Obesity increases the risk of development of atherosclerosis. However, this risk significantly depends on adipose tissue distribution in the body and ectopic accumulation of visceral adipose tissue (VAT). Recent evidence suggests that each visceral fat deposit is anatomically and functionally different. Due to proximity to the organ, each visceral fat deposit exerts a local modulation rather than a systemic effect. Because of its unique location and biomolecular properties, a “non-traditional” fat depot–the epicardial adipose tissue–has been considered to play a causative role in atherosclerosis. Epicardial adipose tissue may be measured with imaging techniques and is clinically related to left ventricular mass, coronary artery disease, and metabolic syndrome. Therefore, epicardial fat measurement may play a role in stratification of cardiometabolic risk and may serve as a therapeutic target.
La obesidad aumenta el riesgo de desarrollar arteriosclerosis; sin embargo, el riesgo depende significativamente de la distribución del tejido adiposo en el cuerpo y la acumulación ectópica de tejido adiposo visceral (TAV). Evidencia reciente indica que cada depósito de grasa visceral es anatómica y funcionalmente diferente. Dada la proximidad al órgano, cada depósito de TAV ejerce una modulación local más que un efecto sistémico. Debido a su peculiar localización y sus propiedades biomoleculares, un tejido adiposo no tradicional, el tejido adiposo epicárdico, se ha abierto campo como causante de arteriosclerosis. Este tejido puede ser medido con técnicas de imagen y está clínicamente relacionado con la masa del ventrículo izquierdo, la enfermedad arterial coronaria y el síndrome metabólico. Por tanto, la medición de la grasa epicárdica puede tener un papel en la estratificación del riesgo cardiometabólico y servir como blanco terapéutico.
Obesity is associated with insulin resistance and atherosclerotic cardiovascular disease. However, the risk of these depends on adipose tissue distribution in the body, and mainly on the increase and ectopic accumulation of visceral fat.1–3 Increased visceral adipose tissue (VAT) not only involves greater adipocyte size, but also an increased expression of pro-inflammatory adipocytokines with harmful effects at both local and systemic levels.4 The quantification of VAT has therefore gained importance in recent years because it allows for a better stratification of both individual and overall cardiometabolic risk.
Recently, scientific interest has focused on the study of certain extra-abdominal visceral fat deposits, including epicardial adipose tissue (EAT), which because of its close relation to the myocardium and coronary arteries has provided a new understanding of the association between obesity and cardiovascular disease.5 This review article will address the morphological, biochemical, and clinical characteristics that make EAT a valuable tool for the comprehensive evaluation of cardiovascular risk.
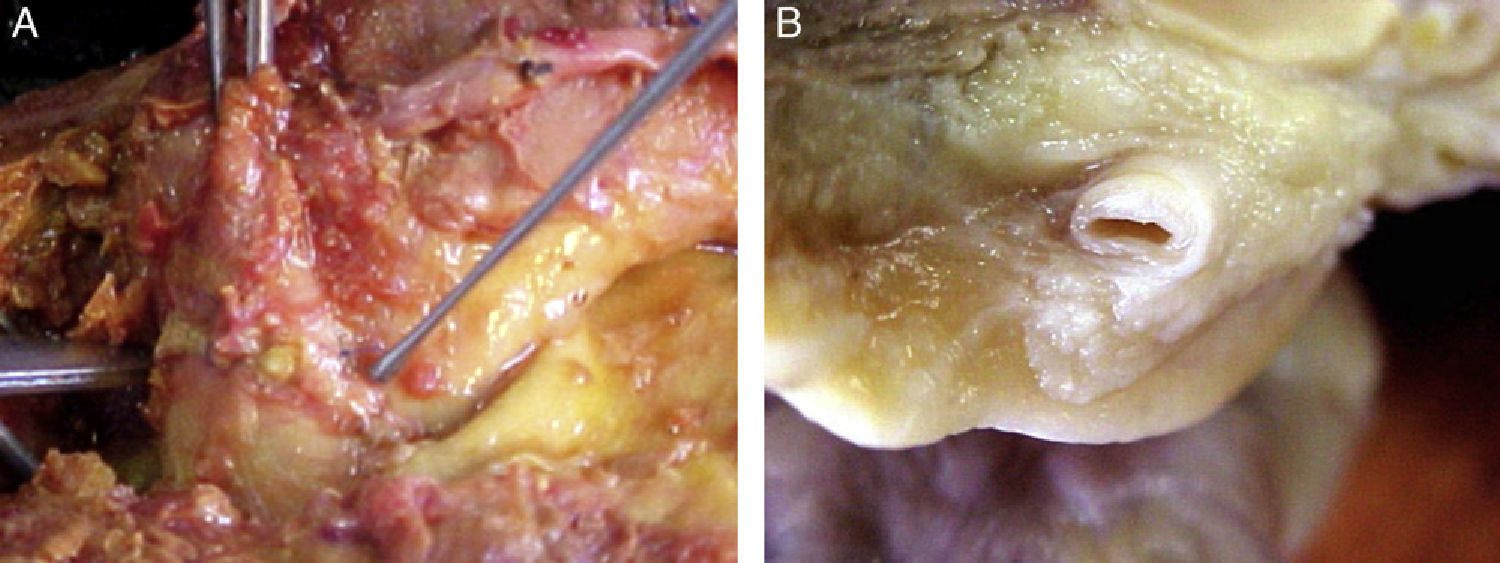
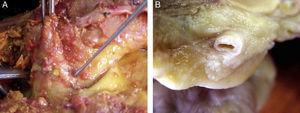
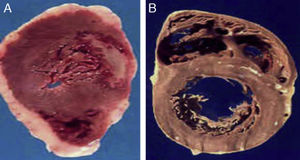
Morphological characteristics of epicardial adipose tissueThe presence of EAT on the myocardium and around the coronary arteries was recognized by anatomists in the mid 19th century.6 In adults, this tissue tends to be located in the atrioventricular and interventricular grooves and to extend toward the apex. Minor fat foci are located at subepicardial level along the free atrial wall.7 EAT increases in obese people or those with ventricular hypertrophy, and may therefore cover the spaces between the ventricles and sometimes completely covers the epicardial surface. In addition, a small amount of adipose tissue extends from the epicardial surface to the myocardium, often following the adventitia of coronary artery branches.7,8 It should be noted that there is no fascia or similar tissue separating epicardial fat from the myocardium or even from coronary vessels (Fig. 1), which means that a marked interaction exists between these structures.5,8 An anatomical distinction between epicardial fat in the myocardium and pericoronary epicardial fat has recently been suggested.9 However, it is not known whether these two components of EAT are functionally different.
Location of epicardial adipose tissue. (A) The close anatomical relation between epicardial fat and the myocardium is seen. (B) Epicardial adipose tissue around one coronary artery. Note the absence of fascia or similar tissues separating epicardial adipose tissue from these structures.
Histologically, EAT consists of adipocytes, nervous and nodal tissue, and inflammatory, stromal, and immune cells.10 Adipocytes in EAT are smaller than subcutaneous adipocytes and those in other VAT deposits, with size being a particularly important determinant of adipocytokine expression by EAT.11,12
Pericardial adipose tissue (PAT) is another cardiac fat deposit but, unlike EAT, it is located outside the visceral pericardium and on the outer surface of the parietal pericardium.13 The embryological origin of both tissues is different: while EAT originates in the splanchnopleure of the mesoderm, PAT originates in the primitive thoracic mesenchyma, which divides to form the parietal pericardium and the outer chest wall. Local circulation is also different in both tissues. The blood supply to epicardial fat comes from branches of coronary arteries (it shares the same circulation as the myocardium), while pericardial fat is supplied by the pericardiophrenic branches of the internal mammary artery.13,14 These anatomical and embryological differences make EAT the true visceral fat deposit of the heart.
Biochemical characteristics of epicardial adipose tissueEAT has a number of biochemical properties that differentiate it from other visceral fat deposits. Such properties include a high rate of free fatty acid uptake and release, which is particularly important because the myocardium uses and metabolizes fatty acids through the β-oxidation process, which accounts for 50–70% of cardiac muscle energy.15 In addition, EAT expresses fatty acid-binding protein 4, which may be involved in fatty acid transport from EAT to the myocardium.16 Interestingly, subjects with metabolic syndrome have an increased expression of this protein,16 and increased EAT has clinically been shown to be related to increased intramyocardial lipid content, which leads to cardiac steatosis and, eventually, to loss of cardiomyocyte function.17,18 In fact, fatty acid overload in the heart causes hyperactivation of β-oxidation which leads to the excess formation of reactive oxygen species (ROS), resulting in the modulation of sarcoplasmic reticulum ATPase, which is an early contributor to diastolic myocardial dysfunction with insulin resistance.19 Similarly, in animal models with overexpression of the enzyme acetyl CoA synthetase, left ventricular dysfunction occurs in parallel to overstimulation of oxidation and the formation of ROS and ceramide.20 These findings suggest that, under physiological conditions, EAT acts as a buffer that protects the heart from lipotoxicity and, in addition, provides the myocardium with the lipids needed to obtain energy through β-oxidation of fatty acids. Under pathological conditions, as in metabolic syndrome, EAT dysfunction occurs, leading to the loss of its cardioprotective effect.21
Epicardial adipose tissue and thermogenesisBrown adipose tissue specializes in the dissipation of energy through heat production. Recent research has shown that even adults have metabolically active brown adipose tissue which may play a significant role in energy homeostasis.22
The main characteristic of brown adipocytes is their high content in mitochondria.23 Such organelles produce energy by a proton gradient through the internal mitochondrial membrane. This energy is used to synthesize adenosine triphosphate (ATP) through the enzyme ATP synthetase. Brown adipocytes are essential for the thermogenic process based on activity of the uncoupling protein 1 (UCP-1), which is responsible for uncoupling oxidative phosphorylation and the respiratory chain, causing heat production due to proton loss.24 UCP-1 expression has recently been reported to be greater in EAT as compared to other fat deposits, which suggests that this tissue may act to defend the myocardium and coronary arteries against hypothermia.25 This hypothesis is supported by animal models such as polar bears, which have significant cardiac adiposity that may be used as a deposit and source of energy during hibernation periods, and may similarly protect the myocardium and the coronary arteries from low polar temperatures.26
Epicardial adipose tissue as an endocrine organEAT is a metabolically active organ that secretes a number of cytokines, collectively called adipocytokines, which are able to substantially modulate cardiovascular morphology and function.10,27,28 Because of its anatomical proximity to the heart and the absence of fascia or similar tissues, EAT may interact locally with coronary arteries through paracrine secretion mechanisms. Paracrine secretion of cytokines from periadventitial EAT may possibly pass through the coronary wall by diffusion from the outside to the inside and interact with cells in each of its layers.29 Atherosclerosis by “diffusion from outside to inside” has been proposed since 1989 based on the observation of leukocyte migration from outside the vessel wall.30 In addition, in vivo studies in pigs have shown that the external application of inflammatory cytokines such as interleukin-1β (IL-1β) and monocyte chemoattractant factor type 1 (MCP-1) to the coronary arteries induces increased intimal thickness and arterial remodeling.31,32 Another feasible factor is the direct release of adipocytokines and free fatty acids from the EAT to the vasa vasorum to be transported in the arterial wall by a vasocrine secretion mechanism.29
The metabolic profile of EAT is clearly different depending on the metabolic context of the patient. Under physiological conditions, EAT is able to synthesize and secrete adiponectin, which has antiatherogenic and anti-inflammatory properties, many of them mediated by AMP-activated protein kinase (AMPK),33,34 and has been related in various studies to a decreased risk of acute myocardial infarction.35,36 Consistent with the above, decreased adiponectin expression by EAT,37 which could be a contributing factor in the genesis of the atherosclerotic process, has been reported in patients with coronary artery disease. EAT also expresses adrenomedullin, a peptide hormone with pleiotropic effects at vascular level38 which is increased in diseases such as atherosclerosis,39 high blood pressure,40 heart failure,41 diabetes mellitus,42 and chronic renal disease,43 possibly as a compensatory mechanism for the endothelial dysfunction process occurring in these conditions. We have recently reported in patients with metabolic syndrome a significant association between EAT thickness measured by echocardiography and plasma adrenomedullin levels.44 However, conflicting evidence is available in patients with coronary artery disease, as Iacobellis et al.45 reported decreased adrenomedullin expression in the EAT of patients with coronary artery disease, while Silaghi et al.46 found increased adrenomedullin expression in this tissue in the same clinical condition. The reason for such differences could be that the patients studied by Iacobellis et al. were older and thinner as compared to those studied by Silaghi et al., as it is likely that age and fat mass interfere with the expression of this adipocytokine by EAT.
On the other hand, under pathological conditions such as obesity, EAT expands, becomes hypoxic and dysfunctional, and is invaded by phagocytic cells.47,48 Size changes in epicardial adipocytes and an increased number of macrophages and T lymphocytes increase the secretion of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), MCP-1, IL-1β, IL-6, resistin, and many others which contribute to the inflammatory environment characteristic of atherogenesis.10,49,50 Similarly, pericoronary EAT is able to secrete leptin and induce endothelial dysfunction by inhibiting nitric oxide synthetase through pathways dependent on protein kinase C (PKC).51,52 These findings confirm that EAT may play a determinant role in the start of the atherosclerotic process by virtue of the close anatomic relationship between these structures. It is postulated that a mechanism dependent on EAT mass regulates the metabolic profile of this tissue (Fig. 2).27 However, other factors may also influence this balance. It has recently been reported that in animal models, vitamin D deficiency is associated with an increased expression of the inflammatory markers in EAT53. It is unknown whether this mechanism also operates in humans.
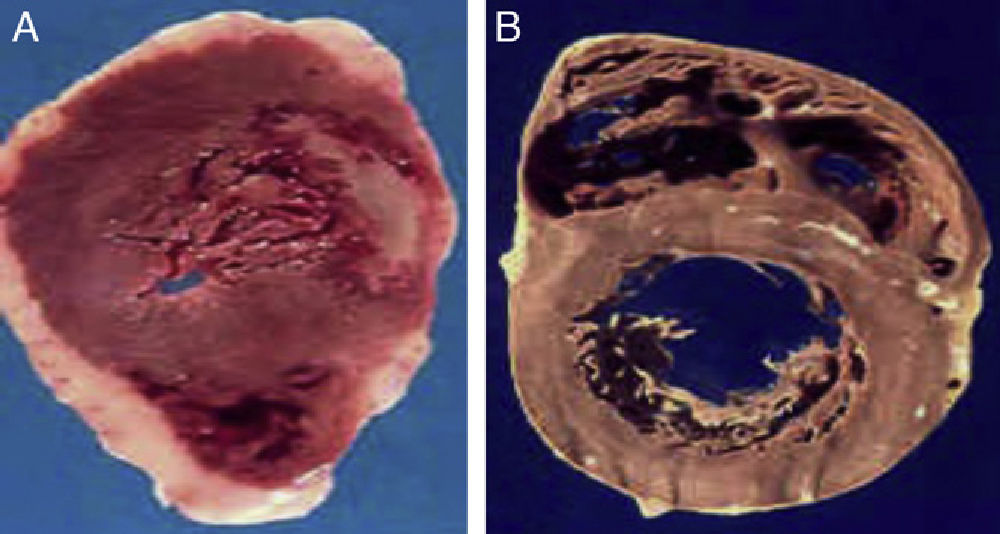
(A) The heart of a 48-year-old obese patient with type 2 diabetes who died from acute myocardial infarction. (B) The heart of a 45-year-old patient with no risk factors who died from violent causes. Note the great thickness of epicardial adipose tissue in patient A as compared to patient B.
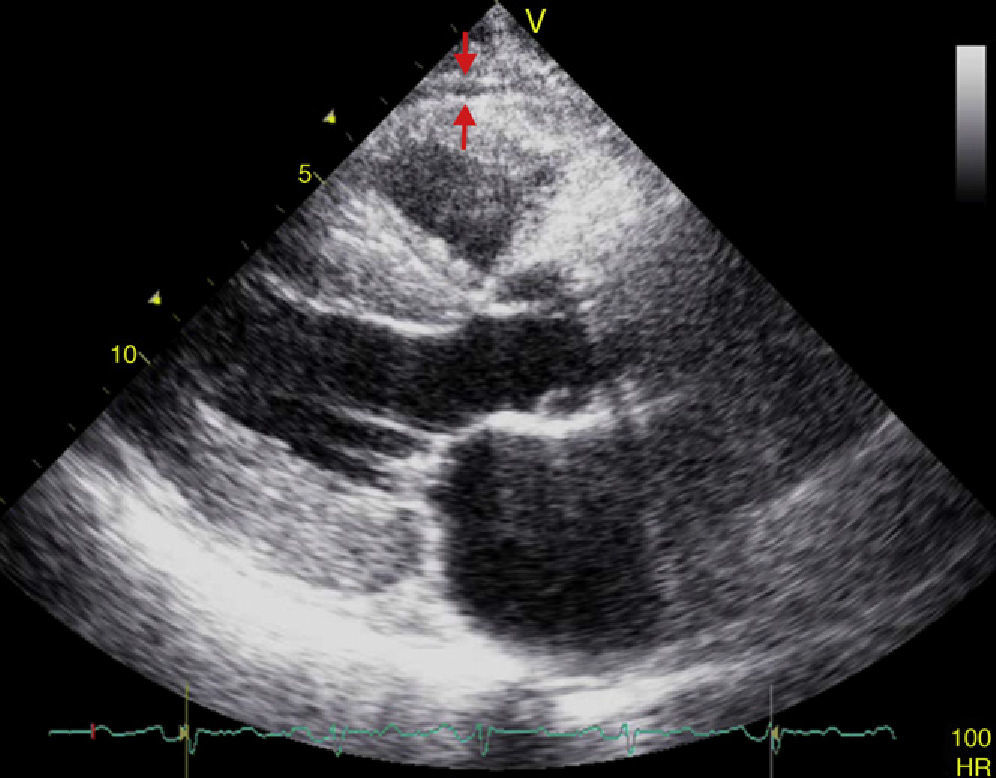
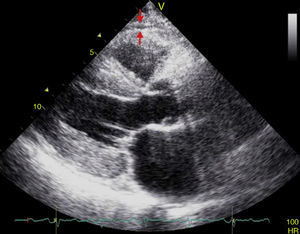
EAT thickness may be measured with standard two-dimensional transthoracic echocardiography using commercial equipment, as proposed and validated by Iacobellis et al.54,55 Two-dimensional views in the parasternal long and short axes allow for a more accurate measurement of epicardial fat in the right ventricle.56 Echocardiographically, EAT is identified as the space between the outer myocardial wall and the visceral layer of the pericardium. This thickness is measured perpendicularly on the free right ventricular wall at the end of systole in three cardiac cycles (Fig. 3).57 The reason why epicardial fat should be measured at the end of systole is that it is compressed during diastole, which causes inaccurate measurement. The mean value resulting from echocardiographic measurement of EAT in three cardiac cycles is then calculated. This value is considered to be the thickness of epicardial fat in the concerned patient.56,57
Population studies have shown little intra- and inter-observer variability.55,58 In addition, echocardiographic measurement of EAT thickness is a noninvasive, reliable, and easily reproducible procedure that represents a direct measurement of true visceral fat in the heart. It may routinely be done in patients considered at high cardiometabolic risk at no additional cost, as it does not require prior preparation and is performed at parasternal long or parasternal short echocardiographic views which are often used to assess other traditional cardiovascular parameters. The measurement of abdominal circumference is undoubtedly the most inexpensive and accessible visceral fat marker. However, it has little sensitivity and specificity for measuring visceral adiposity because it includes subcutaneous adiposity, which is not associated with cardiometabolic risk.59 Despite these advantages, echocardiography may not be an optimal procedure for EAT quantification because it does not allow for obtaining linear measurements at a single location and does not reflect EAT volume, unlike other more sensitive and specific imaging techniques, such as multislice computed tomography (MCT) and magnetic resonance imaging (MRI), which are considered to be the gold standard tests for VAT quantification because of the accuracy of their measurements, their low variability, and the high reproducibility of their results, with few advantages of one method over the other.60 MCT allows for quantifying EAT in terms of volume, and also for collecting information about coronary artery calcification and for visualizing stenotic sites and their distribution along these vessels.61 The measurement of EAT volume by MCT is often performed by tracing regions of interest in a short axis view. Adipose tissue voxels are usually identified between −190 and −30 Hounsfield units, and EAT volume is obtained by adding traced areas measured from the apex of the heart to the middle of the left atrium. Despite the high spatial resolution of MCT, this procedure has significant disadvantages such as exposure to ionizing radiation, its laboriousness and, above all, its high cost, which makes it an impractical and poorly accessible procedure for clinicians in daily practice. As with MCT, with MRI a tracing is obtained of EAT contours and adipose tissue voxels in slices are added to calculate the volume of this tissue. However, this is also a highly cumbersome imaging technique, and even more costly than MCT, and studies such as the one reported by Iacobellis et al.62 have shown a good correlation between measurements of EAT by ultrasound and VAT by MRI.
Epicardial adipose tissue and metabolic syndromeThe heart and coronary arteries are surrounded by a significant amount of adipose tissue. EAT thickness at the right ventricular free wall is normally less than 7mm in healthy, thin subjects57; however, fat volume around the heart is greater in males as compared to females and, like abdominal circumference, varies depending on ethnic group.57,63
Metabolic syndrome is a group of clinical and biochemical findings with a common pathogenetic mechanism, namely increased visceral adiposity and insulin resistance.64,65 A positive relationship has been shown between EAT and metabolic syndrome components.44,62 In fact, epicardial fat volume gradually increases with the number of components of metabolic syndrome,61,66 and even when other cardiometabolic parameters are separately considered, EAT is independently associated with blood pressure,62 low density lipoprotein cholesterol (LD),62 fasting blood glucose,67 and insulin resistance.68 It should be stressed that, in children with obesity, EAT has been shown to be a good marker of visceral adiposity, but it is not an independent predictor of metabolic syndrome, which suggests that the prognostic value of this tissue varies depending on the age group.69
Epicardial fat and changes in cardiac morphologyThe relationship between EAT and changes in cardiac morphology and function has been studied in recent years. A strong association has been shown between left ventricular hypertrophy and EAT thickness regardless of the overall adiposity of the subject.70 Several mechanisms may explain this association, including:
- 1.
Excess EAT represents a load for the heart, which may lead to compensatory cardiac remodeling.71
- 2.
Increased EAT is associated with a greater intramyocardial lipid content and thus with myocardial steatosis and lipotoxicity, which may induce adverse structural and functional adaptations, including myocardiopathy.17,18,72
- 3.
EAT may affect cardiac morphology through both the local and systemic effects of the adipocytokines it synthesizes, as some of them are able to induce cardiac remodeling.10,29 Moreover, at the systemic level EAT may induce insulin resistance, which acts as an intermediary between visceral fat and left ventricular hypertrophy through the direct mitogenic action of insulin on myocardial cells, activation of the sympathetic nervous system and renin–angiotensin system, particularly angiotensin II, whose action upon AT1 receptors is able to produce myocardial cell proliferation, and at the glomerular layer of the adrenal cortex it may stimulate aldosterone synthesis and secretion, causing water and sodium reabsorption, extracellular volume expansion and, finally, ventricular hypertrophy.73,74
EAT thickness is also significantly related to right ventricular cavity size and even with atrial dimensions and the risk of atrial fibrillation in obese subjects.75–77
Epicardial adipose tissue and its relation to coronary artery diseaseIn most clinical studies, increased EAT has been associated with coronary artery stenosis. In the study conducted by Jeong et al.78 in 203 patients with angiographic criteria of coronary artery disease, the Gensini score was used to assess disease extent and severity. Patients with greater epicardial fat thickness as measured by echocardiography (≥7.6mm) were found to have a higher Gensini score (p=0.014). Moreover, Yun et al.79 assessed 153 patients admitted for coronary angiography due to chest pain, excluding from the study those with prior acute myocardial infarction, congestive heart failure, and cardiomyopathy. EAT was measured in these patients using transthoracic echocardiography. EAT thickness was 1.76±1.36mm in patients with no significant stenosis, as compared to 3.39±1.64mm in patients with single vessel coronary disease and 4.12±2.03mm in those with multivessel coronary disease (p<0.001).
Patients with type 2 diabetes mellitus are known to have an increased risk of coronary artery disease. In this regard, Wang et al.80 compared 49 patients with type 2 diabetes mellitus and 78 nondiabetic controls. EAT volume (by MCT), Gensini score, and coronary artery calcification were determined and related to the clinical and biochemical criteria of metabolic syndrome. Patients with type 2 diabetes were found to have a greater EAT volume as compared to nondiabetic controls (166.1±60.6cm3 vs 123.4±41.8cm3, p<0.0001). In addition, EAT volume was associated with metabolic syndrome components and with greater severity of coronary atherosclerosis.
Two recent longitudinal studies81,82 appear to support the “outside to inside” signaling hypothesis as the cause of atherosclerosis. These studies measured the volume of intrathoracic and epicardial adipose tissue and found that increased volumes were associated with a higher incidence of coronary artery disease and with an increase in adverse cardiac events. The recent finding that the relationship between EAT thickness and coronary artery disease is independent of the presence or absence of obesity should be stressed.83In vivo studies have also shown a strong association between carotid intima-media thickness, as a marker of subclinical atherosclerosis, and EAT thickness measured by echocardiography.84,85 It should be noted that EAT volume is an independent determinant of the occurrence of total coronary artery occlusion,66,86 and since total coronary occlusion causes plaque instability, EAT may be associated with greater plaque vulnerability. This hypothesis is supported by the fact that a greater epicardial fat volume has been shown in patients with non-calcified plaques as compared to those with calcified plaques.87 This influences the development of acute coronary syndrome because non-calcified plaques often tend to be more vulnerable.
Despite these conclusive study results, it is still unclear whether EAT plays a causative role in the development of coronary atherosclerosis because, for example, patients with generalized congenital lipodystrophy develop coronary atherosclerosis even in the absence of excess visceral adiposity, including EAT.8 However, both humans and animals have anatomical variants called intramyocardial bridges, consisting of coronary arteries with an intramyocardial tract not surrounded by perivascular adipose tissue, which remain free from atherosclerosis, while the segment proximal to the bridge shows significant atherosclerosis, which is much more marked when the bridge is long and thick, possibly due to hemodynamic factors.88,89 Moreover, a recent meta-analysis involving 2872 patients showed greater EAT thickness and volume in patients with coronary disease, and also showed that patients in the higher EAT tertile were more prone to experience coronary artery disease than patients in the lower tertile.90
Epicardial adipose tissue, a new therapeutic target?The growing interest in EAT is not only due to its significance as a marker of cardiometabolic risk, but also to its potential use as a therapeutic target. Weight loss is associated with a substantial decrease in VAT, which improves the cardiometabolic profile of obese patients. This weight reduction may be achieved through nutritional programs based on low-calorie diets, aerobic exercise, bariatric surgery and, to a lesser extent, by drug treatment.64,91 In this regard, Kim et al.92 showed that a 12-week low-calorie diet (with a 26.8% reduction in daily calorie intake) caused in obese subjects a 17.2% reduction (p<0.001) in EAT thickness as measured by transthoracic echocardiography. It should be stressed that in this study, the reduction in epicardial fat thickness was faster and greater than the decrease in other traditional adiposity indices such as abdominal circumference (−9%) and the body mass index (BMI) (−11%), whose results were similar to those reported by other studies.93 Aerobic exercise also significantly decreases EAT thickness in obese patients, and the decrease is also related to improvements in systolic blood pressure and insulin sensitivity in this patient group.94
Bariatric surgery has also been shown to be effective in reducing EAT thickness. Willens et al.95 showed in 23 patients with morbid obesity who lost on average 40±14kg after surgery that EAT thickness decreased from 5.2±2.4mm to 4.0±1.6mm (p<0.001), showing the benefit of this surgical procedure for the cardiometabolic profile of obese patients.
It is interesting to note that drugs with proven benefits in cardiovascular risk reduction such as atorvastatin have been shown to be able to decrease EAT thickness, although the mechanism of this effect is unknown,96 and that in patients with metabolic syndrome and type 2 diabetes with coronary disease, pioglitazone is effective in the modulation of pro- and anti-inflammatory genes in EAT.97
The use of EAT as a therapeutic target has also been evaluated in other diseases such as growth hormone deficiency in both children and adults, in which replacement therapy with recombinant growth hormone is able to reduce EAT thickness due to its lipolytic effect.98,99 Patients infected with the human immunodeficiency virus (HIV) on antiretroviral therapy also have greater EAT thickness as compared to untreated HIV-infected patients.100
ConclusionsAlthough the presence of EAT in the myocardium and coronary arteries has been known since the 19th century, it has only recently started to attract attention as a new tool for the stratification of cardiometabolic risk. Even with the most recent technological advances, much is still unknown about this extraordinary visceral fat deposit, and its future study will continue to provide both clinicians and researchers with new insights into our understanding of obesity as a causative agent of cardiovascular disease.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Lima-Martínez MM, et al. Tejido adiposo epicárdico: ¿más que un simple depósito de grasa? Endocrinol Nutr. 2013;60:320–8.