Sporting activity is becoming a common practice in patients with diabetes mellitus (DM). This situation requires both a preliminary medical assessment and a wide range of changes in treatment which have scarcely been addressed in medical literature.
ObjectiveTo prepare a clinical guideline on the medical approach to patients with diabetes who practice sport regularly.
MethodsAn expert panel from the Diabetes Mellitus Working Group of the Spanish Society of Endocrinology and Nutrition (SEEN) reviewed the most relevant literature in each of the sections. Based both on this review and on data from the experience of a number of athletes with DM, a number of recommendations were agreed within each section. Finally, the Working Group and representatives of the SEEN jointly discussed all these recommendations.
ConclusionThe guideline provides recommendations ranging from medical assessment before patients with DM start to practice sport to actions during and after physical activity. Recommendations are also given on aspects such as the impact of sport on blood glucose control, training schemes, or special risk situations.
La práctica de deporte con un nivel de exigencia alto es cada vez más habitual en pacientes con diabetes mellitus (DM). Esta situación aconseja realizar tanto una valoración previa como una amplia serie de modificaciones en el tratamiento, escasamente referidas en la literatura médica habitual.
ObjetivoElaborar una guía clínica que oriente sobre la actitud médica a seguir ante un paciente con DM que realiza deporte de forma habitual.
MétodosUn grupo de expertos del Grupo de Trabajo de DM de la Sociedad Española de Endocrinología y Nutrición (SEEN) ha revisado la literatura médica relevante en cada uno de los apartados. En base a esta revisión, y con los datos aportados por la experiencia de una serie de deportistas con DM, se han consensuado una serie de recomendaciones dentro de cada apartado. Tras la formulación de las recomendaciones, estas se han discutido conjuntamente por el Grupo de Trabajo y por representantes de la SEEN.
ConclusiónLa guía ofrece unas pautas que abarcan desde la valoración previa a la práctica deportiva en paciente con DM, como a la actuación durante y después del deporte, pasando por aspectos como la repercusión del deporte en el control de la DM, pautas de entrenamiento o situaciones de especial riesgo.
As in many other countries across the world, the number of people with diabetes mellitus (DM) in Spain is increasing. Fortunately, doctors now have increasingly effective treatments and monitoring systems at their disposal, and these have allowed us not only to control, but almost normalise various metabolic disorders that accompany this disease. However, we must be more ambitious: to restore patients to health, we need to help people with DM to develop lifestyle habits similar to those who do not have DM. Among these lifestyle habits, the regular practice of different types of sport has been extended to include people with DM, for example, people who do such demanding activities as marathons, diving and mountaineering. Many of these people have been monitored by their attending doctors, who advise them in aspects as diverse as nutrition, therapeutic changes (especially insulin regimens), glycaemic control and complications, among others. Nevertheless, when it comes to helping patients with DM practise sport, it is not always easy to find scientific documents that facilitate these tasks in a practical way. To bridge this gap, the Spanish Society of Endocrinology and Nutrition's (SEEN) Working Group on DM has considered the development of this guide as a way to help professionals who treat people who, in addition to having diabetes, are concerned to practise sport and who pose new challenges not addressed in the usual clinical practice guidelines.
Because there is a lack of scientific data needed to establish an evidence-based consensus, the guide puts forward some clinical recommendations based on the consensus criteria of the group of experts. Moreover, the authors considered it important to contact different athletes with DM who have contributed their experience and daily practice, and these contributions serve to enrich the document.
1MEDICAL EVALUATION BEFORE UNDERTAKING ANY SPORTThe scientific societies recommend that patients with DM undergo a medical evaluation before they start an exercise programme or a sporting activity.1,2
Objectives of the evaluation- •
To determine whether there is any disease or complication that may occur or be aggravated by the sport.
- •
To plan and schedule the exercise and performance-related options.
It is recommended that each athlete with DM have a diabetes care plan to follow while undertaking any sporting activity. The plan should include the following:3
- •
Blood glucose monitoring. A frequency for the monitoring and the figures that contraindicate a sport should be established.
- •
Insulin therapy. Type of insulin and dose, and strategies for adjusting and correcting it according to the type of activity planned.
- •
Recommendations for recognising and treating hypoglycaemia, including instructions on the use of glucagon.
- •
Contact details in case of emergency (telephone numbers).
- •
Identification for patients with DM that indicates their condition (medical alert).
For athletes with diabetes-related complications, doctors should determine the limitations or restrictions regarding the practice of sport.
- •
Although glycated haemoglobin is not used to make immediate decisions in regard to exercising, athletes with type 1 DM should have their blood tested for their HbA1c level every 3 to 4 months to evaluate their glycaemic control.
- •
People who have diabetes with any related cardiovascular disease or microvascular complications and who wish to engage in some type of sport should undergo a medical evaluation. The evaluation must include medical history, physical examination (including a dilated eye exam, a foot examination and a check for neuropathy), resting electrocardiogram (ECG) and possibly an exercise stress test.4
Routine screening for coronary heart disease in asymptomatic DM patients remains controversial. The American Diabetes Association does not recommend systematic screening for coronary heart disease in asymptomatic patients,2 but it does recommend the screening of high-risk patients;5 the American College of Cardiology takes the same stance on this, recommending the screening of patients with high-risk DM (Table 2) by means of an exercise stress test before the start of any sport that is of moderate to high intensity (Table 3).6 In general, patients with DM who have established coronary disease but do not have significant ischaemia or arrhythmias should not participate in high-intensity exercises. They may engage in less intense sporting activities, however. Patients with angina should show a target heart rate at least 10 beats below their ischaemic threshold.6
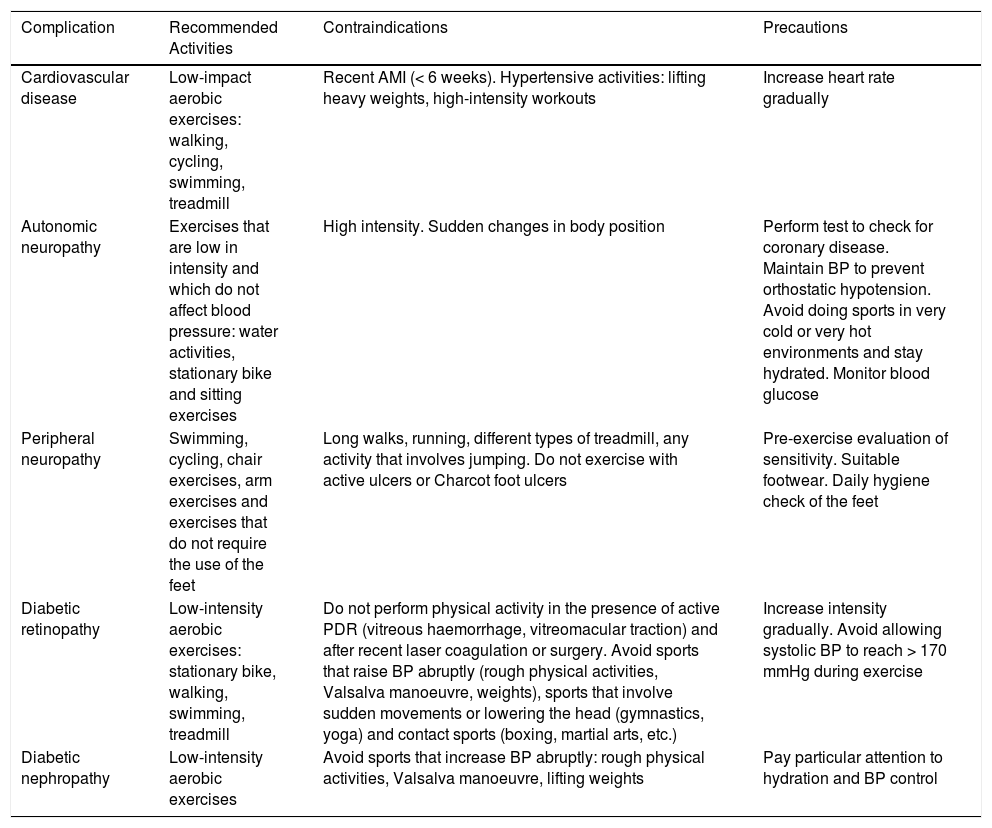
Sport and complications of diabetes mellitus
| Complication | Recommended Activities | Contraindications | Precautions |
|---|---|---|---|
| Cardiovascular disease | Low-impact aerobic exercises: walking, cycling, swimming, treadmill | Recent AMI (< 6 weeks). Hypertensive activities: lifting heavy weights, high-intensity workouts | Increase heart rate gradually |
| Autonomic neuropathy | Exercises that are low in intensity and which do not affect blood pressure: water activities, stationary bike and sitting exercises | High intensity. Sudden changes in body position | Perform test to check for coronary disease. Maintain BP to prevent orthostatic hypotension. Avoid doing sports in very cold or very hot environments and stay hydrated. Monitor blood glucose |
| Peripheral neuropathy | Swimming, cycling, chair exercises, arm exercises and exercises that do not require the use of the feet | Long walks, running, different types of treadmill, any activity that involves jumping. Do not exercise with active ulcers or Charcot foot ulcers | Pre-exercise evaluation of sensitivity. Suitable footwear. Daily hygiene check of the feet |
| Diabetic retinopathy | Low-intensity aerobic exercises: stationary bike, walking, swimming, treadmill | Do not perform physical activity in the presence of active PDR (vitreous haemorrhage, vitreomacular traction) and after recent laser coagulation or surgery. Avoid sports that raise BP abruptly (rough physical activities, Valsalva manoeuvre, weights), sports that involve sudden movements or lowering the head (gymnastics, yoga) and contact sports (boxing, martial arts, etc.) | Increase intensity gradually. Avoid allowing systolic BP to reach > 170 mmHg during exercise |
| Diabetic nephropathy | Low-intensity aerobic exercises | Avoid sports that increase BP abruptly: rough physical activities, Valsalva manoeuvre, lifting weights | Pay particular attention to hydration and BP control |
AMI: acute myocardial infarction; BP: blood pressure; PDR: proliferative diabetic retinopathy.
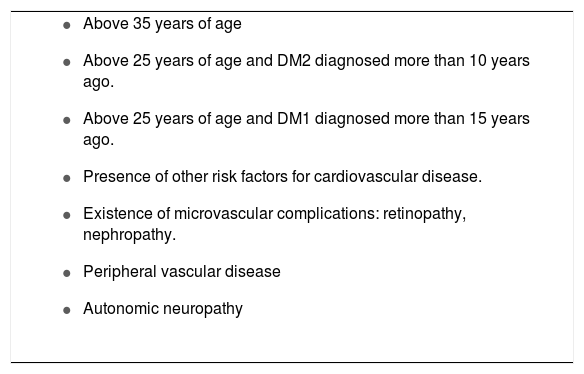
Patients with diabetes mellitus and a high risk of cardiovascular disease
|
DM2: diabetes mellitus type 2 DM1: diabetes mellitus type 1.
Intensity of exercise/sport
| % of MHR | % VO2 max | METs | EXAMPLES | |
|---|---|---|---|---|
| Low | < 60% | < 50% | < 3 | Cycling (< 10 km/hr), slow horseback riding, volleyball, archery, light gymnastics, stationary bike |
| Moderate | 60-79.9% | 50-74.9% | 3-6 | Cycling (10-20 km/hr), padel, tennis, basketball, football (training), swimming sets with changes in style/rhythm/intensity, rowing, diving, trotting, downhill skiing, skating, weights (light), dinghy sailing |
| High | ≥ 80% | ≥ 75% | > 6 | Cycling (> 20 km/hr or mountain-biking), mountaineering, running (> 7 km/hr), cross-country skiing, galloping, basketball, football (competitions), swimming by changing styles/rhythm/intensity, Basque pelota, handball, weights (intense), boxing/karate |
% of MHR: percentage of maximum heart rate; % VO2 max.: Percentage of maximal oxygen uptake; METs: metabolic equivalent (1 MET: 3.5 ml of O2/kg/min; 1 Kcal/kg/hr).
Cardiac autonomic neuropathy is difficult to diagnose in clinical practice because it can be a subclinical condition, and therefore asymptomatic, in many patients with DM. Because of its impact on exercise tolerance, screening for cardiac autonomic neuropathy should be part of the pre-exercise examination in patients who are about to start a new exercise programme or sport involving a moderately or highly intense physical activity. What's more, patients with autonomic neuropathy should undergo a pre-exercise stress test before starting a sport. In case of gastroparesis, carbohydrate absorption can be delayed, which may predispose patients to hypoglycaemia. To control the intensity of an exercise, athletes with autonomic neuropathy should use perceived exertion rather than heart-rate response. Patients who have DM with autonomic neuropathy may be more susceptible to the adverse effects of heat. Reduced sweating and blood flow to the skin decrease the body's ability to maintain its internal temperature at safe levels, especially during prolonged exercise in the heat. In these cases, whenever possible, the exercise should be performed in a cool environment.
Peripheral neuropathyPatients with severe peripheral neuropathy lose protective sensibility in their feet, which can be detected using the 10-g monofilament test. In these cases, it is advisable to limit load-bearing sports and suggest safer activities, such as swimming, cycling or arm exercises. The use of special footwear is also important.
Diabetic retinopathyPatients should be given a pre-exercise evaluation in which a check for diabetic retinopathy is included. If patients have proliferative diabetic retinopathy of any grade of severity or severe non-proliferative diabetic retinopathy, they should be told to avoid activities that increase intraocular pressure, such as weight lifting, jumping or high-intensity aerobics, due to the risk of vitreous haemorrhage or retinal detachment. Precautions should be taken to keep systolic blood pressure (SBP) from rising to 20 to 30 mmHg above the basal during each training session.
Diabetic nephropathyThe presence of microalbuminuria does not mean that patients should be restricted from practising sport. In more advanced stages, activities that increase SBP above 180-200 mmHg should be avoided (for example, the Valsalva manoeuvre, high-intensity aerobics or resistance exercises), because an increase in systemic pressure could potentially worsen the progression of this disease. In more advanced stages of kidney disease, patients should participate in lower intensity physical activities, since cardiorespiratory and health benefits have been observed with this level of exercise.
Special examinationsExercise stress test. What is it for?Table 2 shows cases in which a stress test is recommended, and it would be useful in the following situations:
- •
For a better evaluation of the sport to be performed. The intensity of the sport to be performed could be evaluated more accurately when the actual maximum heart rate or maximal oxygen uptake is determined based on a stress test, compared with an estimation of the target heart rate or work rate of the predicted calculations according to age. In addition, in cases where ischaemia or arrhythmias are induced at higher exercise intensities, the results of stress tests may be used to keep the exercise intensity below the ischaemic threshold.
- •
For risk stratification. Bear in mind that a low level of physical fitness and the presence of ischaemic changes on an ECG are associated with an increased risk of cardiovascular and overall morbidity and mortality.
- •
To detect previously unsuspected coronary heart disease.
- •
To detect abnormal hypertensive responses, and thus prevent them by recommending appropriate physical activities.
Spirometry is a way to test respiratory function at rest; it measures respiratory capacity and volume. It should be done systematically before a cardiopulmonary exercise test (ergospirometry). The values obtained at rest enable the maximum respiratory rate a person can reach to be predicted. Under normal physiological conditions, a person exerting their maximum effort should not reach their maximum respiratory rate without a reserve of approximately 20%. Spirometry also helps in diagnosing other respiratory diseases that may limit performance during exercise (asthma, obstructive pulmonary disease, etc.). Cardiopulmonary exercise testing (ergospirometry) provides a direct measurement of the body's oxygen uptake and CO2 production during exercise, and it is the most important test for determining a person's level of fitness. This testing also allows for monitoring cardiac output during exercise by means of continuous ECG recording and periodic blood pressure (BP) readings. By using all the data obtained during exertion that is made progressively more intense until it reaches maximum capacity, several aspects can be calculated: the aerobic threshold (the level of effort at which muscles produce lactic acid); the anaerobic threshold (the level of effort at which alveolar ventilation is not sufficient to compensate for the metabolic acidosis caused by progressively higher concentrations of lactate); and maximal oxygen uptake and actual maximum heart rate. It is essential to know at which heart rates the thresholds occur in order to design exercise programmes not only for elite athletes but also for people who exercise regularly. To improve performance in a resistance sport (running or cycling), it is advisable to train by using the intensities of effort that fall predominantly between the two thresholds. There are variations on training that include intervals above the anaerobic threshold, but these are meant to be used for competitive athletes.
Resting electrocardiogram (in all cases)As in any ECG, the following aspects must be assessed:
- •
Heart rate, and PR, QRS and QT intervals.
- •
The P wave morphology of the QRS complex and the T wave.
- •
Determination of the cardiac axis.
There are electrocardiographic abnormalities, rhythm control, blockages and ischaemic lesions that contraindicate the performance of a stress test.
RECOMMENDATIONS 1- •
People who have diabetes with possible related cardiovascular disease or microvascular complications and who wish to engage in any type of sport should undergo a medical evaluation, which must include a medical history, a physical examination (including a dilated eye examination, a foot examination and a check for neuropathy), a resting electrocardiogram and possibly a stress test.
- •
All patients considered to be at a high risk for cardiovascular disease should undergo a stress test (Table 2).
- •
A stress test is useful in multiple ways:
- ○
For assessing the sport to be practised.
- ○
For risk stratification.
- ○
For detecting unsuspected coronary heart disease.
- ○
For detecting abnormal hypertensive responses.
- ○
- •
A spirometry test should be done systematically before a cardiopulmonary exercise test (ergospirometry).
- •
An ECG must be performed in all cases.
- •
Patients with DM and cardiovascular disease must limit their exercise to low-intensity sports.
In recent years, there has been considerable interest in identifying the type and properties of exercise (or sport) that has greater benefits on glycaemic control in patients with DM. Table 3 shows a classification of the intensity of dynamic exercise.
Diabetes mellitus type 2 (DM2)Effect on blood glucose and insulin sensitivityAny kind of increase in daily physical activity has been associated with a short-term improvement in insulin sensitivity (IS).7 Moderately intense sport or aerobic exercise (AE) results in a drop of blood glucose that lasts between 2 and 72 hours afterwards.5 Based on the results of a recent meta-analysis of studies that used continuous glucose monitoring (CGM), this decrease mainly affects postprandial glucose levels.8 In clinical practice, lack of time is one of the arguments used to justify low adherence to a prescribed exercise. In this regard, short-term, high-intensity interval training (HIIT) has been shown to be an effective alternative to prolonged, moderately intense exercise,1 although, to date, there have been no studies that directly compare both types of training. Furthermore, although not commonly practised by patients with DM2, very intense aerobic sports may be associated with a transient increase in blood glucose (1-2 hours), which is due to an increase in catecholamine release.5
The practice of resistance sports or exercises (RE) has also shown satisfactory results and is considered as a very useful therapeutic option in a large percentage of patients who have trouble being active. In studies of CGM, researchers have seen an increase in IS and a decrease in blood glucose, which, as with AE, appears predominantly to affect postprandial glucose levels.5,7,8 Last, doing both AE and RE, or combined exercise (CE), might be more effective in increasing IS than practising an isolated aerobic activity.5
Effect on HbA1cSeveral meta-analyses have shown that both AE and RE have a beneficial effect by decreasing HbA1c levels by approximately 0.6%.9 A systematic review of 12 clinical trials comparing the efficacy of AE versus that of RE in patients with DM2 has recently been published. Although a greater reduction in HbA1c levels (-0.18%) was seen with AE, the authors conclude that the absolute differences are very small and of little clinical significance; they therefore recommend doing exercise regardless of which type is performed.10 The influence of the intensity, frequency and duration of exercise on controlling HbA1c levels has also been analysed. Doing more than 150 minutes a week of exercise and practising more intense AE have been associated with greater a decrease in HbA1c levels.9 In a recent meta-regression of clinical trials, the decrease in HbA1c levels seems to be related more to an increase in the frequency and duration of AE than to a higher intensity of the exercise itself.11 Last, the results of some studies suggest that CE may be superior with regard to decreasing HbA1c levels than AE or RE alone.10 Each weekly RE session combined with AE has been associated with an additional 0.02% drop in HbA1c levels.11
Diabetes mellitus type 1 (DM1)Effect on blood glucoseExercise-induced changes in blood glucose levels in patients with DM1 depend mainly on insulin levels when the exercise is performed. In patients with adequate plasma concentrations of insulin, a significant decrease in blood glucose levels occurs. This decrease is associated mainly with the practice of moderately intense AE, whereas with other exercises (RE, CE, HIIT) there have been conflicting results regarding blood glucose levels.12
Occasionally, the physical activity itself may cause a paradoxical increase in blood glucose levels in people with poor metabolic control and a hypoinsulineamia. High-intensity sports, the psychological stress of competition, and mistakes in insulin administration or carbohydrate supplementation may be associated with the onset of this increase.
Practising a sport, especially for children and adolescents, is associated with an increased risk of hypoglycaemia, which may occur during or after doing the sport. The time during which the sport is performed, as well as the type of sport, may influence the onset of hypoglycaemic events. In this regard, doing a sport towards the end of the day is associated with a two-phase increase in IS (during the exercise itself and from 7-11 hours afterwards) that may lead to an elevated risk of nocturnal hypoglycaemia.13 Also, doing a physical activity that lasts longer and is more intense is associated with an increased risk of hypoglycaemia, whereas practising exercises that combine AE and HIIT may be associated with a decrease in episodes of post-exercise hypoglycaemia.14
Effect on HbA1cIn contrast to the data on DM2, there is insufficient evidence to state conclusively that the practice of sport is associated with a significant improvement in HbA1c levels in patients with DM1. Consistent with some previous studies which suggest an inverse correlation between HbA1c levels and weekly hours of exercise, a recent systematic review suggests that doing a sport is associated with a significant decrease in HbA1c levels (-0.78%) and the units of insulin needed (-0.4 U/kg).15 In a previous meta-analysis, published in 2012, simply performing an AE regularly was associated with a significant drop in HbA1c levels (-0.23%), whereas an RE, CE or HIIT was associated with an insignificant decrease in HbA1c levels.12
RECOMMENDATIONS 2In terms of the practice of sport by patients with DM2, we should consider the following:
- •
Aerobic and resistance sports are associated with improved glycaemic control (postprandial blood glucose, IS, HbA1c).
- •
These changes are significantly associated with the duration, intensity and frequency of the exercise performed.
- •
Sports that combine aerobic and resistance exercises may be associated with greater benefits than aerobic or resistance sports done alone.
- •
HIIT can be an effective and safe alternative for certain types of patient.
In terms of the practice of sport by patients with DM1, we should consider the following:
- •
The performance of moderately intense aerobic sports is associated with a significant decrease in blood glucose levels in patients with optimal insulin levels. This decrease leads to an increased risk of hypoglycaemia during or after the sport.
- •
There are conflicting results with regard to the effect on blood glucose levels of other types of sport or exercise (RE, CE, HIIT). The practice of high-intensity sports under conditions of hypoinsulineamia or psychological stress may be associated with episodes of hyperglycaemia and ketosis.
- •
There is insufficient evidence to ensure that doing a sport is associated with a significant decrease in HbA1c levels. In the case of children or adolescents, the practice of an aerobic sport with longer-term exercise protocols may lead to a benefit in this sense.
Physical exercise and sport involve metabolic stress, and, in response to such stress, there is an endocrine- and metabolic-related component. In the initial stages, glucose plays a leading role.16 Glucose is converted from muscle glycogen, but once glucose is used up, hepatic glycogenolysis comes into play.17 The longer the exercise lasts (cycling, endurance running, etc.), the greater the glucose uptake into muscle, as muscle cells translocate GLUT4 receptors to cell surfaces, which is stimulated by physical exercise. This also decreases insulin secretion and increases catecholamines, which causes increased lipolysis; free fatty acids are then used as substrate for gluconeogenesis.
In sports where fast-twitch muscle fibres are often used (football, rugby, sprinting, etc.), most of the exercise takes place in anaerobiosis, mainly using lactate.18 This contributes to muscle fatigue, although tolerance develops with training. At the same time, hepatic gluconeogenesis is increased.
On finishing the exercise, insulin increases and catecholamines drop. This causes hyperglycaemia and hyperinsulinaemia, which contribute to muscle glycogen repletion and recovery from fatigue.16
In patients with an insulinopenic form of DM1 or DM2, the above sequence is reproduced, with the exception of insulin self-regulation. Therefore, nutritional management is essential for preventing metabolic imbalances.
Individual nutritional needsNo ideal and unique composition has been demonstrated in the evidence regarding the ideal percentage of calories that macronutrients should provide in patients with DM.19 Calorie distribution must be tailored to dietary habits, preferences and goals.
The current recommendations for athletes regarding carbohydrate (CH) intake vary according to the intensity (Table 3) and duration of training:20
- •
In cases of low-intensity exercise (30’-45’/ day), 3-5 g/kg/day are recommended.
- •
In the case of daily one-hour, moderately intense exercise, 5-7 g/kg/day are recommended.
- •
In the case of moderate to high intensity exercise lasting between 1 and 3 hours, a CH intake of 7-10 g/kg/day is recommended.
- •
For exercises that exceed this length of time, a CH intake of 10-12 g/kg/day is recommended.
In terms of protein intake, it is recommended that athletes in general consume 1.2-1.4 g/kg/day, and strength athletes16 1.2-1.7 g/kg/day for sports such as weightlifting or bodybuilding.
To date, there are no other recommendations for athletes in regard to other nutrients, and general recommendations should be followed.19
Nutrition before practising sportsSeveral studies suggest that diets with a low glycaemic index (GI) combined with physical exercise in patients with DM improve basal blood glucose levels, decrease insulin resistance, improve muscle endurance and promote the burning of fat.21
The benefits of carbohydrate-loading diets during the days before a competition to delay glycogen depletion have been shown with regard to events lasting longer than 90 minutes. These benefits have also been demonstrated in athletes with DM1.22 The usual diet consists of an intake between 8 and 12 g/kg of body weight/day of CHs during the three days before an event. This intake should account for 70% to 85% of energy in the diet. The benefit of this intake has not been shown in patients with DM2.
Drinking 5 mg/kg of caffeine before exercise (one espresso or two cups of coffee with milk) decreases the incidence of hypoglycaemia during and after exercising.23
Before starting a sport, capillary blood glucose should be measured. If it is below 100 mg/dl, taking a supplement of 10-20 g of slow-burning CHs is advisable in patients treated with insulin or secretagogues.2 In general, patients who are on other treatments and who have a low risk of hypoglycaemia do not require pre-exercise CH supplementation. If a patient's blood glucose level is below 70 mg/dl, practising a sport should be postponed until their levels rise above 100 mg/dl.
Nutrition during sportsSome recommended guidelines are shown in Table 4. As a general rule, we can say the following:
- •
In exercises that last less than 60 minutes it is essential to maintain a good state of hydration, preferably by drinking water.2 Drinks containing CHs should be avoided, unless the exercise is of high intensity. In these cases, CH supplements that provide 30-60 g of glucose would be suitable.
- •
In terms of exercises lasting from one to two and a half hours, in addition to maintaining good hydration, patients should take supplements that provide from 30-60 g of glucose per hour of exercise.
- •
In terms of exercises that last more than two and a half hours (marathons, triathlons, cycling, etc.), the recommendations are similar to those in the previous case. It is a good idea to mix sources of CHs by adding slow-burning CHs.
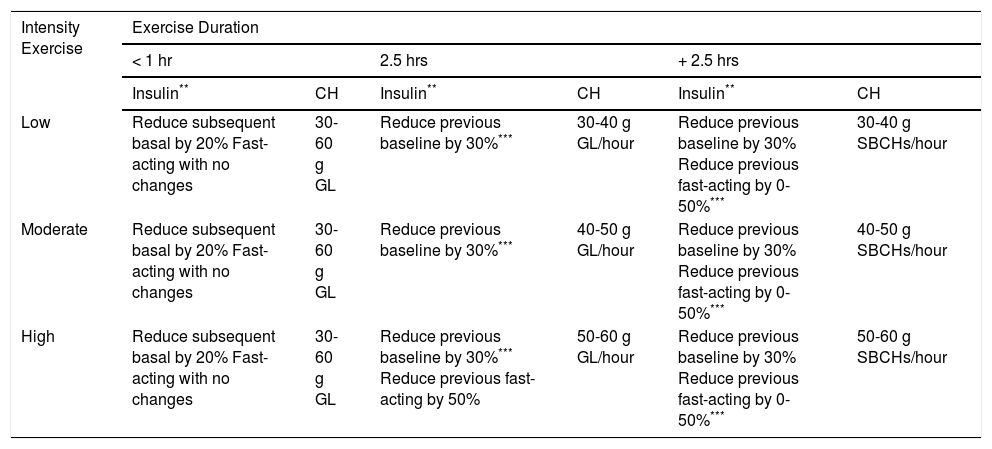
Adjusting insulin and carbohydrate supplements during sport*
| Intensity Exercise | Exercise Duration | |||||
|---|---|---|---|---|---|---|
| < 1 hr | 2.5 hrs | + 2.5 hrs | ||||
| Insulin** | CH | Insulin** | CH | Insulin** | CH | |
| Low | Reduce subsequent basal by 20% Fast-acting with no changes | 30-60 g GL | Reduce previous baseline by 30%*** | 30-40 g GL/hour | Reduce previous baseline by 30% Reduce previous fast-acting by 0-50%*** | 30-40 g SBCHs/hour |
| Moderate | Reduce subsequent basal by 20% Fast-acting with no changes | 30-60 g GL | Reduce previous baseline by 30%*** | 40-50 g GL/hour | Reduce previous baseline by 30% Reduce previous fast-acting by 0-50%*** | 40-50 g SBCHs/hour |
| High | Reduce subsequent basal by 20% Fast-acting with no changes | 30-60 g GL | Reduce previous baseline by 30%*** Reduce previous fast-acting by 50% | 50-60 g GL/hour | Reduce previous baseline by 30% Reduce previous fast-acting by 0-50%*** | 50-60 g SBCHs/hour |
GL: glucose
CHs: carbohydrates
SBCHs: slow-burning carbohydrates
Once the sport has been finished, blood glucose levels should be monitored, and, if they are less than 120 mg/dl, patients with DM1 or DM2 treated with insulin or secretagogues should take 15-20 g of low-GI CHs.24 For competitive athletes, the recovery period is very important, because muscle glycogen must be re-synthesised, which is an insulindependent process. These athletes should take 1-1.5 g/kg of CHs as soon as possible after finishing their exercises, since muscle glycogen replenishment is more effective that way.16
In sports where fast-twitch muscle fibres are used often, post-exercise hyperglycaemia can be a problem in the first 60 minutes following the end of the activity. These cases should be managed on an individual basis, by preventing subsequent hypoglycaemia, if necessary using an extra CH intake together with bolus insulin only if hyperglycaemia persists beyond those 60 minutes.16
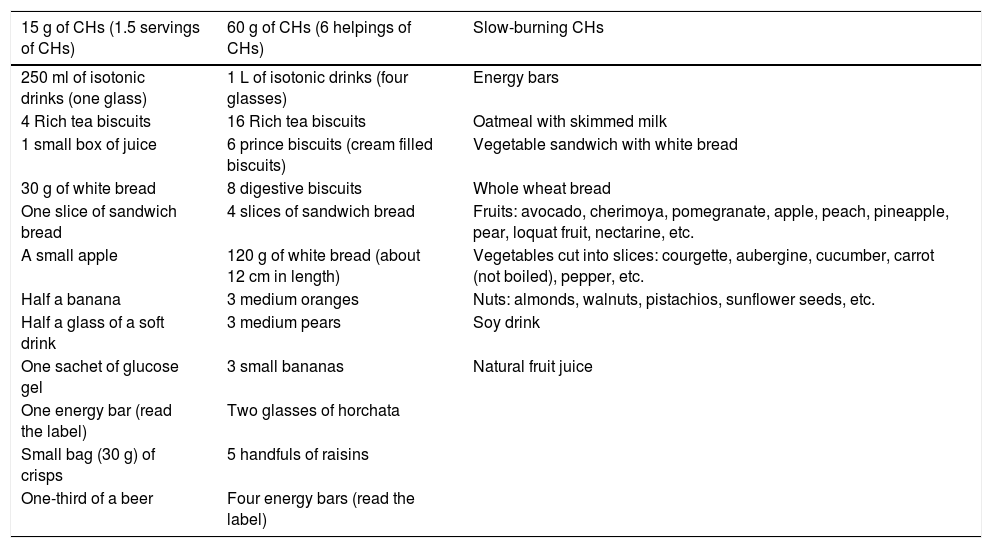
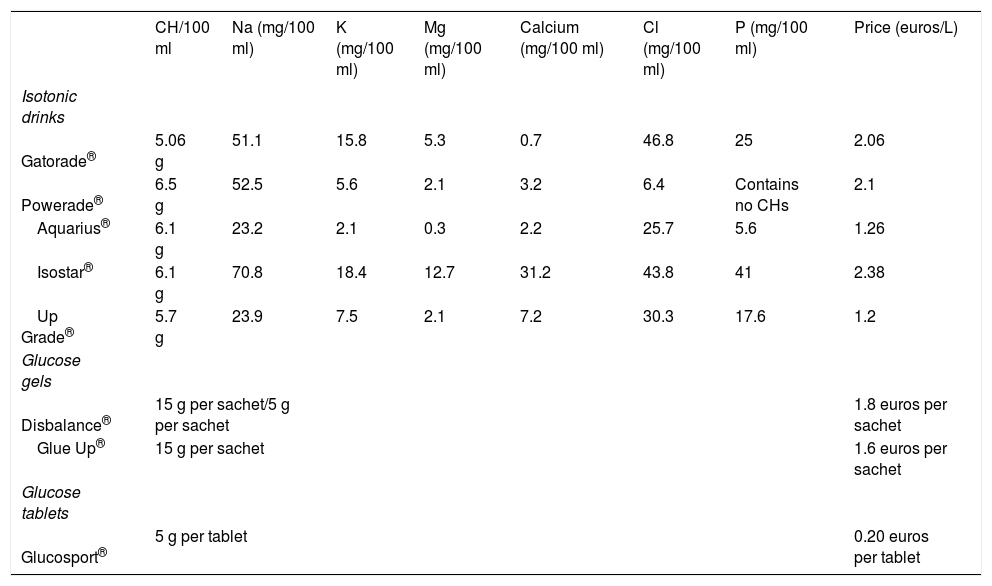
Products available on the marketIn addition to the traditional fruit supplements, there are other options for providing CHs: isotonic drinks, glucose tablets and glucose gels (Tables 5 and 6).
Isotonic drinks, also called sports drinks, are characterised by being isotonic or slightly hypotonic. They not only hydrate but also provide CHs. They contain no more than 10% CHs. They have not demonstrated favourable effects in exercises lasting less than 60 minutes, unless these exercises happen to be very intense.2 The composition of an isotonic drink is similar to that of traditional alkaline lemonade. The labels should be read, because the contents of the drinks vary.
There is a wide variety of glucose gels on the market with similar characteristics in terms of composition, but with different amounts of CHs, and so it is advisable to read the labels carefully. They should be taken slowly and not mixed with isotonic drinks at the same time, to prevent an overload of CHs. They are usually flavoured. They should be mixed with water, except for Gluc Up® and Diabalance®. These last-named gels contain 15 g of glucose per pack.
Glucose tablets are marketed in generic forms under this name or with brand-names, containing 5 g of CHs.
RECOMMENDATIONS 3- •
There is no ideal recommendation concerning macronutrients. General recommendations and a balanced diet should be followed.
- •
Patients who are on insulin or secretagogues should have their capillary blood glucose checked before exercising. If it is less than 100 mg/dl, a supplement containing slowrelease CHs should be taken.
- •
While practising a sport, it is important to maintain an adequate state of hydration.
- •
During a sport, CH supplements must be taken, depending on the intensity and duration of the sport, as shown in Table 4.
- •
After doing a sport, CH replenishment should be ensured, preferably by taking with low-GI CHs. If blood glucose levels at the end of practising a sport are below 120 mg/dl, 15-20 g of CHs should be taken.
- •
Persistent hyperglycaemia after exercise should be monitored.
The immediate and subsequent impact of physical activity on glycaemic control in people with diabetes is not easy to quantify and predict. It depends on individual factors (level of fitness, type of treatment, type of diabetes, etc.) and on the characteristics of the particular sport.25 Physical exercise leads to hormonal changes aimed at getting the energy needed for muscle contraction without impairing the availability of substrates for other vital organs such as the brain. The body adjusts in this way during physical activity and during the post-exercise recovery period. In fact, in the hours following exercise, major changes in glycaemic control may occur, in particular hypoglycaemia.26,27 The goal for people with diabetes is to see their treatment simulate these physiological changes, both during sports practice and in the recovery period.13
In addition to the type of diabetes, the type of drug treatment used is the most determining factor of the risk of exercise-induced decompensation, with insulin and oral hypoglycaemic agents (sulfonylureas and glinides) being the most important types of drug for practising sport.28 Despite their shorter half-life, there are no scientific data or clinical experiences showing that it may act differently with glinides.
Common clinical situations associated with sports/physical activityPhysical exercise in the context of hyperglycaemiaBy practising physical exercise, people with very low levels of insulin and ketosis may worsen their decompensation by stimulating the secretion of counterregulatory hormones.
Hypoglycaemia during physical exerciseThis risk is greater in cases of long-duration physical exercise and during the peak effect of insulin's action.
Late-onset hypoglycaemia after exerciseThis condition may occur hours later, and there are several possible triggering factors: excess insulin in the context of glucose intake, increased insulin sensitivity and peripheral glucose uptake (primarily in skeletal muscle) for replenishing stores. Patients with long-standing diabetes may be especially vulnerable due to the presence of autonomic neuropathy. In these cases, recovery from hypoglycaemia is especially impaired.
Changes in insulin absorptionPhysical activity may increase insulin absorption by stimulating the subcutaneous blood flow, lymphatic drainage and involuntary pumping due to muscle contraction. It is therefore advisable to avoid injecting insulin into areas that will be exercised immediately. Likewise, intense heat/cold (both local and ambient) may increase or decrease, respectively, insulin absorption.
Practical aspects to be consideredSelf-monitoring of capillary blood glucoseA glycaemic-response profile should be made the first few times the sport is practised. Based on the doctor's opinion, this may include the following:
- •
Pre-measurement.
- •
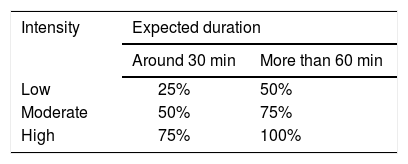
During the activity: every 20’-30’ if it is a moderate-high intensity activity (Table 3) or every hour if it is a low-intensity aerobic sport.
- •
At the end and during the post-exercise recovery period: every 2-3 hrs in the 8-12 hrs afterwards, before going to bed and at least one measurement during the following night.
No sport should be performed when the patient is hypoglycaemic (< 70 mg/dl). Nor should any sport be done in cases of clear hyperglycaemia (greater than 300 mg/dl or greater than 250 mg/dl with positive ketonaemia), especially patients with DM1.
Changes in insulin therapyLong-acting analogues should be used as basal insulin. A reduction of basal insulin is the most advisable option for minimising the risk of hypoglycaemia, but it is a recommendation that needs to be tailored and that may depend on changes in dietary intake and supplements on that day29 (Table 7). In general, many competitive sports (padel, indoor football, etc.) are anaerobic regardless of their intensity, and they generate stress and elevate counterregulatory hormones. Therefore, the dose of insulin should not be reduced before exercising, but should afterwards. On the other hand, if a sport is aerobic, the dose of insulin should be reduced before exercising, because it involves glucose consumption during the activity. Some recommended guidelines are given in Table 4. In general, the following can be said:
- •
For short-duration sports (< 60’), reduce basal insulin by 20-30% in the 12 hrs after exercising, regardless of the intensity of the sport. Generally speaking, when practising a high-intensity, short-duration sport, it is not necessary to change the rapid-acting insulin of the following dose (though basal insulin should be reduced in the 12 hours after exercising).
- •
For low to moderate intensity sports that last more than an hour and a half, especially if they are aerobic, reduce the dose of basal insulin by 20-30% before the sport is performed. The doses of rapid-acting insulin may also be adjusted, although these changes should be tailored according to any changes in dietary intake made just before or after exercise, and which may not be very effective in preventing post-exercise hypoglycaemia.30
- •
For aerobic sports, especially long-duration ones (more than two and a half hours), doses must be tailored. Some people with DM do not include rapid-acting insulin in their pre-exercise dose and try to start the activity with a blood glucose level greater than 180 mg/dl (see Table 7).
Example of changes in treatment for patients practising sports (real testimony): 42-year-old patient with type 1 diabetes mellitus; runs a half-marathon (21 km); on usual treatment with insulin glargine 0-0-18 IU and insulin glulisine 1 IU/serving of CHs
|
CHs: carbohydrates.
When doing physical exercise sporadically, it is impractical to try to modify the dosage of sulfonylureas due to their long half-life. Taking CH supplements before a physical activity, adjusted to the intensity, duration and blood glucose level at the start, may be the most advisable way to go. This also applies to glinides.
When starting a medium- or long-term physical activity, the following guideline may be used:31
- •
Patients with very strict control or a high risk of hypoglycaemia: reduce dose by 50% to 100%.
- •
Patients with moderate control: reduce dose by 25% to 50%.
- •
Patients with control above the target: reduce dose minimally or not at all.
- •
A glycaemic-response profile should be made the first few times a new sport is practised, while the intensity of the sport should be increased gradually.
- •
Long-acting analogues should be used as basal insulin.
- •
For a sport of moderate to high intensity that lasts a short time: pay special attention to the risk of hypoglycaemia in the post-exercise recovery period (self-monitor blood glucose levels in the following 12 hrs and reduce basal insulin in that period by at least 20% initially).
- •
For a sport of low intensity but which lasts a long time (> 2.5 hrs): self-monitor blood glucose levels during the activity, reduce the previous basal insulin by at least 30% and consume CHs that have a medium-high GI regularly.
- •
For patients on oral hypoglycaemic therapy who engage in sports regularly, the dose reduction may be as much as 100% (depending on the usual degree of control).
Patients with DM who practise sports should include the following material in their equipment:3,32
- •
Capillary blood glucose monitor and test strips. Make sure to check the expiry dates.
- •
Ketone body assay kit (in the blood glucose monitor with specific test strips or urine strips).
- •
Ensure an adequate amount of water for proper hydration.
- •
Fast-acting CHs: 10-30 g of CHs that must be administered every 30-45 minutes, especially during long-duration exercise. These CHs may be consumed in liquid or solid form, according to the athlete's preference. Different dosage forms are marketed, but foods such as fruits or biscuits may also be eaten (Table 5 and 6).
- ○
Isotonic drinks: these contain a proportion of sugars from 5-8%. They also provide electrolytes that help to replenish mineral losses. These are recommended for activities lasting more than one hour.
- ○
Refreshing drinks: they provide 10% of CHs. Coca-Cola soft drinks also contain caffeine, which may increase dehydration during exercise.
- ○
Energy drinks: these have a high CH content (> 10%) and substances, such as ginseng or taurine, that are thought to have effects against mental or physical fatigue. The high content of sugars and stimulants mean that they are not recommended as supplements during physical exercise.
- ○
Fruit juices: it is necessary to distinguish between natural and processed juices. Natural fruit juices have a relatively low CH content (between 4% and 6%). Some processed juices may be added to this group, i.e., those “with no added sugars”. Processed fruit juices that have added sugar have a CH content of approximately 10%.
- ○
Glucose tablets: these raise blood sugar more quickly. They should be taken very slowly and with plenty of liquids to facilitate their absorption.
- ○
Glucose gels: these contain a mixture of glucose (or other sugars) with water and fruit flavours that give the emulsion a more pleasant taste than that of glucose tablets.
- ○
Energy bars: they are usually made from cereals or flour, to which a certain amount of sugars or proteins is added. They fulfil a double function in regard to exercise, because in addition to maintaining blood glucose levels, they help to suppress the appetite in long-duration exercises. There are also dried-fruit bars.
Table 5.Examples of foods by carbohydrate intake
15 g of CHs (1.5 servings of CHs) 60 g of CHs (6 helpings of CHs) Slow-burning CHs 250 ml of isotonic drinks (one glass) 1 L of isotonic drinks (four glasses) Energy bars 4 Rich tea biscuits 16 Rich tea biscuits Oatmeal with skimmed milk 1 small box of juice 6 prince biscuits (cream filled biscuits) Vegetable sandwich with white bread 30 g of white bread 8 digestive biscuits Whole wheat bread One slice of sandwich bread 4 slices of sandwich bread Fruits: avocado, cherimoya, pomegranate, apple, peach, pineapple, pear, loquat fruit, nectarine, etc. A small apple 120 g of white bread (about 12 cm in length) Vegetables cut into slices: courgette, aubergine, cucumber, carrot (not boiled), pepper, etc. Half a banana 3 medium oranges Nuts: almonds, walnuts, pistachios, sunflower seeds, etc. Half a glass of a soft drink 3 medium pears Soy drink One sachet of glucose gel 3 small bananas Natural fruit juice One energy bar (read the label) Two glasses of horchata Small bag (30 g) of crisps 5 handfuls of raisins One-third of a beer Four energy bars (read the label) CHs: carbohydrates.
Table 6.Composition (in CHs) of supplements most commonly used in sport
CH/100 ml Na (mg/100 ml) K (mg/100 ml) Mg (mg/100 ml) Calcium (mg/100 ml) Cl (mg/100 ml) P (mg/100 ml) Price (euros/L) Isotonic drinks Gatorade® 5.06 g 51.1 15.8 5.3 0.7 46.8 25 2.06 Powerade® 6.5 g 52.5 5.6 2.1 3.2 6.4 Contains no CHs 2.1 Aquarius® 6.1 g 23.2 2.1 0.3 2.2 25.7 5.6 1.26 Isostar® 6.1 g 70.8 18.4 12.7 31.2 43.8 41 2.38 Up Grade® 5.7 g 23.9 7.5 2.1 7.2 30.3 17.6 1.2 Glucose gels Disbalance® 15 g per sachet/5 g per sachet 1.8 euros per sachet Glue Up® 15 g per sachet 1.6 euros per sachet Glucose tablets Glucosport® 5 g per tablet 0.20 euros per tablet CHs: carbohydrates.
- ○
- •
Emergency kit with glucagon.
- •
Medical alert bracelet.
- •
If travelling in order to practice a sport, it is a good idea to have a special bag ready for regular use. The bag should contain the usual medicines, a blood glucose monitor with test strips, needles and lancets, information on where to go in an emergency and a copy of one's diabetes management plan. Batteries or chargers for the capillary blood glucose monitor.
- •
For people treated with continuous insulin infusion systems (insulin pumps), it is advisable to have replacement parts on hand (spare catheters, reservoirs, etc.).
- •
Footwear suitable for sports and cotton socks. Feet should be cared for appropriately, by staying hydrated and by watching for any irritations, blisters and wounds.
- •
Weather-appropriate clothing is needed, wearing clothes that keep the body dry. Material such as polypropylene and silk help to absorb sweat and also prevent irritation.
- •
It is important to carry contact information.
- •
Hypoglycaemia in patients who practise sports is a common complication that may occur for different reasons: excessive doses of insulin or oral hypoglycaemic agents (secretagogues), mistakes in calculating the doses, increase in the intensity or duration of the exercise, insufficient or delayed food intake, and alcohol intake during or immediately after exercise.33,34
- •
Athletes should be able to recognise the symptoms early and know how to treat them. It is important to know that the symptoms of hypoglycaemia are not specific and may vary from person to person.
- •
Athletes and their activity partners should receive appropriate training to treat episodes of hypoglycaemia.1
Strategies to prevent hypoglycaemia include:34
- •
Capillary blood glucose monitoring.3,34 This must be done according to the guidelines in Section 4.
- ○
Athletes who practise sports in extreme temperatures, at high altitudes or who have experienced late-onset hypoglycaemia (6-24 hours after the end of exercise) may require additional measurements. In this case, blood glucose should be measured at 30-minute intervals during exercise, if possible, and every 2-4 hours after exposure. In cases where there is a history of nocturnal hypoglycaemia, blood glucose levels should be measured before going to bed and at least once during the night.
- ○
Exercise should be avoided for 24 hours after an episode of hypoglycaemia because of the risk of a relapse of hypoglycaemia.
- ○
- •
CH supplementation. This is explained in Section 3.
- •
Drug-treatment adjustments per Section 4.
- •
10-second sprint before or after exercise. The findings of some studies on a small number of DM1 patients suggest that a 10-second sprint, right before or after a moderately intense sport, decreases the risk of post-exercise hypoglycaemia for about 60’ without affecting the risk of late-onset hypoglycaemia.35
Treatment will vary depending on the severity of the hypoglycaemia.36,37
- •
Mild hypoglycaemia (blood glucose < 70 mg/dl, and with the athlete aware of it and able to treat it):
- ○
Stop the sporting activity.
- ○
Administer 10-15 g of fast-acting CHs (4 glucose tablets, half a cup of juice or a glass of milk).
- ○
Measure capillary blood glucose level and repeat after 15 minutes.
- ○
If blood glucose levels remain low, repeat the intake of 10-15 g of fast-acting CHs and measure blood glucose again after 15 minutes.
- ○
If low blood glucose levels persist, activate the emergency medical system by calling a medical service.
- ○
If blood glucose has normalised, take slow-burning CHs.
- ○
- •
Severe hypoglycaemia (athlete is unconscious or does not respond to guidelines):
Activity partners should be alerted to the condition of the diabetic athlete and trained to take the following actions:
- ○
Activate the emergency medical system by notifying a medical service if available; otherwise, do the following:
- ○
Use glucagon (1 mg given subcutaneously or intramuscularly).
- ○
If the patient's glycogen stores have been depleted by intense exercise, glucagon will not be effective and an intravenous glucose supply will be required.
- ○
Once the patient is able to swallow, give them some food.
- ○
- •
Clear hyperglycaemia in athletes occurs mainly in patients with DM1 and generally responds to low levels of circulating insulin.
- •
It may also be related to an inadequate insulin administration, excessive intake, inactivity, disease, stress or injury.
- •
If pre-exercise blood glucose levels are greater than 250 mg/dl, measure ketone bodies in blood or urine. If the test is positive, do not practise the sport until the levels are normalised.
- •
A significant reduction in blood glucose levels is recommended to prevent ketoacidosis by administering additional doses of rapid-acting insulin.
- •
If blood glucose levels are between 250-300 mg/dl and there are no ketone bodies, the sport may be done, as long as blood glucose is monitored every 15 minutes until capillary blood glucose drops. Sports avoid should be avoided if blood glucose levels are greater than 300 mg/dl in patients with DM1.
- •
For patients with DM2, exercise should be avoided if blood glucose levels are greater than 400 mg/dl.
- •
For patients with diabetes to practise a sport, some essential materials are necessary, including a glucose monitor and fast-acting CHs, among others, without which no sport should be started.
- •
To prevent hypoglycaemia, it is essential to monitor capillary blood glucose levels and to adjust not only CH supplements but also doses of hypoglycaemic drugs. For DM1, it may be useful to perform a 10-second sprint before or after a sport.
- •
Treating hypoglycaemia requires athletes to take CHs and check capillary blood glucose levels every 15’. To manage severe hypoglycaemia, a trained partner must accompany the athlete.
- •
Capillary blood glucose monitoring before exercising will help to detect hyperglycaemia and prevent diabetic ketoacidosis.
- •
Athletes with DM1 and blood glucose levels greater than 250 mg/dl and ketone bodies or with blood glucose levels greater than 300 mg/dl without ketone bodies, as well as athletes with DM2 with blood glucose levels greater than 400 mg/dl, should not start a sporting activity.
Treatment with continuous subcutaneous insulin infusion (CSII) provides clear advantages to patients with DM1 who regularly or sporadically practise sports, because CSII allows for continuous and more finely tuned dose adjustments.38,39 Patients who practise sports should receive specific training, including how to adjust the basal and prandial insulin doses needed before, during and after exercise. If patients also routinely or occasionally use CGM (with or without combined insulin infusion), they should receive specific training so as to act according to their actual and predicted blood glucose levels.
Insulin adjustments to prevent hypoglycaemiaDuring sport- •
Adjustments to bolus insulin.
If the exercise is meant to start while the bolus insulin is expected to act, do not decrease the bolus insulin dose. Since most patients use fast-acting insulin analogues, this period of time is 2-3 hours. The percentage decrease in the preprandial bolus dose varies according to the duration and intensity of the physical exercise to be performed, and a decrease in the bolus dose has been suggested,38,40 as shown in Table 8. Because all insulin pumps have a built-in bolus calculator, it should be calculated by using the usual method and then applying the estimated percentage reduction. Some models of pumps have exercise variables 1 and 2 built in to the calculator, and the percentage by which to decrease the bolus can be programmed for each type of exercise. A decrease of 20-50% for low-intensity exercises and 50-100% for moderate- to high-intensity exercises is recommended.
- •
Basal rate settings
- ○
All insulin pumps are made so that patients with DM can temporarily decrease the dose of basal insulin (temporary basal rate). In general, the dose of basal insulin should be decreased during exercise by 20-50%. A percentage reduction in the basal rate has been proposed based on capillary blood glucose levels 60 minutes before starting the exercise (Table 9). To achieve an effective decrease in insulin levels during exercise, the dose should be lowered between 60 and 90 minutes before starting physical activity.38,41
Table 9.Percentage reduction in basal rate in continuous subcutaneous insulin infusion before planned exercise
Capillary blood glucose (60 min. before) Reduction of basal rate CH supplements < 70 mg/dl Decrease by 50% Take 10-20 g (without bolus) 70-150 mg/dl Decrease by 30-50% As needed, if taken 1/2 bolus > 150 mg/dl Decrease by 20-30% Not required CHs: carbohydrates.
- ○
For children and adolescents, discontinuing an insulin infusion during one hour of moderate exercise has been shown to decrease the risk of hypoglycaemia, thus increasing the frequency of hyperglycaemia but without causing ketosis.42
- ○
If the exercise has not been planned, capillary blood glucose levels should be checked when the exercise is started, and the basal rate should be decreased accordingly (Table 10). In this case, CH supplements may be necessary, given that the dose reduction will be done once the exercise has started.
Table 10.Percentage reduction in basal rate in continuous subcutaneous insulin infusion before unplanned exercise
Capillary blood glucose/mg/dl Reduction of basal rate CH supplements < 70 Decrease by 70-80% Take 20 g (without bolus) 70-150 Decrease by 50% Take 10-20 g (without bolus) > 150 Decrease by 30% Not required CHs: carbohydrates.
- ○
At the end of the exercise, blood glucose should be checked, and the insulin pump should be resumed if blood glucose levels are greater than 100-120 mg/dl. If the levels are < 100 mg/d, it is recommended to continue with a temporary basal rate of 70-80% for the next 2-4 hours. The more intense the activity, the longer this reduction should be maintained.
- ○
Late-onset hypoglycaemia after sports is very common and can occur up to 24 hours later, owing to an increase in insulin sensitivity; however, most cases of hypoglycaemia occur between 2.5 and 12 hours after aerobic exercise.41
For the sake of prevention, the dose of basal insulin should be decreased the night following the performance of the sport. The percentage and time of the decrease of the basal dose depend on the intensity and duration of the sport, and there is little evidence supporting any specific recommendation.
A 20% reduction in the basal rate for 6 hours (from 9 p.m. to 3 a.m.) was shown to cause a significant decrease in nocturnal hypoglycaemia in a group of 16 adolescents after 1 hour of aerobic exercise in the afternoon.43
Insulin adjustments to prevent post-exercise hyperglycaemiaEarly-onset hyperglycaemia may occur after an intense physical activity; this is more common after anaerobic exercise. If capillary blood glucose levels after exercise are elevated (greater than 250 mg/dl), a correction bolus should be administered, decreasing it to half the dose calculated with the usual sensitivity factor, given the increase in insulin sensitivity that occurs after exercise.
Temporarily disconnecting the pump during sportsIn some sports, such as swimming and diving, the pump needs to be stopped and disconnected from the catheter.
Depending on how long the pump is stopped, the patient may need to take an extra dose of insulin before disconnecting. The following algorithm has been suggested:44
- •
Short-term temporary stop (< 3 hours): calculate the basal rate that would occur in the hours during the disconnection, and administer it in a bolus before disconnecting:
- •
Temporary disconnection of moderate duration (3-9 hours). Two options:
- ○
Every 3 hours, reconnect the pump and use a bolus with the dose calculated according to the previous formula.
- ○
Use a total dose calculated with the above formula of NPH insulin or insulin detemir, according to the expected length of disconnection. This dose should be given one hour before disconnecting the pump.
- ○
Once this theoretical dose has been calculated, a reduction of 20-50% should be applied, as when setting a temporary baseline rate. In sports where hypoglycaemia can be especially dangerous, such as diving or climbing, this initially calculated dose may be reduced somewhat more.
Utility of real-time CGMReal-time CGM is a tool with great potential to help maintain normal blood glucose levels during sports, although its utility has been questioned because of the time (10-15 min.) needed for sensor glucose to equilibrate with plasma glucose, which may be higher during the periods of rapid change in glucose concentrations that occur during exercise. One recent study showed that the sensor's accuracy (Medtronic's CGM system) is better during exercise than during rest.45 This is probably due to an increase in subcutaneous blood flow, which causes plasma and sensor glucose to equilibrate earlier.
Insulin pumps with automatic shut-off in hypoglycaemiaThe use of insulin pumps that automatically shut off the insulin infusion on detecting hypoglycaemia (Paradigm VEO) has been shown to reduce the severity and duration of nocturnal hypoglycaemia46 and exercise-induced hypoglycaemia,47 without causing significant rebound hyperglycaemia.
The blood glucose value at which the pump stops automatically should be tailored to each patient, depending on the frequency and severity of the hypoglycaemia. Usually the shut-off threshold is set between 60-70 mg/dl, but it may be appropriate to set it at higher values (80-90 mg/dl) during sports that may be life-threatening if hypoglycaemia occurs (mountaineering, skiing, cycling, etc.).
Also, some CGM systems provide patients with information not only on their actual blood glucose levels but also on the trend of the levels, by estimating the rate at which their glucose falls or rises, and therefore the chances of having hypoglycaemia and hyperglycaemia. An algorithm for the intake of fast-acting CHs has been proposed48 if during exercise (even without hypoglycaemia) there has been a rapid or slow decrease in glucose. This algorithm also takes into account actual blood glucose levels (Table 10).
Technical issuesWhile practising sports, the same areas for inserting a catheter can be used normally, avoiding areas restricted by clothing or materials used during the sport performed. In contact sports, it is safer to insert the catheter closer to the umbilical area because it will be better protected there from any inadvertent jerking or pulling. The area around the hips and thighs may also be used, while bearing in mind that exercise may increase the absorption rate in these areas in a more intense way.
Using pumps at high altitudesIn any sport that involves climbing high mountains or any activity at high altitudes, people with diabetes must watch for the presence of bubbles in the catheter or the insulin reservoir. The changes in pressure that occur in an ascent can cause the air that normally dissolves in the insulin solution to escape and form bubbles or increase any bubbles already there. The presence of large bubbles displaces the insulin in the catheter and causes errors in insulin administration, thereby promoting both hypoglycaemia and hyperglycaemia. To prevent these problems, all visible bubbles in the catheter and reservoir must be removed prior to an ascent, and one should check for bubbles every 1000 meters during the ascent.
In high mountains, a delay in the gastrointestinal absorption of CHs has also been reported, which promotes postprandial hypoglycaemia. To avoid this, it may be useful to give preprandial insulin by using a square- or dual-wave bolus.
It is a good idea to carry the pump in a way that protects it from the cold, holding it against the skin to maintain a suitable temperature that prevents the insulin from freezing.
RECOMMENDATIONS 6- •
If planning on doing a physical activity two to three hours after a preprandial or correction bolus, the dose should be reduced by 25-100% depending on the duration and intensity of the exercise to be performed (Table 8).
- •
If the sport has been planned, the dose should be reduced 60-90 minutes before the basal rate. We recommend setting a temporary basal rate of 50% in the first sessions; then the rate will be adjusted according to the blood glucose level obtained with this guideline. Alternatively, reduce the basal rate according to the capillary blood glucose level obtained one hour earlier (Table 9).
- •
If the sport has not been planned, capillary blood glucose levels should be checked, and the basal rate should be decreased by 30-70% (Table 10).
- •
To prevent nocturnal hypoglycaemia, we recommend setting a temporary basal rate at night, thus reducing the usual dose by 10-20%.
- •
The automatic shut-off threshold should be tailored to patients with pumps that have this option, and we suggest a threshold that is higher than usual (80-90 mg/dl) for patients doing high-risk sports.
Training is a planned and complex process that involves progressive and increasing workouts that stimulate the development of different physical capacities (resistance, strength, speed, flexibility and others) to promote and enhance sporting performance. Its effects are reversible.
- •
A workout is the training done in each session, and this is the stimulus that sets in motion the mechanisms of the body's adaptation. A workout has to start small and be increased little by little; it has to be progressive so that the stimulus continues and the training can be effective (with tolerance to greater workouts and improved performance).
- •
Scheduled rests or recovery periods are essential for the training to be fully effective, because rest and recovery consolidate the adaptation changes made by the workout.
- •
The training volume is the amount of training performed (total of exercises and repetitions in each workout). This relates to the total amount of time spent training.
- •
The intensity of the training is the quality of the workout, and it is related to the speed at which movements/exercises or sets are performed, with rest intervals between sets or, in the case of strength training, with changes made to the weight to be lifted. The intensity of exercise can be expressed in numerous ways (Table 3). At the user level the most commonly used way, owing to its simplicity, is to calculate a person's maximum heart rate (MHR) by using the following formula: MHR = 220 - age (which, however, is not the one that best relates to oxygen uptake and it can be calculated by other equations and methods). In strength training, intensity is defined mainly by the % of the maximum force (a test that defines the maximum weight that can be lifted).
- •
The training should progress gradually to let the body adjust properly, thereby preventing undesirable effects (aches, cramps, muscle pain, tachypnoea) and minimising the risk of injury. The rate of progression will be slower when the physical fitness level is lower at the outset. As a general rule, people should start with a low intensity, first increasing the frequency (number of sessions/week), then the volume of exercise and time of each session, and finally the intensity. For people with DM, in addition to the initial physical fitness level, their clinical condition must be taken into account (e.g., history and stability of the disease, existence and type of complications and related drug treatments).
Each training session should consist of 3 stages: first, warm-up; second, core training (where workouts are done); and third, cool-down and stretching.
Recommendations for training with regard to adults with DMThese recommendations include the stance taken jointly by the American Diabetes Association (ADA) and the American College of Sports Medicine for people with DM2 who wish to practise sports.5 More specific studies of people with DM1 are needed to be able to make evidence-based recommendations, but we have recently published results, and there are many studies in progress. Many of the recommendations can be extrapolated to general training guidelines and from the data derived from studies on DM2.
The benefits of exercise are greater if aerobic exercises are combined with strength training (for example, performing each group of exercises every other day) than if only one group is done.5,2 The benefits are even greater if, in addition to exercise (i.e., structured physical activity), unstructured physical activity is increased (walking, climbing stairs, leisure activities, etc.).2,4,5
Cardiorespiratory endurance training (aerobic workouts)- •
Type of exercise. It is a good idea to choose to do aerobic exercises that involve several muscle groups working together over long periods (swimming, running, walking, cycling). These exercises are essential for getting into good physical shape, and they have significant cardiorespiratory benefits. They have an effect on blood glucose levels during exercise (especially if they last a long time).5,49
- •
High-impact aerobic exercises (athletics, running, jumping, basketball, volleyball, alpine skiing, high-impact aerobics) may be associated with an increased cardiovascular risk (to be taken into account for patients with long-standing DM and/or complications), a higher risk of injury (especially if the patient is overweight) and less adherence.5,50
- •
Frequency, duration and intensity: A minimum of 3 days/week (no more than 2 consecutive days without activity), for 150 min./week with at least a moderate intensity (50-70% MHR, Table 3) (level of evidence A, ADA 20152). If doing high-intensity exercise (> 80% MHR), 75 min./week may be sufficient, since the same benefits are obtained in less time.5
- •
Progression. There are no specific studies of progression in DM, but it seems logical to assume the general progression guideline to be valid. Follow the training cycle of each session (warm-up, core training, stretches), and start the core with light exercises (< 40% of MHR).2,5 For example, 1-2 sessions/week for 20-40 min. × 2-6 weeks;5 increase first in frequency, then in volume and duration, and finally in intensity (MHR).
- •
Type of exercise. Patients with DM should perform exercises that strengthen a muscle group by lifting weight or using resistance training. These exercises can make use of the body itself as resistance or different types of equipment (elastic bands, weights, bars or machines that provide resistance by pulleys, hydraulic cylinders or electromagnetic systems) to work all the muscle groups. Breathing should be coordinated well with the muscular movement, and the Valsalva manoeuvre should be avoided if health-oriented strength exercises are being performed. Caution should be used with exercises in cases of apnoea, and proper control of BP is essential. This type of exercise has little impact on blood glucose levels during exercise (except that large muscle groups such as the quadriceps are worked). Each training session should consist of several exercises (each with several repetitions) for each muscle group, with a rest of 1-2 minutes after each set of repetitions.
- •
Frequency, duration and intensity. A minimum of 2 sessions per week (preferably 3) on non-consecutive days is recommended. Add aerobic exercise in between on the days of rest from strength training (recommendation level A of the ADA for DM2 and increasingly robust data on DM1).49,51 The final objective, in the absence of contraindications, is as follows: 75-80% of maximum strength,5 which is the figure reported to have the best cardio-metabolic benefits.49,51 For each muscle group, ensure a recovery period of at least 48 hours (essential for anabolic repair).
- •
Progression. Most plans begin with adaptation exercises with very little or no weight, and these plans must progress very slowly by first increasing the weight and then the number of repetitions. Each session should consist of 3-10 repetitions of 3-10 exercises, which should cause the muscle group that is being worked to come close to tiring out. A good goal might be to progress up to 3 weekly sessions of 8-10 exercises with 8-10 repetitions performed at 75-80% maximum strength in 6 months’ time.5
This can be included in a physical exercise programme, but it should not replace other training. Flexibility training increases the range of motion around the joints, which facilitates technique, helps to achieve goals and decreases injuries from strength exercises and from some aerobic exercises (swimming, skiing). Adding flexibility training to a programme increases effectiveness in regard to seeing benefits from physical exercise.2,5
RECOMMENDATIONS 7For people with DM, training should include advice from the different professionals involved in the physical activity, as well as from the professionals on their therapeutic team. Practice has to be tailored (which does not mean that it cannot be done in homogeneous groups).
- •
Do any aerobic exercise, with a minimum final goal of 150 min./week and at least a moderate intensity (> 50-70% MHR). The same benefits can be achieved in less time if patients set more intense goals (75 min./week at a MHR > 80%).
- •
Do strength training: 2 sessions/week (preferably 3) on non-consecutive days, with a final goal of 75-80% maximum strength (unless contraindicated).
- •
If possible, combine aerobic exercises with strength exercises every other day, as this combination increases the benefits of exercise.
- •
Whenever possible, include flexibility exercises with aerobic and strength exercises (but do not use them as a substitute). This allows for goals to be reached earlier and in a better way, so that benefits may be obtained more efficiently.
- •
Whenever possible, do more unstructured physical activity, because it leads to extra health benefits.
- •
Progress through these exercises slowly and gradually. There are no specific studies of progression for DM patients, and so it is best to follow general standards of progression. Progression will be slower when a patient's level of physical fitness is low, always depending on their clinical condition.
Nowadays in Spain the practice of diving in relation to patients with diabetes is not clearly defined in the current legislation, so that DM could be interpreted as a relative contraindication. In other countries around the world, such as the United Kingdom, there are practically no limitations; so, patients with DM may dive outside Spain.
Patients with DM who want to dive must deal with two fundamental hazards: a possibly greater predisposition to decompression sickness due to peripheral vasculopathy, and the risk of hypoglycaemia. In addition, hypoglycaemia while diving involves the added risk of drowning, and the condition is more difficult to notice underwater than at the surface or it can be confused with the symptoms of a deep diving (dizziness, fatigue, cold tremor, mild confusion, etc.). In spite of this, different studies have shown that patients with DM can dive safely if they always follow a series of conditions. In 2005, Divers Alert Network52 established a series of standards for subjects with DM who wish to dive; there are also Swedish recommendations in this regard.53Table 11 summarises the recommendations based on both publications.
Recommendations for patients with diabetes who wish to dive
Criteria to be met before diving
|
Type of diving to be performed
|
Blood glucose management on the day of the dive
|
To prevent decompression sickness, good hydration is very important, and subjects with DM should readjust their dive computers to more conservative safety limits. Still, the limited evidence available does not support a higher risk in this situation for people with DM.
Aspects to be taken into account by mountaineersThere are no data that contraindicate the practice of mountaineering in regard to people with well-controlled DM1 or DM2 with no chronic complications, even at altitudes above 5000 meters. Note that at altitudes, however, people with DM are at a greater risk than other mountaineers of dehydration, hypothermia (due to hypoglycaemia and impaired thermogenesis), frostbite or cold-related injuries (due to vascular problems and neuropathy). Below are some specific aspects of this sport.
- •
Altitude sickness (AS): no increased risk of AS has been reported in patients with DM1 or DM2. Acetazolamide, occasionally used to prevent AS, is not recommended in patients with DM1 due to a theoretical risk of acidosis, though there is no evidence of this risk. The treatment and prevention of AS, as well as of possible pulmonary or cerebral oedema, must be the same as for people without DM.54
- •
Glycaemic control: high altitudes decrease caloric intake due to an appetite-suppressing effect. Nevertheless, and in spite of an increase in energy expenditure, at altitudes above 3500-4000 meters, insulin requirements and blood glucose levels are increased, possibly due to the effect of the counterregulatory hormones that are released at altitudes,55,56 and so decreasing the dose of insulin or oral drugs in mountaineers with DM is not recommended. Furthermore, above 5000 meters gastric emptying is delayed, which can lead to postprandial hypoglycaemia and late-onset hyperglycaemia; prandial insulin should therefore be given after eating. It should be borne in mind that cold can reduce the subcutaneous absorption of insulin and contribute to increasing insulin needs. The symptoms of hypoglycaemia may be confused with typical AS, and so a target of 110-220 mg/dl should be maintained with frequent self-tests and supplements taken every hour. After a day of heavy physical exertion, glycogen stores may have been depleted, and glucagon administration would be ineffective.
- •
Dehydration: to prevent dehydration (caused by hyperglycaemia and hyperventilation at altitudes), patients must drink plenty of fluids (more than 4 litres/day).
- •
Frostbite: there is an increased risk of frostbite due to the trouble perceiving the cold caused by possible neuropathy. Frostbite should be prevented by means of proper nutrition, hydration, monitoring the feet daily and proper footwear and gloves.
- •
Retinopathy: the onset of asymptomatic retinal haemorrhage, which resolves spontaneously, has been described in patients with underlying diabetic retinopathy. It is therefore advisable to have an eye examination before and after climbing to high altitudes. This can be prevented by taking the ascent slowly.57
- •
Practical aspects: insulin and glucagon should be kept from freezing by storing them in bags attached to the body.57 The ascent itself can cause bubbles to appear in the insulin, and these must be purged. Needles should be removed from the pen after injections, as variations in pressure may cause the fluid to be expelled and alter the consistency of the insulin solution. At high altitudes, glucose monitors may show readings that are either under- or over-estimated, but with no significant variation.58 Test strips, batteries and glucose monitors should also be kept from freezing.
Regarding people with diabetes who sail, the risk of hypoglycaemia must be taken into account, mainly for those who pilot small boats with only two-crew members (only 1 partner) and where a greater physical effort is required (dinghy sailing). In these cases, a drop in blood glucose must be expected, and so patients with DM may either reduce the dose of basal insulin or take supplements before sailing. Checks should be done prior to sailing, and the activity should not begin if blood glucose levels are below 120 mg/dl. People with diabetes should take CH supplements and self-monitor while sailing. The partner should be trained on how to give a glucagon injection if necessary. Going solo is not recommended.
All equipment required for glycaemic control (insulin, reflectometers, test strips, glucagon, etc.) should be placed in a sealed and buoyant pack; to prevent moisture, add desiccants. Because this pack may be exposed to the sun and reach high temperatures, it should be refrigerated.
Aspects to be taken into account by cross-country runners/skiers/cyclistsIn endurance sports lasting more than one hour or even several hours, there are a number of things to keep in mind: an increased risk of hypoglycaemia (during the activity itself and for several hours after finishing), possible dehydration and a risk of heat stroke or hyperthermia.
In endurance sports, the risk of hypoglycaemia (in people on hypoglycaemic drugs) that is common to all sports is increased because of the duration of the activity. Further, the depletion of muscle glycogen makes it very likely that late-onset hypoglycaemia will occur within several hours of stopping the sport. It is therefore necessary to take CH supplements and to check blood glucose levels on an hourly basis during the activity. The glycaemic target during exercise is thought to be blood glucose levels of 120-180 mg/dl.59 The CH intake needed to maintain this figure varies considerably and would be between 30 and 60 g/hour,60 but always based on the results of the checks. The following should be carried during the activity: reflectometer, test strips, fast-acting insulin and CH supplements.
The precise dosage adjustments are those discussed above in Section 4.
Hyperglycaemia may also occur if the exertion is very intense, due to an adrenergic discharge. Before starting, if blood glucose levels exceed 250 mg/dl (in DM1), ketosis should be ruled out, while there is no contraindication in well-hydrated DM2 patients.5
These sports increase hydration needs; therefore, if there is osmotic diuresis caused by hyperglycaemia, dehydration may occur. People with DM should drink between 0.4 and 0.8 L of water or isotonic drinks per hour. CHs may be added if the exercise lasts more than one hour.34 People with DM should also try to limit sweating by avoiding wearing too many layers and using ventilated clothing.
If practising in high temperatures, symptoms of heat stroke can occur. There are some data suggesting that adults with DM2 as well as those with DM1 might have more difficulty than patients without DM in releasing the heat generated by sustained exercise, possibly due to microvascular changes. These patients should therefore use special protection against the heat (wearing caps to keep the sun off, cooling off as frequently as possible, staying hydrated, avoiding the hottest times of the day, wearing breathable and ventilated fabrics, etc.).
Aspects to be taken into account by swimmersSwimmers with DM who are on hypoglycaemic treatment should be especially careful to prevent hypoglycaemia, since it might lead to a risk of drowning. Also, given the difficulty of performing self-monitoring while swimming, they lack readings that would alert them to any problems. Blood glucose levels should therefore be above 180 mg/dl before entering the water, as recommended for diving. If swimming for more than half an hour, CH supplements should be taken (in glucose gels that can be stowed in a bathing suit). The activity should be stopped at the slightest symptom of hypoglycaemia; the swimmer should take CHs and get out of the water quickly.
It is a good idea for swimmers to be constantly monitored by someone who knows their condition. After swimming, blood glucose levels should be checked and a CH supplement taken if necessary.
RECOMMENDATIONS 8- •
Divers: start at a blood glucose level ≥ 180 mg/dl. With any suspicious sign of hypoglycaemia: alert the partner and do not dive. Dive only according to the recommended safety margins for patients with DM. Go with a partner who is informed and trained in managing hypoglycaemia.
- •
Mountaineers: do not reduce the dose of drugs; stay hydrated; protect against the cold, especially the feet; and check blood glucose frequently. Keep insulin and glucagon from freezing. No increased risk of AS. Acetazolamide not recommended in DM1.
- •
Endurance athletes: for prevention, reduce doses of hypoglycaemic agents. Adequate hydration. Scheduled supplements and self-monitoring. Prevent heat stroke
- •
Sailors: do not go sailing alone. Supplements and self-monitoring every hour.
- •
Swimmers: start a blood glucose level ≥ 180 mg/dl. With suspected hypoglycaemia: stop exercising, take supplements and get out of the water.
This consensus document has received external funding in the form of a grant from the Spanish Society of Endocrinology and Nutrition, by means of an unrestricted grant from the company Sanofi. The sponsors did not influence any stage of the drafting of this document, nor did they have prior access to its contents.
Contributions in drafting this manuscriptCoordination: Dr Escalada - Dr Gargallo. 1. Medical evaluation before undertaking any sport: Dr J Escalada. 2. Effects of different exercises on glycaemic control: Dr P Rozas. 3. Dietary changes to be made: Dr C Tejera. 4. Glycaemic control: self-monitoring and drugs: Dr F Gomez-Peralta. 5, General recommendations and approach to glycaemic excursions for athletes with diabetes: Dr A Marco. 6. Recommendations for patients treated with insulin infusion: Dr M Botella. 7. Training guidelines for patients with diabetes mellitus: Dr J Fernández. 8. Special situations (special risk): Dr M Gargallo. The entire text has been reviewed and approved by all the authors.
Conflict of interestThe authors state that there is no conflict of interest related to the drafting of this document.
AcknowledgementsThe authors would like to thank the following athletes with DM1 for their cooperation in the development of this guide: José Ignacio García de Castro (marathon runner), Luis Ríos Fernández (multi-sport athlete), Ismael Escobar Rego (diver), Susana Ruiz Mostazo (mountain climber), David Jiménez Román (long-distance runner), José García Durán (dinghy and yacht sailing) Antonio Ortega Rivas (triathlete) and Xabi Garralda Zelay (football player).
Dr Judith Fernandez would like to thank Alejandro Gómez and Irma Bermúdez for their collaboration on her chapter.