Type 2 diabetes and depressive disorder are 2 chronic diseases highly prevalent in developed countries and with a negative impact on quality of life and life expectancy. In recent years, both conditions have been shown to be strongly associated. Thus, diabetics have an increased risk of suffering depressive disorder, as well as impaired glucose homeostasis, if they experience depression. In diabetic patients, concurrent depression is associated to greater difficulties in disease management and metabolic control, increased risk of developing chronic complications, decreased quality of life, and higher healthcare expenses. As a result, the interest of diabetic scientific societies in this association has increased, and they recommend regular mood assessment in diabetic patients. However, the limited clinical experience available and the conflicting results reported to date make it difficult to draw conclusions.
La diabetes tipo 2 y el trastorno depresivo son 2 enfermedades crónicas con una alta prevalencia en los países desarrollados, y con un impacto negativo sobre la calidad y la esperanza de vida. En los últimos años se ha demostrado que ambas entidades se hallan fuertemente asociadas, existiendo un mayor riesgo de desarrollar un trastorno depresivo entre la población diabética, así como de presentar alteraciones en la homeostasis de la glucosa si existe un síndrome depresivo. La coexistencia de una depresión en los pacientes diabéticos condiciona una mayor dificultad en el manejo de la enfermedad y en el control metabólico, un riesgo incrementado de desarrollar complicaciones crónicas, una disminución de la calidad de vida y un aumento del gasto sanitario. Ello ha suscitado el interés de las sociedades científicas, que ya aconsejan evaluar periódicamente el estado de ánimo. Sin embargo, la escasa experiencia a nivel clínico y los resultados contradictorios a nivel científico hacen que la elaboración de conclusiones resulte prematura.
Type 2 diabetes mellitus (T2DM) and depressive disorder (DD) are two chronic diseases that have a negative impact on both quality of life and life expectancy.1
Data from the International Diabetes Federation suggest that some 366 million adult individuals currently have T2DM. This represents approximately 8.3% of the worldwide population.2 It is also estimated that 23 million quality-adjusted life years are lost every year because of diabetes complications.3
On the contrary, approximately 340 million people have DD worldwide. In Europe, specifically, DD prevalence is estimated at 7% of the population.4 This is a highly relevant fact because DD is responsible for the majority of adverse non-fatal consequences related to health and accounts for 12% of total years lived with disability. DD is also associated with work absenteeism and decreased productivity, and also to a significant increase in healthcare resource utilization.5 In fact, DD is the second and tenth leading cause of disability-adjusted life years lost in females and males, respectively.6
Relationship between diabetes and depressive disorderMore than 300 years ago, the British physician Thomas Willis suggested a probable relationship between T2DM and DD by stating that DM was the consequence of a “period of sadness”.7
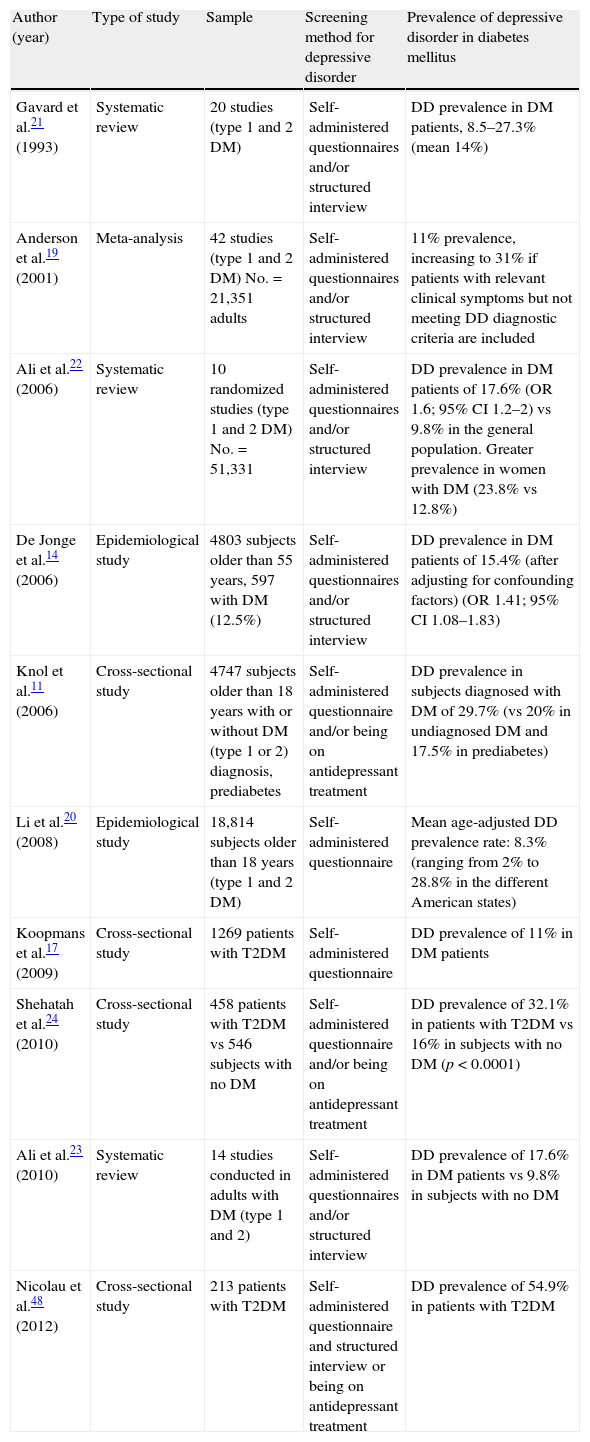
Since then, many studies have agreed that an association exists between T2DM and DD.8–24 In line with this, an Anderson et al. meta-analysis estimated an 11% prevalence of DD, diagnosed using a structured interview, in T2DM patients. This prevalence increased to 31% if subjects with clinically relevant depressive symptoms were included, even if they did not meet DD diagnostic criteria.19 DD prevalence may of course vary depending on the socioeconomic level of the country, the ethnic subgroup, diagnostic criteria, and the clinical characteristics of the diabetic population.9,14,17,19–24 On the contrary, most of these studies had a cross-sectional design that did not allow any temporal or causal relationship to be established. However, various prospective studies suggest an increased risk of DD in patients with T2DM, and of T2DM in depressed patients.10,13,16,17 Some meta-analyses have also detected a two-directional relationship between these conditions.9,11,12 In a systematic review, Renn et al.10 recently reported a relative risk (RR) of T2DM in patients with DD of 1.60 (95% confidence interval [CI] 1.37–1.88). A similar but lower increase in RR of DD was found in diabetic patients (1.15 [95% CI 1.02–1.30]). Table 1 summarizes the different studies on DD prevalence in diabetes.
Prevalence of depressive disorder in the diabetic population in different studies conducted.
| Author (year) | Type of study | Sample | Screening method for depressive disorder | Prevalence of depressive disorder in diabetes mellitus |
| Gavard et al.21 (1993) | Systematic review | 20 studies (type 1 and 2 DM) | Self-administered questionnaires and/or structured interview | DD prevalence in DM patients, 8.5–27.3% (mean 14%) |
| Anderson et al.19 (2001) | Meta-analysis | 42 studies (type 1 and 2 DM) No.=21,351 adults | Self-administered questionnaires and/or structured interview | 11% prevalence, increasing to 31% if patients with relevant clinical symptoms but not meeting DD diagnostic criteria are included |
| Ali et al.22 (2006) | Systematic review | 10 randomized studies (type 1 and 2 DM) No.=51,331 | Self-administered questionnaires and/or structured interview | DD prevalence in DM patients of 17.6% (OR 1.6; 95% CI 1.2–2) vs 9.8% in the general population. Greater prevalence in women with DM (23.8% vs 12.8%) |
| De Jonge et al.14 (2006) | Epidemiological study | 4803 subjects older than 55 years, 597 with DM (12.5%) | Self-administered questionnaires and/or structured interview | DD prevalence in DM patients of 15.4% (after adjusting for confounding factors) (OR 1.41; 95% CI 1.08–1.83) |
| Knol et al.11 (2006) | Cross-sectional study | 4747 subjects older than 18 years with or without DM (type 1 or 2) diagnosis, prediabetes | Self-administered questionnaire and/or being on antidepressant treatment | DD prevalence in subjects diagnosed with DM of 29.7% (vs 20% in undiagnosed DM and 17.5% in prediabetes) |
| Li et al.20 (2008) | Epidemiological study | 18,814 subjects older than 18 years (type 1 and 2 DM) | Self-administered questionnaire | Mean age-adjusted DD prevalence rate: 8.3% (ranging from 2% to 28.8% in the different American states) |
| Koopmans et al.17 (2009) | Cross-sectional study | 1269 patients with T2DM | Self-administered questionnaire | DD prevalence of 11% in DM patients |
| Shehatah et al.24 (2010) | Cross-sectional study | 458 patients with T2DM vs 546 subjects with no DM | Self-administered questionnaire and/or being on antidepressant treatment | DD prevalence of 32.1% in patients with T2DM vs 16% in subjects with no DM (p<0.0001) |
| Ali et al.23 (2010) | Systematic review | 14 studies conducted in adults with DM (type 1 and 2) | Self-administered questionnaires and/or structured interview | DD prevalence of 17.6% in DM patients vs 9.8% in subjects with no DM |
| Nicolau et al.48 (2012) | Cross-sectional study | 213 patients with T2DM | Self-administered questionnaire and structured interview or being on antidepressant treatment | DD prevalence of 54.9% in patients with T2DM |
DM, diabetes mellitus; CI, confidence interval; OR, odds ratio; DD, depressive disorder.
One possible reason for the increased risk of DD in diabetic patients is the psychosocial impact involved in being diagnosed with a chronic disease.11 The need for self-care to maintain good metabolic control, the fear of experiencing chronic complications in the mid or short term, etc., may lead to an anxious-depressive state, especially in people with little social support and a low cultural level.12 However, the observation of a greater prevalence of depressive symptoms compared to the general population, even in undiagnosed diabetic patients, makes the psychosocial theory insufficient to explain per se the increased risk of DD in T2DM.18 On the contrary, various biochemical changes secondary to T2DM form the basis of a biological hypothesis that could explain the higher risk of DD among the diabetic population.8,11 According to this hypothesis, increased levels of circulating proinflammatory molecules, hyperglycemia, and probably hyperinsulinism contribute to a low-grade chronic inflammation state. The passage of these proinflammatory cytokines to the central nervous system through the blood–brain barrier would facilitate the development of DD by activating different pathways, such as cytokine synthesis by microglial cells, the activation of macrophage-like cells in periventricular areas, changes in neurotransmitter levels, decreased neuroplasticity, and adrenal axis hyperactivation.8 Indeed, a decreased volume of the brain areas implicated in the etiopathogenesis of DD, such as hippocampus and amygdala, has been shown in patients with T2DM.25 Finally, it is currently unknown whether the relationship between T2DM and DD could be explained through a genetic mechanism. DD is known to have a polygenic inheritance, with up to 40–50% heritability based on twin studies. Multiple genetic abnormalities have been suggested, including polymorphisms in the serotonin transporter promoter region, genes related to neurotrophic processes, polymorphisms in the brain-derived neurotrophic factor gene, etc., none of which have been confirmed or related to genetic defects of T2DM.26
Depressive disorder as a predisposing factor for type 2 diabetes mellitusIt has been suggested that the increased risk of developing T2DM in subjects with DD may be attributed to the lifestyles adopted by most of them. DD is significantly associated with a higher body mass index, poorer dietary habits and a more sedentary lifestyle.10 Golden et al. examined the potential relation between DD and a higher incidence of T2DM, as well as its influence on lifestyle. De novo T2DM rates were significantly higher in the group with DD. However, these differences disappeared after adjustment for lifestyles (smoking, alcohol consumption, mean daily calorie consumption, and physical activity).27 It is also known that drug treatment for DD may have harmful effects on blood glucose control, although this in itself does not fully explain the increased risk of T2DM, as this risk is also greater in individuals with undiagnosed or untreated DD.8,28 On the contrary, the biological hypothesis also attempts to explain the increased incidence of T2DM in subjects with DD. According to this theory, the increased synthesis of proinflammatory cytokines (interleukin-1β, tumor necrosis factor-α, interleukin-6) in the central nervous system results in systemic inflammation due to the passage of these substances through the blood-brain barrier. This leads to insulin resistance and β cell dysfunction and, finally, T2DM.8 In addition, hyperactivation of the corticotroph axis in DD induces a proinflammatory state by depressing the immune system, as well as insulin resistance due to the counterregulatory effect of cortisol.29 This would appear to suggest that diabetic patients with concomitant DD have higher levels of proinflammatory cytokines than those with no DD, and therefore have an increased risk of chronic complications. However, this hypothesis is yet to be proven.27
Impact of depressive disorder on metabolic controlIn a four-year prospective study, Richardson et al. noted that glycosylated hemoglobin (HbA1c) levels were persistently higher in patients with T2DM who also had DD compared to that in those with no concomitant DD.30 Sufficient evidence is now available to show that DD impairs the care needed to maintain adequate control and drug treatment adherence and that it is associated with less healthy life habits (sedentary lifestyle and diets with high calorie contents).31–33 This is partly attributable to the fact that a positive attitude of patients with T2DM is crucial for disease management, and that DD is associated with a negative perception of the capacity for self-management.34
Effect of depressive disorder on chronic complications of diabetesAs DD has been shown to impair metabolic control, it is only natural to think that diabetic patients with concomitant DD will experience more chronic complications. De Groot et al. published a meta-analysis of 27 studies conducted in T1DM and T2DM patients. There were more chronic complications among diabetic patients diagnosed with DD, including diabetic retinopathy, diabetic nephropathy, diabetic polyneuropathy, and erectile dysfunction.35 As regards macrovascular complications, an increased prevalence of cardiovascular disease has been noted in diabetic postmenopausal women with concomitant DD.36
As regards the question of whether mortality is higher in patients with both T2DM and DD, epidemiological studies reported to date agree that such subjects have a more than 50% greater risk of all-cause death compared to healthy individuals and to patients with only one of the diseases.37 Egede et al., in a cohort of 10,025 subjects from the National Health and Nutrition Examination Survey I followed up for eight years, showed an RR of all-cause death of 2.50 (95% CI 2.04–3.08).38 The risk of all-cause death and cardiovascular death was also significantly increased in 78,282 women from the cohort of the Nurses Health Study (2.07 [CI 1.79–2.40] and 2.72 [CI 2.09–3.54] respectively).39
Effect of depressive disorder on quality of life and work environmentT2DM and DD are two chronic diseases associated with a loss of functional capacity. In fact, the odds ratio for functional disability is up to seven times higher among subjects with T2DM and depression compared to that in those with none of these conditions, and it is also higher, although not significantly, compared to that in subjects with only one of the diseases.40 This has an impact on work, and more than seven workdays per year may be lost on average.41 This functional loss also has a negative impact on quality of life.42 All of the foregoing shows that the coexistence of T2DM and DD in a same individual has a negative synergistic effect on functional capacity and quality of life.
Health care resource consumptionA greater frequency of outpatient visits to primary and specialized care has been shown for patients with both T2DM and DD.43 On the contrary, if a coexistent DD causes an increase in chronic complications in diabetic patients, health care expenses in these subjects may also be expected to be influenced by DD diagnosis and treatment. However, no cost studies supporting such assumption are yet available.
Impact of treatmentThere is no doubt that DD has both metabolic and psychologically harmful effects on patients with T2DM, and treatment is directed at improving both areas. There are three types of interventions for ameliorating depressive symptoms: psychosocial, pharmacological, and mixed.44
The psychosocial intervention most commonly used in patients with T2DM is cognitive-behavioral therapy (CBT). Studies reported to date show that CBT is effective for treating depressive symptoms in diabetic patients. However, there are conflicting results as to whether this type of intervention contributes to improvements in self-care and blood glucose control.44,45 Moreover, it is difficult to draw conclusions because of sample heterogeneity, different definitions of DD, the lack of subject stratification based on metabolic control, etc. In this regard, the only randomized study reported to date, conducted by Lustman et al., found that at six months of treatment, and despite the fact that no differences were seen immediately after the intervention, patients with T2DM randomized to CBT had lower HbA1c levels than patients who had only received diabetes education (9.5% vs 10.9% (p=0.03). Interestingly, patients with T2DM on CBT had a lower self-testing frequency.46
As regards drug treatment, selective serotonin reuptake inhibitors are the class of antidepressants most commonly used because of their efficacy and safety profile. They have also been shown to be able to decrease glucose levels and promote weight loss, and are therefore the drugs of choice for the treatment of DD in the population with T2DM. By contrast, the use of tricyclic antidepressants and monoamine oxidase inhibitors in diabetic subjects is less popular. The reason for this is that their use has been associated with hyperglycemia and weight increase respectively.45 Other antidepressants, such as bupropion, have shown significant reductions in both depressive symptoms and body mass index and HbA1c. However, this improvement in HbA1c values was only maintained if adequate control of the depression was maintained.47 In most studies reported to date, DD improvement and/or remission was evident in patients with T2DM on drug treatment, but this appeared to be ineffective for optimizing metabolic control. Our group similarly noted that treatment with citalopram (a selective serotonin reuptake inhibitor) for six months in a sample of patients with T2DM with DD criteria improved quality of life and depressive symptoms, but not metabolic control.48
Similar results are found when CBT and drug therapy to treat DD are combined. That is to say, the depressive symptoms are controlled, but no positive results are seen in blood glucose levels.44,45
It should be noted that the studies reported to date have all had significant limitations, such as small sample size and short duration (which may make it difficult to show metabolic improvement) and between- and within-study heterogeneity of the samples with regard to the type of diabetes and the antidepressive treatment being assessed. On the contrary, changes in depressive symptoms have usually been assessed based on statistical significance, rather than actual clinical impact. In other words, since improvements are assessed based on depression test scores, the degree of personal impact and its potential positive influence on lifestyle habits, T2DM management, or even a change in clinical severity of DD is unknown.
With regard to whether or not improved metabolic control reduces depressive symptoms, this is a question that has not been elucidated yet. Nor do we know if different effects may be exerted on depressive symptoms depending on the hypoglycemic drug used to improve metabolic control. In this regard, it should be kept in mind that, unlike other hypoglycemic agents, some of the new glucagon-like peptide 1 receptor agonists cross the blood-brain barrier and affect cerebral physiology.49
Future linesThe various societies of specialists in diabetes are becoming aware of the clinical association of T2DM and DD. In fact, their recommendations for the management of diabetes include an initial evaluation of the patient's mood, the stress caused by diabetes, and the social environment of the patient, and state that DD should be ruled out if any impairment in blood glucose control occurs which is otherwise difficult to explain.50 Despite this, the understanding by professionals of the diagnosis and treatment of DD in diabetes is still suboptimal. Because of this, treatment is most often inadequate or, in the worst of cases, nonexistent. Thus, in a series of patients with T2DM, we found that a significant proportion were undiagnosed and, even more significantly, a high proportion of patients, even when diagnosed, received inadequate treatment.51 This area of knowledge should therefore be reinforced in training programs on diabetes, and adequate patient management should be promoted. It should not be forgotten that, although the treatment of depression has not been shown to improve metabolic control, it does achieve significant improvements in both mood and quality of life scales.
As regards research, further studies are needed to identify DD mediators in diabetes and vice versa, particularly modifiable or treatable mediators. Toward this end, the conduct of longitudinal and generalizable studies using homogeneous diagnostic criteria should be promoted. Thus, few data are available regarding the reliability and validity of different tools to diagnose depression in T2DM. Little information is also available about the applicability of these instruments in different sociocultural situations.52 On the contrary, it is very important to identify, define, and homogenize the diagnosis of comorbid conditions and any associated sociodemographic factors that may influence the results.53 Both approaches will allow us to better identify the different risk groups and will contribute to the design of better therapeutic strategies. As regards the latter, it should be noted that the use of different antidepressants, prevents us from making a comparison of their results. Larger and longer studies are needed to quantify the impact of the different antidepressant treatments not only on depression scales, but also on personal life, quality of life, and the management of diabetes. Special mention should be made of the few data available on health economics. It should finally be emphasized that the impact of improved metabolic control and the different hypoglycemic agents on DD remains unknown.
In conclusion, it may be stated that T2DM and DD are two closely related conditions. They very often coexist, and DD is not uncommonly underdiagnosed and undertreated in diabetic patients. In addition, the coexistence of both diseases is associated with poorer metabolic control and more difficult management. It also involves an increased risk of chronic complications and a substantial increase in morbidity and mortality. It may therefore be stated that both further research into this undesirable combination and improvements in clinical practice will be well worth the effort. As regards research, the need for prospective studies using homogeneous criteria that make it possible to advance in different therapeutic strategies should be emphasized. As regards clinical practice, it is essential to promote training and increase awareness in the different health care professionals involved.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Nicolau J, Masmiquel L. Diabetes mellitus y trastorno depresivo, un mal binomio. Endocrinol Nutr. 2013;60:583–589.