To assess plasma renin and aldosterone levels in obese and non-obese women with polycystic ovary syndrome (PCOS).
MethodsObese women (body mass index [BMI]>30kg/m2; group A, n=34) and non-obese women (BMI<25kg/m2; group B, n=13) with PCOS were selected. The control group (group C, n=47) consisted of age-matched women with regular menses and normal ultrasonographic ovaries. Luteinizing hormone, follicle-stimulating hormone, androstenedione, testosterone, sex hormone-binding globulin, serum glucose, insulin, renin, plasma renin activity, and aldosterone levels were measured.
ResultsObese and non-obese women with PCOS had higher luteinizing hormone, follicle-stimulating hormone, androstenedione, testosterone, and insulin levels as compared to women in the control group (p<0.05). Women with PCOS had significantly higher renin levels (group A: 50.2±4.9picoU/mL, group B: 39.9±2.7picoU/mL, and group C: 24.6±2.6picoU/mL), plasma renin activity (group A: 3.7±0.3ng/mL/h, group B: 3.6±0.3ng/mL/h, and group C: 2.2±0.4ng/mL/h), and aldosterone levels (group A: 31.2±3.3ng/dL, group B: 29.3±2.9ng/dL, and group C: 22.2±3.9ng/dL) as compared with controls.
ConclusionSignificant differences exist in plasma renin and aldosterone levels between obese and non-obese women as compared with polycystic ovary syndrome and normal controls.
Determinar las concentraciones plasmáticas de renina y aldosterona en mujeres obesas y no obesas con diagnóstico de síndrome de ovarios poliquísticos (SOPQ).
MétodosSe seleccionaron mujeres con SOPQ obesas (índice de masa corporal (IMC)>30 Kg/m2; grupo A, n=34) y no obesas (IMC<25kg/m2; grupo B, n =13). El grupo control (grupoC, n=47) consistió en mujeres de edades similares, con menstruaciones regulares y ovarios normales por ecografía. Se analizaron las concentraciones de lutoprina, folitropina, androstendiona, testosterona, globulina fijadora de hormonas sexuales, glucosa sérica, insulina, renina, actividad de la renina plasmática y aldosterona.
ResultadosLas mujeres con SOPQ obesas y no obesas presentaron concentraciones más elevadas de lutoprina, folitropina, testosterona, androstendiona e insulina comparado con las mujeres del grupo control (p<0,05). Se observó que las mujeres con SOPQ presentaron concentraciones significativamente más altas de renina (grupo A: 50,2±4,9 picoU/ml, grupo B: 39,9±2,7 picoU/ml y grupo C: 24,6±2,6 picoU/ml; p<0,05), actividad de la renina plasmática (grupo A: 3,7±0,3ng/ml/h, grupo B: 3,6±0,3ng/ml/h y grupo C: 2,2±0,4ng/ml/h; p<0,05) y aldosterona (grupo A: 31,2±3,3ng/dl, grupo B: 29,3±2,9ng/dl y grupo C: 22,2±3,9ng/dl grupo C; p<0,05) comparado con los controles.
ConclusiónExisten diferencias significativas en las concentraciones plasmáticas de aldosterona y renina entre las mujeres con SOPQ obesas y no obesas respecto a los controles normales.
Polycystic ovary syndrome (PCOS) is an endocrine condition characterized by chronic oligo-ovulation or anovulation, characteristic ovarian findings in ultrasound examination, and hyperandrogenism.1 PCOS is the most common endocrine disease in females, affecting 5–10% of child-bearing women.2 PCOS is commonly associated with different risk factors for cardiovascular disease such as insulin resistance, obesity, dyslipidemia, and hypertension.3,4 Controversy exists as to whether insulin resistance is the result of PCOS or obesity.4
Renin, previously considered an enzyme, is a hormone that increases the production of plasminogen activator inhibitor-1 and induces cell hypertrophy and vascular fibrosis.5–7 It has been suggested that high plasma renin activity could be an additional risk factor for cardiovascular disease in patients with essential hypertension.8 The pathophysiological mechanisms of this association are still unknown, but experimental and clinical data have revealed the relationship between high plasma renin activity and vascular damage.9
Aldosterone is a recognized cardiovascular risk factor because of its significant role in the pathophysiology of hypertension, left ventricular hypertrophy, and heart failure.10 It has been shown to promote myocardial and valvular fibrosis, impair cardiac remodeling, and induce perivascular inflammation.11 The mechanism by which aldosterone causes these changes may be activation of an inflammatory status characterized by the presence of oxidative stress and inflammation.12 High aldosterone levels are associated with a high prevalence of glucose intolerance, diabetes mellitus, and metabolic syndrome.13,14
Since patients with PCOS are considered to be at a high risk of suffering cardiovascular diseases, the purpose of the research was to assess plasma renin and aldosterone levels in obese and non-obese women diagnosed with polycystic ovary syndrome.
MethodsFrom September 2009 to January 2011, women attending the outpatient clinics of internal medicine, endocrinology, and gynecology of Hospital Central Dr. Urquinaona diagnosed with PCOS were recruited into the study. The ethics committee of the hospital approved the study, and written consent was obtained from all patients.
Diagnosis of PCOS was confirmed using the following criteria: evidence of oligoanovulation (less than six menstrual periods in the previous year), clinical or biochemical signs of hyperandrogenism (plasma testosterone levels above the upper limit of normal and an abnormal LH [luteinizing hormone]/FSH [follicle-stimulating hormone] ratio>2), and normal or enlarged ovaries (>10mL) with the presence of subcapsular microcysts (12 or more) 2–9mm in diameter in the abdominal ultrasound examination.1
Women with PCOS, both obese (body mass index>30kg/m2; group A, n=34) and non-obese (body mass index<25kg/m2; group B, n=13), were recruited. Hormone tests and abdominal ultrasound were performed during the early follicular phase, between the third and fifth days of the spontaneous menstrual cycle. The control group (group C, n=47) consisted of age-matched women with regular menses and ultrasonographically normal ovaries attending the clinic for reasons other than PCOS. All controls were studied on days 3 to 5 of their menstrual cycles. Women with thyroid or adrenal disease, hyperprolactinemia, secondary or severe hypertension (defined as diastolic blood pressure>120mmHg), renal failure with creatinine clearance<30mL/min by 1.73m2 body surface area, urinary protein excretion greater than 1g/day, angina pectoris, recent myocardial infarction or cerebrovascular disease, as well as those who did not agree to participate were excluded from the study. Women with secondary arterial hypertension were excluded based on clinical and laboratory tests. Patients taking antihypertensive drugs were excluded from the study, and those taking lipid lowering drugs were asked to discontinue them four weeks before the study was due to begin. No patient was taking drugs which could have affected aldosterone or renin levels.
Hypertension was defined as systolic blood pressure>140mmHg and/or diastolic blood pressure>90mmHg. Measurements were done at least twice at two different times. Blood pressure was measured using a mercury sphygmomanometer after resting for 15min in a supine position, using a cuff of an adequate size. Systolic and diastolic blood pressures were measured at the first and fifth Korotkoff sounds, respectively. The average of three measures taken in 5min was calculated.
Primary hyperaldosteronism was defined as an increased aldosterone–renin ratio in the presence of aldosterone levels>150pg/mL, and confirmed by the absence of aldosterone suppression after the administration of intravenous saline.
Ultrasound examination was performed with General Electric Logiq Pro 3 ultrasound equipment using a convex 3.5MHz abdominal transducer and a 5MHz vaginal transducer. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2), while waist-hip ratio (WHR) was calculated by dividing waist circumference by hip circumference. Waist and hip circumferences were measured at the narrowest abdominal region and at the widest part of the gluteal region, respectively.
All venous blood samples were drawn under fasting conditions within one week of spontaneous or induced menstruation. All samples were similarly handled and were stored at −8°C for 1–3 days. FSH, LH, estradiol, androstenedione, and testosterone levels were measured by radioimmunoassay and chemiluminescence using commercial kits (Immulite 2000, Diagnostic Product Corp., USA). Intra-assay and inter-assay coefficients of variation were 4% and 7% for FSH, 6% and 7% for LH, 7% and 9% for estradiol, 6% and 10% for androstenedione, and 4% and 7% for testosterone, respectively. Sex hormone binding globulin (SHBG) was quantified by immunoassay (Perkin-Elmer Auto-DELFIA Immunoassay analyzer); inter-assay and intra-assay coefficients of variation were 3% and 4%, respectively. Serum glucose was quantified by the glucose oxidase method (Pointe Scientific Inc., USA). Intra-assay and inter-assay coefficients of variation were 1.4% and 1.9%, respectively. Insulin was measured using a radioimmunoassay (Coat-a-Count, Diagnostic Products Corp., USA). Intra-assay and inter-assay coefficients of variation were 1.6% and 5.5%, respectively.
Blood samples for measuring renin and aldosterone levels were drawn between 8 and 9 in the morning at a separate room where patients remained in a decubitus position for 1h. Dietary sodium intake was not standardized. The venous plasma sample was collected 10min after needle insertion in a precooled glass tube containing EDTA-potassium and was immediately centrifuged at −4°C. Radioimmunoassay was used for measuring aldosterone and renin (Coat-a-Count Aldosterone, Diagnostic Products Corp., USA and OBI-DSLCherwll innovation Centre, United Kingdom, respectively) with highly specific rabbit polyclonal antibodies. Coefficients of variation were lower than 9% and 10%, respectively. All procedures were performed at room temperature within 2h of sampling to avoid the cryoactivation of renin. Plasma renin activity (Renin Riabead, ABBOTT Laboratories, Diagnostics Division, Illinois, USA) was measured as angiotensin I generation rate according to the Sealey method15 and expressed as nanograms of angiotensin I formed per milliliter of plasma and hour of incubation (ng/mL/h). The coefficient of variation was less than 10%.
Data are given as mean±standard deviation. Statistical analysis was performed using an ANOVA test with a Dunnett's post hoc test between groups of women with PCOS (groups A and B), using women in group C as controls. Correlation coefficients among renin, plasma renin activity, and aldosterone levels and laboratory parameters were assessed using a Pearson's test. A linear regression analysis was performed between the different laboratory parameters and renin, plasma renin activity, and aldosterone levels. A value of p<0.05 was considered statistically significant.
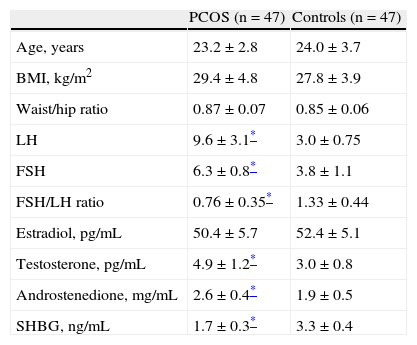
ResultsTable 1 shows the clinical and endocrine characteristics of women with PCOS and controls. Groups were matched for age and body mass index. Findings confirmed the differences between women with PCOS and controls. LH and FSH levels and the FSH/LH ratio were significantly higher in women with PCOS as compared to the control group (p<0.05). No statistically significant differences were found in estradiol levels. Testosterone and androstenedione levels were significantly higher in women diagnosed with PCOS (p<0.05). SHBG levels were significantly lower in women with PCOS as compared to controls.
General characteristics of the study groups.
| PCOS (n=47) | Controls (n=47) | |
| Age, years | 23.2±2.8 | 24.0±3.7 |
| BMI, kg/m2 | 29.4±4.8 | 27.8±3.9 |
| Waist/hip ratio | 0.87±0.07 | 0.85±0.06 |
| LH | 9.6±3.1* | 3.0±0.75 |
| FSH | 6.3±0.8* | 3.8±1.1 |
| FSH/LH ratio | 0.76±0.35* | 1.33±0.44 |
| Estradiol, pg/mL | 50.4±5.7 | 52.4±5.1 |
| Testosterone, pg/mL | 4.9±1.2* | 3.0±0.8 |
| Androstenedione, mg/mL | 2.6±0.4* | 1.9±0.5 |
| SHBG, ng/mL | 1.7±0.3* | 3.3±0.4 |
FSH: follicle-stimulating hormone; BMI: body mass index; LH: luteinizing hormone; SHBG: sex hormone binding globulin; PCOS: polycystic ovary syndrome.
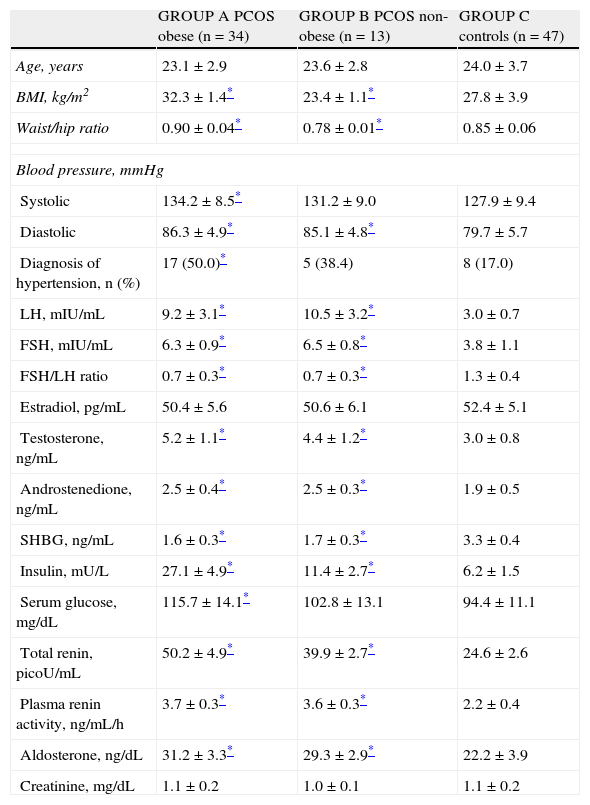
Table 2 shows the characteristics of obese and non-obese women with PCOS and control women. No age-related statistically significant differences were found between the three groups (p=NS). Group A patients had significantly higher systolic blood pressure values as compared to group C controls, while women from groups A and B had significantly higher diastolic blood pressure levels than women in the control group (p<0.05). As regards arterial hypertension in each group, this was diagnosed in 17 women in group A (50.0%), as compared to 8 women (17.0%) in group C (p<0.05). Five women from group B (38.4%) were diagnosed with arterial hypertension, with no statistically significant difference versus group C (p=NS).
Characteristics of patients with PCOS and controls.
| GROUP A PCOS obese (n=34) | GROUP B PCOS non-obese (n=13) | GROUP C controls (n=47) | |
| Age, years | 23.1±2.9 | 23.6±2.8 | 24.0±3.7 |
| BMI, kg/m2 | 32.3±1.4* | 23.4±1.1* | 27.8±3.9 |
| Waist/hip ratio | 0.90±0.04* | 0.78±0.01* | 0.85±0.06 |
| Blood pressure, mmHg | |||
| Systolic | 134.2±8.5* | 131.2±9.0 | 127.9±9.4 |
| Diastolic | 86.3±4.9* | 85.1±4.8* | 79.7±5.7 |
| Diagnosis of hypertension, n (%) | 17 (50.0)* | 5 (38.4) | 8 (17.0) |
| LH, mIU/mL | 9.2±3.1* | 10.5±3.2* | 3.0±0.7 |
| FSH, mIU/mL | 6.3±0.9* | 6.5±0.8* | 3.8±1.1 |
| FSH/LH ratio | 0.7±0.3* | 0.7±0.3* | 1.3±0.4 |
| Estradiol, pg/mL | 50.4±5.6 | 50.6±6.1 | 52.4±5.1 |
| Testosterone, ng/mL | 5.2±1.1* | 4.4±1.2* | 3.0±0.8 |
| Androstenedione, ng/mL | 2.5±0.4* | 2.5±0.3* | 1.9±0.5 |
| SHBG, ng/mL | 1.6±0.3* | 1.7±0.3* | 3.3±0.4 |
| Insulin, mU/L | 27.1±4.9* | 11.4±2.7* | 6.2±1.5 |
| Serum glucose, mg/dL | 115.7±14.1* | 102.8±13.1 | 94.4±11.1 |
| Total renin, picoU/mL | 50.2±4.9* | 39.9±2.7* | 24.6±2.6 |
| Plasma renin activity, ng/mL/h | 3.7±0.3* | 3.6±0.3* | 2.2±0.4 |
| Aldosterone, ng/dL | 31.2±3.3* | 29.3±2.9* | 22.2±3.9 |
| Creatinine, mg/dL | 1.1±0.2 | 1.0±0.1 | 1.1±0.2 |
FSH: follicle-stimulating hormone; BMI: body mass index; LH: luteinizing hormone; SHBG: sex hormone binding globulin; PCOS: polycystic ovary syndrome.
Higher LH, FSH, FSH/LH ratio, testosterone, and androstenedione values were found in women in both study groups (Table 2) as compared to the control group (p<0.05). There were no significant differences between estradiol levels in women from groups A and B and those found in group C (p=NS). On the other hand, SHBG levels were lower in both groups of women diagnosed with PCOS as compared to controls (p<0.05). As regards insulin levels, women in groups A and B had significantly higher values than group C controls. Obese women with PCOS had significantly higher serum glucose levels as compared to controls, while non-obese women with PCOS showed no significant differences.
Obese and non-obese women with PCOS showed higher renin levels as compared to controls. Plasma renin activity was higher in women from groups A and B as compared to group C (p<0.05). Aldosterone levels were also significantly higher in obese and non-obese women with PCOS as compared to control women. Plasma creatinine levels were similar in all three groups.
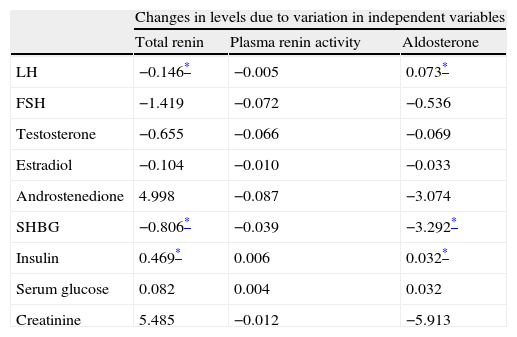
Analysis of PCOS patients, both obese and non-obese, showed a significant correlation of total renin levels with levels of insulin, SHBG (r=−0.189), FSH (r=0.177), and LH (r=0.151). A significant correlation (p<0.05) was found between aldosterone levels and insulin (r=0.032), sex hormone binding globulin (r=−0.124), LH (r=0.114), and FSH (r=0.107) levels. A linear regression analysis (Table 3) showed that androstenedione and insulin levels affected plasma renin concentration, while androstenedione, SHBG, and creatinine levels affected aldosterone concentrations.
Regression analysis between renin, aldosterone, and plasma renin activity values, and laboratory parameters in patients with polycystic ovary syndrome.
| Changes in levels due to variation in independent variables | |||
| Total renin | Plasma renin activity | Aldosterone | |
| LH | −0.146* | −0.005 | 0.073* |
| FSH | −1.419 | −0.072 | −0.536 |
| Testosterone | −0.655 | −0.066 | −0.069 |
| Estradiol | −0.104 | −0.010 | −0.033 |
| Androstenedione | 4.998 | −0.087 | −3.074 |
| SHBG | −0.806* | −0.039 | −3.292* |
| Insulin | 0.469* | 0.006 | 0.032* |
| Serum glucose | 0.082 | 0.004 | 0.032 |
| Creatinine | 5.485 | −0.012 | −5.913 |
FSH: follicle-stimulating hormone; LH: luteinizing hormone; SHBG: sex hormone binding globulin.
Research results show that women with PCOS, both obese and non-obese, have higher renin, plasma renin activity, and aldosterone levels as compared to control women.
In women, 50% of androgens come from the adrenal medulla and 50% from the ovaries. Women with PCOS have, on the one hand, excessive androgen production by ovarian theca cells and, on the other hand, decreased SHBG synthesis in the liver. Both of these increase free testosterone indices, leading to hyperandrogenism.2 The mechanism by which hyperandrogenism predisposes to the development of cardiovascular disease and hypertension has not been elucidated yet, but it has been postulated that testosterone stimulates the renin–angiotensin system in renal proximal tubules, which increases renal sodium reabsorption, with resultant increases in extracellular volume and blood pressure. In addition, testosterone induces insulin resistance in adipocytes.13 Free testosterone levels in blood may, therefore, be considered as a marker of the hyperfunction of the renin–angiotensin system, rather than as a stimulus of such a function per se.2–14
Several animal studies have suggested that a local renin–angiotensin system exists in tissues such as the brain, adrenal glands, and testes.13 The identification of renin and angiotensin receptors in the ovary suggests that there may be a functional ovarian renin–angiotensin system. Patients with PCOS have an increased activity of the renin–angiotensin system in plasma and ovaries (in both theca and granulosa cells) that causes an increased androgen synthesis. This may play a significant role in ovarian physiology and processes such as follicle development, steroidogenesis, oocyte maturation, ovulation, and follicular atresia.16
The results of the investigation suggest that both obese and non-obese PCOS patients have increased plasma renin activity that is associated with high insulin levels. Linear regression analysis also showed that fasting insulin levels induced significant changes in plasma renin activity. The higher insulin levels seen in obese and non-obese PCOS patients may be one of the potential explanations for the changes found in these patients. Elevated plasma renin activity could be due to a concomitant increase in circulating catecholamine levels during hyperinsulinemia.17 The increase in plasma renin activity related to hyperinsulinemia in this study supports the prior observations by Rooney et al.18
The specific mechanism responsible for the association between plasma renin activity and metabolic and cardiovascular risk factors is unknown. However, several studies have failed to demonstrate an association between high plasma renin activity and cardiac complications.19 Alderman et al.8 demonstrated an association between plasma renin activity and myocardial infarction, but did not show a causal relationship. Plasma renin activity may be a cardiovascular risk factor in women with PCOS, or may act as a marker of other risk factors such as increased sympathetic activity or other metabolic abnormalities.
Aldosterone has well-known proinflammatory actions in the cardiovascular system. Several studies have confirmed the hypothesis that high circulating aldosterone levels induce inflammation followed by reparative fibrosis.11,20 This proinflammatory phenotype is based on oxidative stress induction.12 Moreover, it has been shown that aldosterone may directly contribute to oxidative stress and to the occurrence of atherosclerotic lesions.21 This research found that both obese and non-obese PCOS patients had higher aldosterone levels than normal controls. It has been shown that aldosterone may lead to the development of cardiovascular disease by a mechanism which is independent of its effects on blood pressure. The vascular wall not only represents a target organ for aldosterone effects, but vascular cells also appear to be able to produce aldosterone at local level.22
High aldosterone levels in women with PCOS and metabolic syndrome may be in some manner related to insulin levels. High insulin levels have been reported to increase aldosterone secretion in vivo and in experimental studies.23 On the other hand, aldosterone may have a direct effect upon the insulin receptors, and recent experiments suggest that aldosterone may decrease insulin sensitivity in adipocytes.24 Prior studies13 have suggested changes in glucose metabolism and insulin actions induced by high aldosterone levels. A negative effect on glucose levels and an increased prevalence of metabolic syndrome have been reported in subjects with primary hyperaldosteronism.14 All of these data support the hypothesis that elevated levels of insulin or other cytokines act as a secretagogue for aldosterone.
The results of our research are similar to those reported by Uncu et al.,25 who showed higher renin levels in obese and non-obese women with PCOS as compared to controls. The concept that high renin concentrations contribute to insulin resistance may have implications in hormonal and metabolic abnormalities in PCOS. High renin levels in circulation or tissues have been shown to increase the production of angiotensin II, which inhibits the activity of PI 3-kinase, thus aggravating insulin resistance.26 In addition, increased renin levels may also contribute to endothelial dysfunction, a known abnormality in patients with PCOS.27,28
The investigation found different degrees of correlation of renin, aldosterone, and plasma renin activity values with gonadotropin and SHBG levels. The results of the correlation between LH and renin levels have previously been reported.25 Sealey15 showed that the initial elevation of LH levels precedes the initial increase in renin levels during the menstrual cycle. Blood samples were therefore taken at the early follicular phase to avoid fluctuating levels. Prior research has documented that renin production by the ovary is subject to a complex regulation that may involve paracrine and autocrine regulatory factors, which would suggest the possibility of an increased activity of the renin–angiotensin system in the ovary, contributing to excess androgen production.29
In conclusion, these observations provide evidence that plasma aldosterone and renin levels are increased in obese and non-obese women with PCOS. Increases in aldosterone and renin levels are associated with altered fasting insulin levels. These results may be relevant for understanding, at least partly, the underlying mechanism contributing to an increased risk of cardiovascular disease in patients with PCOS, who are known to be hyperinsulinemic.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please, cite this article as: Mejia-Montilla J, et al. Concentraciones plasmáticas de renina y aldosterona en mujeres obesas y no obesas con síndrome de ovarios poliquísticos. Endocrinol Nutr. 2012;59(1):21–7.