Carbohydrates (g) | 244.92±102.01 | 228.97±73.43 | 194.57±25.55 | 229.22±64.69 |
| Fat (g) | 67.32±17.27 | 70.95±39.34 | 67.28±17.27 | 65.55±21.78 |
| Protein (g) | 64.36±18.99 | 63.09±21.63 | 65.42±23.77 | 68.96±15.21 |
| Physical activity (h/week) | 2.44±2.36 | 5.05±3.31** | 2.42±2.81 | 5.50±4.44** |
| Exercise: yes/no (%) | 14(58.3)/10(41.7) | 20(83.3)/4(16.7) | 7(58.3)/5(41.7) | 12(100)/0(0.0) |
| Steps/day | 10,256.1±2649.3a | 21,728.6±4455.1*** | 9061.8±3384.5b | 22,707.5±5586.9*** |
| Hours/day TV and video | 4.54±1.93 | 4.64±2.19 | 4.11±3.30 | 3.28±1.87 |
Data are given as X±SD. BMI: body mass index; DBP: diastolic blood pressure; SBP: systolic blood pressure.
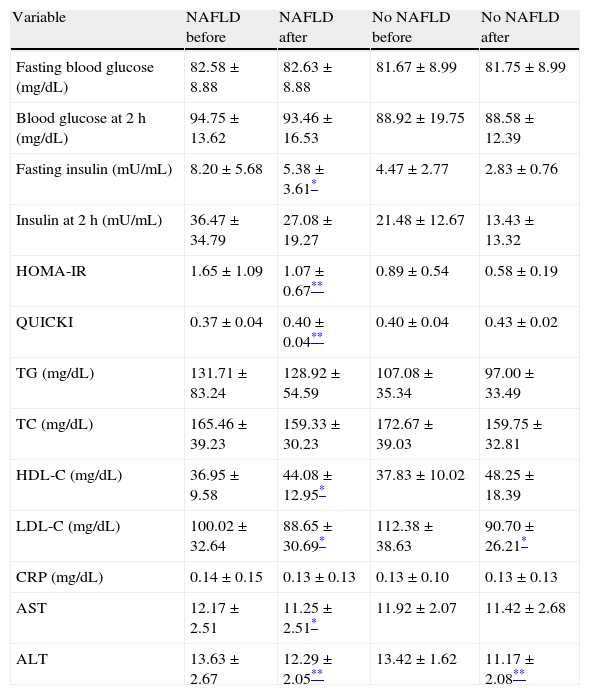
In the NAFLD group, metabolic variables improving with intervention included fasting insulin (p<0.05), HOMA-IR (p<0.005), QUICKI (p<0.005), HDL-C (p<0.005), LDL-C (p<0.005), AST (p<0.05), and ALT (p<0.005). Although improvements in metabolic variables were also seen in patients without NAFLD, the differences were only significant for LDL-C (p<0.05) and ALT (p<0.05) (Table 4).
Levels of metabolic variables, C-reactive protein, and aminotransferases before and after intervention in children and adolescents with and without NAFLD.
| Variable | NAFLD before | NAFLD after | No NAFLD before | No NAFLD after |
| Fasting blood glucose (mg/dL) | 82.58±8.88 | 82.63±8.88 | 81.67±8.99 | 81.75±8.99 |
| Blood glucose at 2h (mg/dL) | 94.75±13.62 | 93.46±16.53 | 88.92±19.75 | 88.58±12.39 |
| Fasting insulin (mU/mL) | 8.20±5.68 | 5.38±3.61* | 4.47±2.77 | 2.83±0.76 |
| Insulin at 2h (mU/mL) | 36.47±34.79 | 27.08±19.27 | 21.48±12.67 | 13.43±13.32 |
| HOMA-IR | 1.65±1.09 | 1.07±0.67** | 0.89±0.54 | 0.58±0.19 |
| QUICKI | 0.37±0.04 | 0.40±0.04** | 0.40±0.04 | 0.43±0.02 |
| TG (mg/dL) | 131.71±83.24 | 128.92±54.59 | 107.08±35.34 | 97.00±33.49 |
| TC (mg/dL) | 165.46±39.23 | 159.33±30.23 | 172.67±39.03 | 159.75±32.81 |
| HDL-C (mg/dL) | 36.95±9.58 | 44.08±12.95* | 37.83±10.02 | 48.25±18.39 |
| LDL-C (mg/dL) | 100.02±32.64 | 88.65±30.69* | 112.38±38.63 | 90.70±26.21* |
| CRP (mg/dL) | 0.14±0.15 | 0.13±0.13 | 0.13±0.10 | 0.13±0.13 |
| AST | 12.17±2.51 | 11.25±2.51* | 11.92±2.07 | 11.42±2.68 |
| ALT | 13.63±2.67 | 12.29±2.05** | 13.42±1.62 | 11.17±2.08** |
Data are given as X±SD.
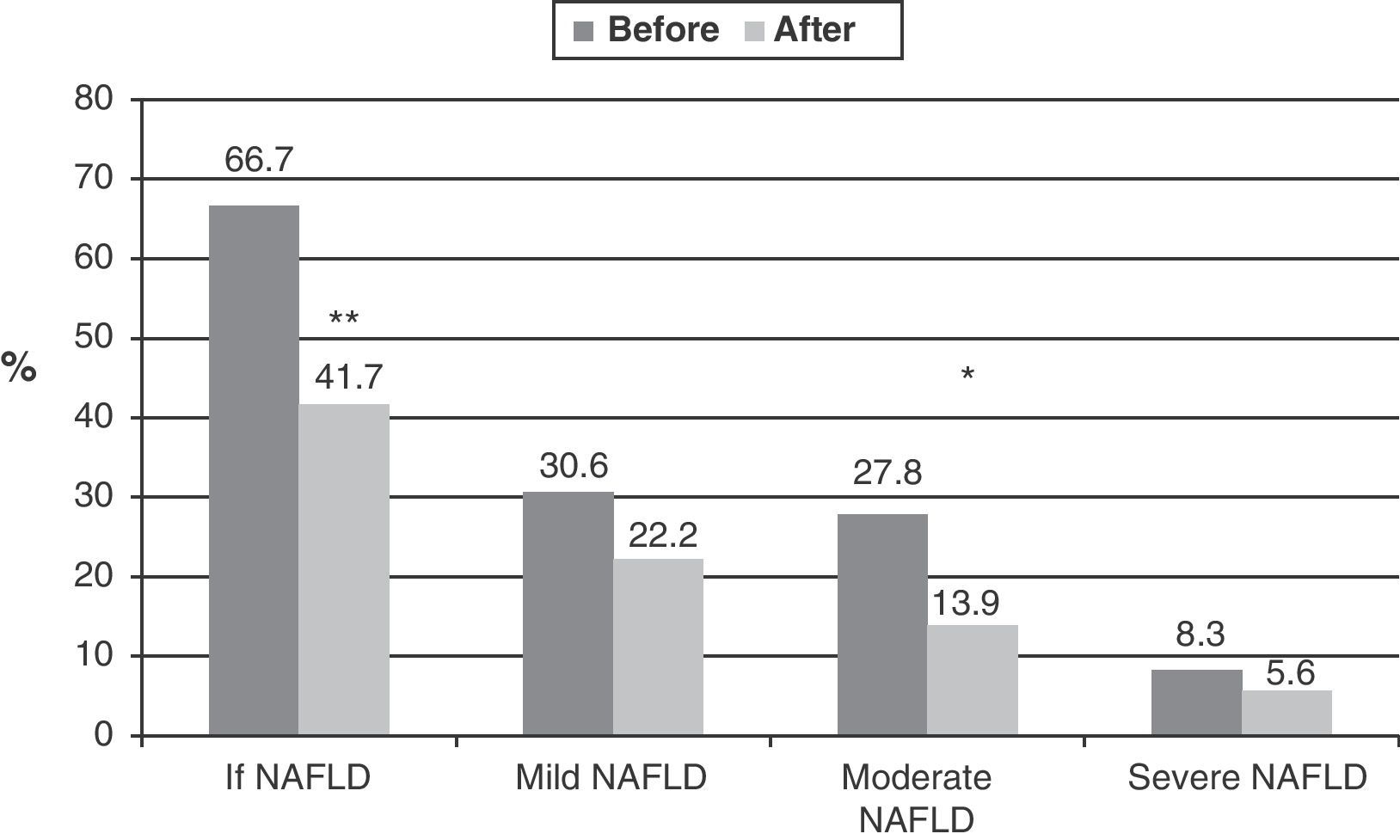
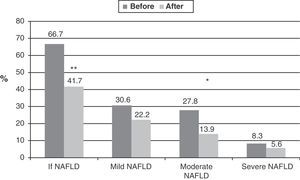
Among the 36 obese children and adolescents, 24 (66.7%) had NAFLD before intervention. After intervention, the condition persisted in 15 (41.7%) and disappeared in 9 (37.5%) (Fig. 1). As regards the severity of the condition, of the 24 patients with NAFLD, 30.6% (n=11) had mild NAFLD, 27.8% (n=10) moderate disease, and 8.3% (n=3) severe NAFLD. A significant improvement (p=0.03) was seen after intervention, when 22.2% of patients (n=8) had mild NAFLD, 13.9% (n=5) moderate disease, and 5.6% severe disease (n=2). In three patients in whom NAFLD did not disappear, the severity of the condition decreased. NAFLD improvement was independent of sex and pubertal stage.
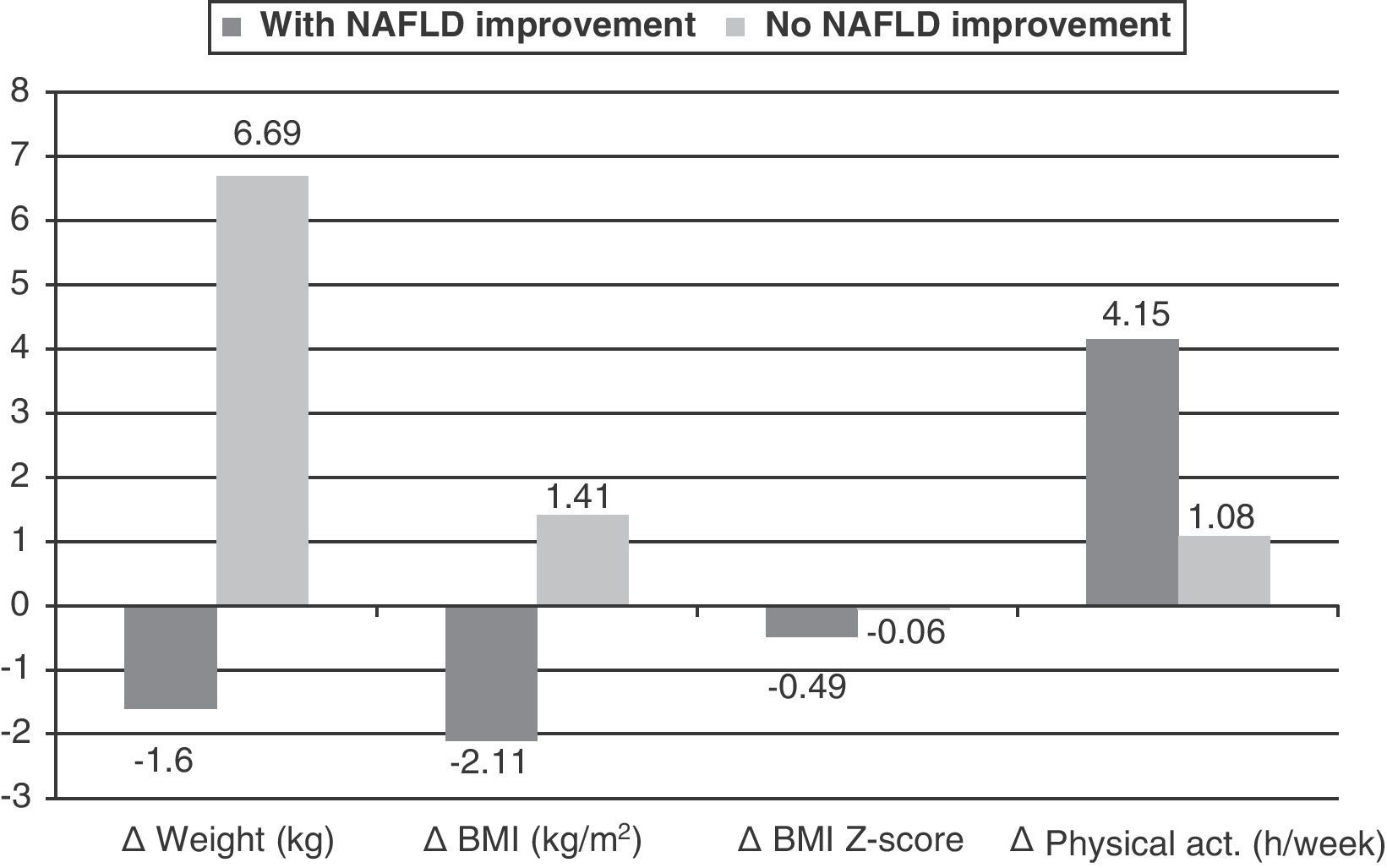
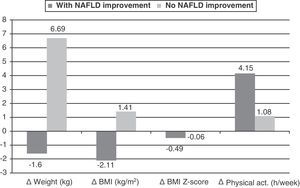
Fig. 2 shows the significant delta changes in the variables seen in participants stratified by improvement (n=12) or no improvement (n=12) in NAFLD with intervention. Changes were Δweight and ΔBMI, decreasing in the group with NAFLD improvement and increasing in the group with no improvement (p=0.0001 for both variables), and ΔBMI Z-score, which showed a greater decrease in the group with NAFLD improvement (p=0.002). Physical activity showed a greater increase in the group with NAFLD improvement (p=0.056). In logistic regression analysis, using NAFLD improvement and non-improvement as the dependent variable, the best explanatory variable was found to be change in weight (Δweight), with an R-squared of 0.634, i.e. an explanation of 63% of change and a 1.68-fold greater chance of NAFLD improvement in the group achieving weight loss (odds ratio: 1.682, CI: 1.072–2.639; p=0.024).
DiscussionNAFLD has become the most common cause of liver disease in children, particularly obese children.5,15,23 NAFLD prevalence varies, depending on whether histology or liver ultrasonography is used for diagnosis. Overall, studies suggest an increase in prevalence from 2.6% one decade ago24 to 5% in children with normal weight, 38% in obese children,8 and 48% in children with type 2 diabetes.25 In 2006, Pacheco et al.7 reported in Cuba a 48% prevalence of NAFLD in obese children and adolescents. In our study, liver ultrasound examination allowed for the identification of a high prevalence of NAFLD, 66.7%, in obese children and adolescents. These results, like others reported in the literature,26 suggest a very significant association between NAFLD and obesity.
While early studies reported a higher frequency of NAFLD in females as compared to males, the opposite was subsequently reported. However, more recent studies have found no sex differences.2,7,27 In agreement with the latter, no relationship was found in our study between NAFLD and sex or pubertal development.
All the children in our study were obese. However, those with NAFLD showed higher weight, BMI, AC, fatty area, and carbohydrate consumption, as well as higher basal insulin levels and greater insulin resistance, with no differences in aminotransferase or CRP levels, as compared to subjects with no NAFLD, showing that NAFLD is related to greater adiposity and insulin resistance and poorer dietary habits. It should be noted that 20% (5/24) of our patients with NAFLD had HOMA-IR values greater than 2.5, while no obese subjects without NAFLD had insulin resistance. These results agree with those published in 2006 by Burgert et al.,28 who reported that an increase in the fatty fraction of the liver in obese adolescents was associated with decreased insulin sensitivity, and differ from those reported by Reinehr et al.,15 who found no relationship between NAFLD and insulin resistance. It should be noted that although HOMA-IR values were higher in patients with NAFLD, in our obese children, just as in previous studies in our city,29,30 HOMA-IR, CRP, and transaminase levels were generally much lower than those reported by other authors in other parts of the world.28 Indeed, unlike in the Burgert et al. study,28 none of our patients had elevated transaminase levels, a finding which has also been reported by other authors.12
The mechanisms by which excess adiposity contributes to NAFLD continue to be controversial. It has been shown that obesity, mainly visceral obesity, and its associated insulin resistance, due to an imbalance in cytokine production (an increase in tumor necrosis factor alpha and adiponectin decrease, among others), lead to lipolysis, which increases circulating fatty acids. Fatty acids enter the liver through the portal vein, inducing an overload of beta oxidation and fatty acid accumulation in hepatocytes.31 Because of this sequence of events, NAFLD is currently recognized as a consequence of insulin resistance.
Most intervention studies combine dietary changes with aerobic exercise at different intervals and with different supervision levels, and support the notion that even small weight losses of approximately 5–10% show a clear benefit.14,30,32 The results of our research are similar to those reported in the international literature in that, after one year of dietary and physical exercise intervention, the subjects showed a decreased BMI Z-score, a significant decrease in fatty area, and a significant increase in physical activity, which led to improvements in metabolic variables such as total cholesterol, HDL-C, LDL-C, insulin, liver enzymes, and insulin sensitivity, as shown by a significant increase in HOMA-IR and an increase in the arithmetic estimate related to insulin sensitivity, QUICKI. These changes were more marked in the group with NAFLD, which was the one with the greatest baseline changes.
Liver echogenicity after intervention revealed the total disappearance of NAFLD in nine patients and an improvement in three, i.e. benefits in 50% of patients with NAFLD. These results are similar to those reported by Ueno et al.33 and Reinher et al.15 Weight and BMI decreases were shown to be the changes making the difference between children and adolescents with NAFLD improvement as compared to those with no improvement. Specifically, weight decrease, which was only 1.7% in the latter subjects, was the best explanatory variable for this improvement according to logistic regression analysis. This finding agrees with the report by Huang et al.34 of patients with non-alcoholic steatohepatitis and liver histology and by Reihner et al.15 of patients with NAFLD diagnosed by ultrasonography.
In adults, a low level of exercise is positively related to greater cardiovascular mortality and morbidity, and such correlation has also been shown in children and adolescents.35 In addition, a sedentary lifestyle with little physical activity, irrespective of diet, represents another determinant factor for NAFLD. Moderate lifestyle intervention using strategies aimed at weight reduction by restricting the intake of total and saturated fat, together with increased physical exercise, is currently considered the first line therapy.12,32,36 In our study, the increase in physical activity, as measured both by hours per week and steps per day, was more evident than changes in calorie intake. The latter did not decrease significantly but remained unchanged. However, this still resulted in an improved diet, as the participants were growing children and adolescents, and so finally led to weight and BMI reduction.
Our study showed a high prevalence of NAFLD in this group of obese children and adolescents, and also showed that a simple counseling program to implement lifestyle changes consisting of diet and physical exercise significantly improves insulin resistance in muscle, reduces visceral fat, and decreases liver echogenicity in ultrasonography, thus demonstrating the potential benefits of lifestyle counseling as a helpful treatment modality. The reported findings allow us to state that weight reduction induced by dietary treatment, combined with increased physical activity, has a favorable effect on NAFLD improvement.
FundingThis study was supported by the Council for Scientific, Humanistic, and Technological Development (CDCHT) of Universidad de Los Andes, M-1013-11-07-AA and ADG-M-10.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Santomauro M, et al. Hígado graso no alcohólico y su asociación con variables clínicas y bioquímicas en niños y adolescentes obesos: efecto de un año de intervención en el estilo de vida. Endocrinol Nutr. 2012;59:346–53.