During myocardial infarction (MI), a variety of mechanisms contribute to activation of cell death processes in cardiomyocytes, which determines the final MI size, subsequent mortality, and post-MI remodeling. The deleterious mechanisms activated during the ischemia and reperfusion phases in MI include oxygen deprival, decreased availability of nutrients and survival factors, accumulation of waste products, generation of oxygen free radicals, calcium overload, neutrophil infiltration in the ischemic area, depletion of energy stores, and opening of the mitochondrial permeability transition pore, all of them contributing to the activation of apoptosis and necrosis in cardiomyocytes. Glucagon-like peptide-1 [GLP-1 (7–36) amide] has gained relevance in recent years for metabolic treatment of patients with type 2 diabetes mellitus. Cytoprotection of different cell types, including cardiomyocytes, is among the pleiotropic actions reported for GLP-1. This paper reviews the most relevant experimental studies that have contributed to a better understanding of the molecular mechanisms and intracellular pathways involved in cardioprotection induced by GLP-1 and analyzes in depth its potential role as a therapeutic target both in the ischemic and reperfused myocardium and in other conditions that are associated with myocardial remodeling and heart failure.
La activación de diferentes procesos de muerte celular en los cardiomiocitos tras un infarto de miocardio (IM) contribuye al tamaño final del infarto, a la mortalidad subsecuente y al remodelado postinfarto en los supervivientes. Los diversos mecanismos deletéreos activados durante las fases de isquemia y reperfusión en el IM incluyen la privación de oxígeno, la disponibilidad reducida de nutrientes y factores de supervivencia, la acumulación de residuos, la generación de especies reactivas del oxígeno, la sobrecarga de calcio, la infiltración por neutrófilos en el área isquémica, la depleción energética, y la apertura del poro de transición de permeabilidad mitocondrial, todos ellos mecanismos de activación de apoptosis y necrosis en los cardiomiocitos. En los últimos años, las terapias basadas en el péptido similar al glucagón tipo 1 [GLP-1 (7-36) amida] han adquirido mayor relevancia como tratamiento metabólico de la diabetes mellitus tipo 2. Entre las acciones atribuidas a GLP-1 destaca la preservación de la viabilidad en diferentes tipos celulares, entre ellos los cardiomiocitos. Este artículo revisa los principales estudios experimentales que han contribuido a una mayor comprensión de la citoprotección inducida por GLP-1 en el miocardio y de sus efectos en la función cardiaca, ahondando en el estudio de su papel como diana terapéutica, no solo en el contexto de la diabetes mellitus sino también en otras patologías que cursan con remodelado cardiaco.
Changes in cardiac metabolism occurring during the ischemic phase of myocardial infarction (MI) include the deprivation of oxygen, nutrients, and survival factors and the accumulation of residues in cardiomyocytes, causing death cell processes and resulting in myocardial stunning and hibernation and, finally, contractile function impairment.1,2 Paradoxically, the sudden restoration of oxygen flow to the ischemic area may increase myocardial injury (so-called “ischemia-reperfusion injury”),3 generating reactive oxygen species, calcium overload, neutrophil infiltration, the depletion of energy stores, and changes in intracellular mechanisms that may lead to the opening of the mitochondrial permeability transition pore (MPTP).2 Thus, oxygen provision increases damage to previously ischemic cardiomyocytes and reduces the benefits of reperfusion.4 As a consequence of the abovementioned processes, mechanisms of necrotic cell death are activated during the ischemic phase and the characteristic changes of apoptosis mainly occur after reperfusion,5 with both types of cell death contributing to the final size of MI.2
The activation of reperfusion injury survival kinases (RISK) confers protection against ischemia-reperfusion injury through their antiapoptotic and antinecrotic actions.6 Specifically, cardioprotection induced after activation of the RISK pathway is mediated by the inhibition of MPTP opening, the blockade of calcium overload, and the activation of several antiapoptotic mechanisms.7 In this regard, it should be noted that therapeutic strategies designed to increase activity of the RISK pathway significantly decrease MI size.6,8
Glucagon-like peptide-1 [GLP-1 (7–36) amide] is a hormone derived from the proglucagon gene which is released from intestinal L cells in response to nutrient intake. Once in the circulation, GLP-1 (7–36) exerts incretin actions, stimulating glucose-dependent insulin secretion by interacting with its receptor (GLP-1R) in pancreatic islet beta cells.9–11 However, GLP-1 (7–36) has a very short half-life in blood (<2min), mainly because of its rapid degradation by the enzyme dipeptidyl peptidase-4 (DPP-4) to the GLP-1 (9–36) peptide, a weak antagonist of GLP-1R with no incretin activity.12,13 Thus, in type 2 diabetes mellitus (T2DM), treatments based on GLP-1 (7–36) have been developed using strategies which increase the presence of GLP-1 (7–36) in blood, using either compounds that inhibit activity of the enzyme DPP-4 (e.g. sitagliptin, vidagliptin, linagliptin, saxagliptin, alogliptin) or GLP-1R agonists resistant to the action of DPP-4 (e.g. exenatide, liraglutide, albiglutide, lixisenatide, taspoglutide).14
Beyond its effects on glucose metabolism, many studies in recent years have reported cytoprotective actions of GLP-1 (7–36) in various cell types. For example, GLP-1 (7–36) has been shown to inhibit cell death processes in cholangiocytes,15 neurons,16 and pancreatic beta cells.17,18 A combination of antiapoptotic and antinecrotic effects has been reported in pancreatic beta cells.19 Such a cytoprotection appears to be due to the direct preservation of mitochondria, as studies in hepatocytes show that GLP-1 (7–36) exerts insulin-like effects through the modulation of oxidative phosphorylation and the inhibition of oxidative stress.20
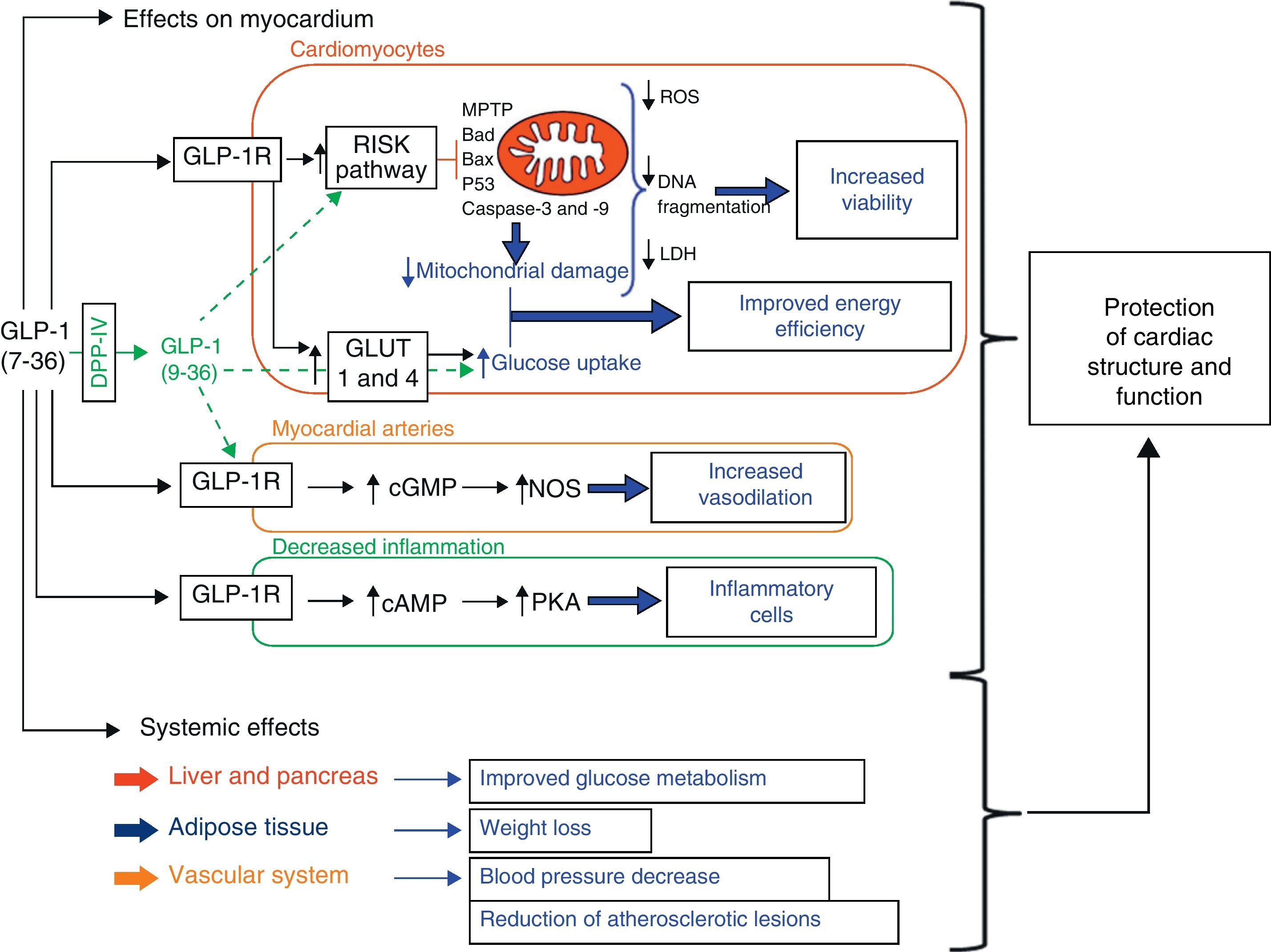
A wide variety of cardiovascular actions of GLP-1 have been reported to date, including hemodynamic system changes.21,22 Specifically, cytoprotective actions of GLP-1 (7–36) have been reported in the heart.21–24 In this regard, the fact that different studies show the presence of GLP-1R in the myocardium of different animal models25,26 and in human cardiac tissue27 suggests that GLP-1 (7–36) may have direct effects on the heart. In fact, several experimental studies show that the direct action of GLP-1 (7–36) on myocardium preserves cardiac function. For example, genetic deletion of GLP-1R results in an impaired left ventricular contractile response and diastolic function in mice.28 The stimulation of GLP-1R in cardiomyocytes has also been shown to increase their viability through activation of the RISK pathway (Fig. 1).29–31 In addition, genetic DPP-4 deficiency preserves cardiac function during endotoxemia32 and ischemia-reperfusion.33,34 Finally, it should be noted that circulating GLP-1 levels have been associated with cardiac function in clinical studies.35,36
Schematic representation of intracellular pathways proposed as mediators of the cardioprotective actions of glucagon-like peptide-1 (GLP-1). The combination of the effects of GLP-1 on myocardium (e.g. inhibition of apoptosis and necrosis in cardiomyocytes through activation of the RISK pathway, increased glucose metabolism, vasodilation, and anti-inflammatory actions) and the systemic metabolic and vascular effects of GLP-1 contributes to improve cardiac survival and function (cAMP: cyclic adenosine monophosphate; cGMP: cyclic guanosine monophosphate; Cyt c: cytochrome c; DPP-4: dipeptidyl peptidase-4; ERK: extracellular signal-regulated kinases; GLUT: glucose transporter; GSK: glycogen synthase kinase-3; LDH: lactate dehydrogenase; MEK ½: MAP kinase kinase; MPTP: mitochondrial permeability transition pore; NOS: nitric oxide synthase; PI3K: phosphatidylinositol 3-kinase; PKA: protein kinase A; protein kinase B; ROS: reactive oxygen species).
This review includes the most relevant studies contributing to a better understanding of the intracellular pathways and molecular mechanisms triggered after GLP-1R stimulation in cardiomyocytes, with a particular focus on the modulation of the RISK pathway and the inhibition of cell death mechanisms. A parallel comparison of the impact of GLP-1 on different intracellular mechanisms related to myocardial survival and the potential functional consequences of these actions during experimental ischemia-reperfusion in both diabetic and non-diabetic models is also provided. Finally, since cardiomyocyte survival is also compromised in heart failure (HF),37–39 an additional discussion of the cardioprotective role of strategies based on GLP-1 and their consequences for myocardial structure and function in experimental HF models have been included.
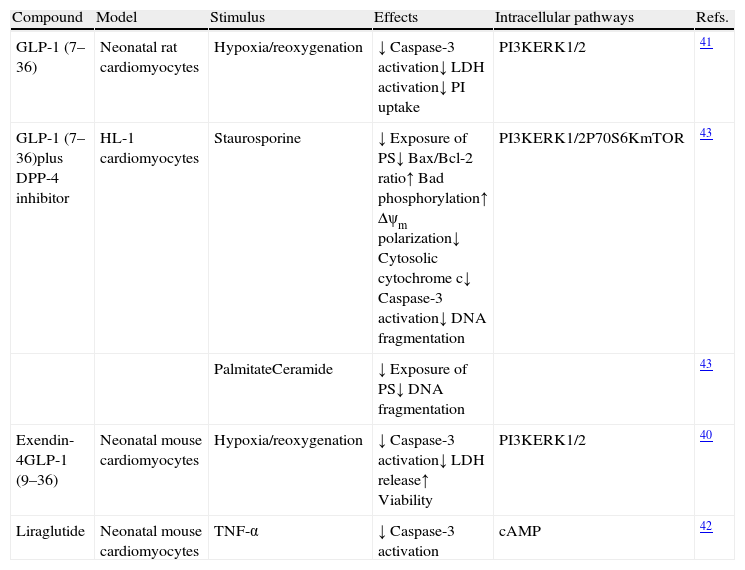
Glucagon-like peptide-1 and cardiomyocytes in experimental in vitro modelsVarious studies have shown that incubation with GLP-1 (7–36), or with agonists of its receptor, preserves the viability of cardiomyocytes cultured in the presence of different cell death stimuli (Table 1). For example, incubation with GLP-1 (7–36) or an analog inhibits the activation of apoptotic and necrotic processes and increases the viability of neonatal cardiomyocytes under ischemia-reperfusion conditions40,41 and in the presence of stimuli characteristic of HF, such as tumor necrosis factor (TNF)-alpha.42 Moreover, incubation of adult mouse cardiomyocytes (HL-1 line) in the presence of GLP-1 (7–36) prevents the activation of mechanisms involved in death cell processes triggered by classical apoptotic stimuli such as staurosporine and by stimuli inherent to the diabetic setting such as palmitate and ceramide.43 In addition, in all these experiments, the cytoprotective effects of GLP-1 (7–36) were shown to be primarily mediated by mechanisms dependent on the activation of the RISK pathway, mainly phosphoinositol 3-kinase (PI3K) and extracellular signal-regulated kinases (ERK1/2) (Fig. 1). On the other hand, it should be noted that some studies assign a cytoprotective role to GLP (9–36), which is considered to have no incretin activity, because it inhibits cell death processes in cardiomyocytes subject to ischemia-reperfusion conditions, also through the activation of intracellular pathways dependent on PI3K and ERK1/1 (Fig. 1).40
Study of the cytoprotective effects of GLP-1 and therapies based on GLP-1 in cardiomyocytes in vitro.
| Compound | Model | Stimulus | Effects | Intracellular pathways | Refs. |
| GLP-1 (7–36) | Neonatal rat cardiomyocytes | Hypoxia/reoxygenation | ↓ Caspase-3 activation↓ LDH activation↓ PI uptake | PI3KERK1/2 | 41 |
| GLP-1 (7–36)plus DPP-4 inhibitor | HL-1 cardiomyocytes | Staurosporine | ↓ Exposure of PS↓ Bax/Bcl-2 ratio↑ Bad phosphorylation↑ Δψm polarization↓ Cytosolic cytochrome c↓ Caspase-3 activation↓ DNA fragmentation | PI3KERK1/2P70S6KmTOR | 43 |
| PalmitateCeramide | ↓ Exposure of PS↓ DNA fragmentation | 43 | |||
| Exendin-4GLP-1 (9–36) | Neonatal mouse cardiomyocytes | Hypoxia/reoxygenation | ↓ Caspase-3 activation↓ LDH release↑ Viability | PI3KERK1/2 | 40 |
| Liraglutide | Neonatal mouse cardiomyocytes | TNF-α | ↓ Caspase-3 activation | cAMP | 42 |
GLP-1: glucagon-like peptide-1; DPP-4: dipeptidyl peptidase-4; TNF: tumor necrosis factor; LDH: lactate dehydrogenase; PI: propidium iodide; PS: phosphatidylserine; Δψm: mitochondrial membrane potential; PI3K: phosphatidylinositol 3-kinase; ERK: extracellular signal-regulated kinases; cAMP: cyclic adenosine monophosphate.
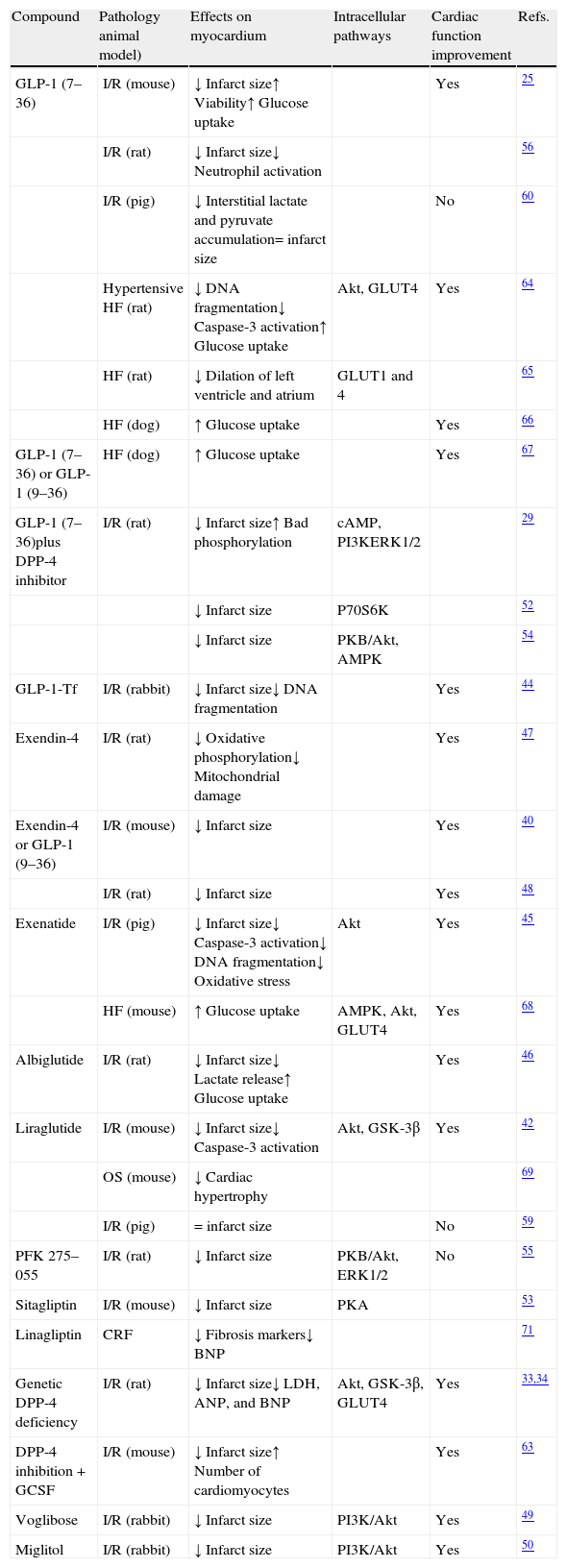
Many studies have shown that the administration of GLP-1 (7–36) and therapeutic strategies based on this peptide (GLP-1R agonists or DPP-4 inhibitors) inhibit cell death processes activated in myocardium in different experimental ischemia-reperfusion models (Table 2). For example, treatment with GLP-1 (7–36) combined with a DPP-4 inhibitor inhibits the activity of the proapoptotic protein Bad and decreases the size of the infarct area in animal models of ischemia-reperfusion.29 In addition, the antiapoptotic effect associated with a reduced infarct size is seen both when GLP-1 (7–36) is administered before myocardial ischemia and at the start of reperfusion.44 GLP-1R agonists such as exenatide, albiglutide, and liraglutide show antiapoptotic and antinecrotic activities in ischemia-reperfusion models. Specifically, Timmers et al.45 showed in an ischemia-reperfusion model in pigs that exenatide decreased the size of the infarct area, inhibited the expression of protease caspase-3 and DNA fragmentation, and reduced oxidative stress. Moreover, the administration of albiglutide preserved myocardial viability and decreased cardiac lactate production after ischemia-reperfusion injury in rats.46 Intraperitoneal administration of liraglutide before the coronary artery occlusion procedure in mice also decreased the size of the infarct area, probably through the inhibition of caspase-3 activation in cardiomyocytes.42 Interestingly, increased cardiac cell survival in response to treatments based on GLP-1 had a significant impact on cardiac function, as was shown in most of the studies mentioned, in the setting of both ischemia-reperfusion and HF (Table 2).25,34,40,42,44–51
Studies of the cytoprotective effects of GLP-1 and therapies based on GLP-1 in experimental ex vivo and in vivo models.
| Compound | Pathology animal model) | Effects on myocardium | Intracellular pathways | Cardiac function improvement | Refs. |
| GLP-1 (7–36) | I/R (mouse) | ↓ Infarct size↑ Viability↑ Glucose uptake | Yes | 25 | |
| I/R (rat) | ↓ Infarct size↓ Neutrophil activation | 56 | |||
| I/R (pig) | ↓ Interstitial lactate and pyruvate accumulation= infarct size | No | 60 | ||
| Hypertensive HF (rat) | ↓ DNA fragmentation↓ Caspase-3 activation↑ Glucose uptake | Akt, GLUT4 | Yes | 64 | |
| HF (rat) | ↓ Dilation of left ventricle and atrium | GLUT1 and 4 | 65 | ||
| HF (dog) | ↑ Glucose uptake | Yes | 66 | ||
| GLP-1 (7–36) or GLP-1 (9–36) | HF (dog) | ↑ Glucose uptake | Yes | 67 | |
| GLP-1 (7–36)plus DPP-4 inhibitor | I/R (rat) | ↓ Infarct size↑ Bad phosphorylation | cAMP, PI3KERK1/2 | 29 | |
| ↓ Infarct size | P70S6K | 52 | |||
| ↓ Infarct size | PKB/Akt, AMPK | 54 | |||
| GLP-1-Tf | I/R (rabbit) | ↓ Infarct size↓ DNA fragmentation | Yes | 44 | |
| Exendin-4 | I/R (rat) | ↓ Oxidative phosphorylation↓ Mitochondrial damage | Yes | 47 | |
| Exendin-4 or GLP-1 (9–36) | I/R (mouse) | ↓ Infarct size | Yes | 40 | |
| I/R (rat) | ↓ Infarct size | Yes | 48 | ||
| Exenatide | I/R (pig) | ↓ Infarct size↓ Caspase-3 activation↓ DNA fragmentation↓ Oxidative stress | Akt | Yes | 45 |
| HF (mouse) | ↑ Glucose uptake | AMPK, Akt, GLUT4 | Yes | 68 | |
| Albiglutide | I/R (rat) | ↓ Infarct size↓ Lactate release↑ Glucose uptake | Yes | 46 | |
| Liraglutide | I/R (mouse) | ↓ Infarct size↓ Caspase-3 activation | Akt, GSK-3β | Yes | 42 |
| OS (mouse) | ↓ Cardiac hypertrophy | 69 | |||
| I/R (pig) | = infarct size | No | 59 | ||
| PFK 275–055 | I/R (rat) | ↓ Infarct size | PKB/Akt, ERK1/2 | No | 55 |
| Sitagliptin | I/R (mouse) | ↓ Infarct size | PKA | 53 | |
| Linagliptin | CRF | ↓ Fibrosis markers↓ BNP | 71 | ||
| Genetic DPP-4 deficiency | I/R (rat) | ↓ Infarct size↓ LDH, ANP, and BNP | Akt, GSK-3β, GLUT4 | Yes | 33,34 |
| DPP-4 inhibition+GCSF | I/R (mouse) | ↓ Infarct size↑ Number of cardiomyocytes | Yes | 63 | |
| Voglibose | I/R (rabbit) | ↓ Infarct size | PI3K/Akt | Yes | 49 |
| Miglitol | I/R (rabbit) | ↓ Infarct size | PI3K/Akt | Yes | 50 |
ANP: atrial natriuretic peptide; BNP: brain natriuretic peptide; cAMP: cyclic adenosine monophosphate; DPP-4: dipeptidyl peptidase-4; ERK: extracellular signal-regulated kinases; CRF: chronic renal failure; GLP-1: glucagon-like peptide-1; GLP-1-Tf: GLP-1 fused to human transferrin; GLUT: glucose transporter; GSK: glycogen synthase kinase; HF: heart failure; I/R: ischemia-reperfusion; PI3K: phosphatidylinositol 3-kinase; PKA: protein kinase A; PKB: protein kinase B; PKF 275–055: DPP-4 inhibitor; LDH: lactate dehydrogenase; OS: obesity syndrome.
In agreement with observations made in in vitro models, various findings support the hypothesis that the cardioprotective effects of GLP-1 (7–36) in ischemia-reperfusion models are mediated by the activation of kinases in the RISK pathway, including PI3K, ERK1/2, cAMP, PKA, Akt, and P70S6K (Table 2).29,34,42,49,50,52–55 On the other hand, it has been suggested that the improvement seen after the administration of GLP-1 (7–36) in MI in terms of increased survival and improved cardiac function could also be due to the decreased activation of inflammatory cells,56 improved myocardial microcirculation,57 and increased myocardial glucose uptake (Fig. 1).46,58
However, the benefits of therapies based on GLP (7–36) in cardiac structure and function have not been supported by certain studies conducted in pig models of ischemia-reperfusion. For example, neither GLP-1 (7–36) infusion nor treatment with liraglutide changed infarct size in such experimental models.59,60 However, it is well known that results may substantially change depending on treatment duration, doses administered, and the animal model used. It should also be noted that blood flow is usually negligible in pig myocardium,61 which may contribute to a decreased cardioprotective efficacy of GLP-1 due to the accumulation of toxic products after MI. However, the demonstration by other authors that treatment with exenatide inhibits apoptosis and oxidative stress and activates survival kinases in pig myocardium after an ischemia-reperfusion procedure45 suggests the need for designing additional studies to clarify the beneficial impact of strategies based on GLP-1 in this experimental model. There are also data showing that the chronic blockade of DPP-4 activity with vidagliptin does not prevent structural and functional remodeling after MI in an experimental model of non-diabetic rats.62 In this regard, findings reported by the Sauvé et al. group33 support the hypothesis that DPP-4 inhibition is a strategy showing greater cardioprotective efficacy in terms of the activation of survival pathways after MI in diabetic as compared to non-diabetic mice. On the other hand, recent observations show that DPP-4 inhibition, combined with the use of granulocyte colony-stimulating factor (G-CSF) under cell cycle activation conditions in cardiomyocytes, enhances myocardial regeneration and improves the function in mice after MI.63 Finally, in agreement with in vitro findings, several studies suggest that administration of the peptide GLP-1 (9–36) decreases myocardial injury after ischemia-reperfusion, which supports the hypothesis that treatments that inhibit the formation of this peptide may have less cardioprotective efficacy in these experimental models.25,40,48
Glucagon-like peptide-1 and cardiomyocytes in experimental models of heart failureThe effects of GLP-1 (7–36) on cardiac viability and function have also been studied in experimental HF models (Table 2). In spontaneously hypertensive rats with HF, for instance, continued administration of GLP-1 (7–36) for three months decreased both the apoptotic index and the activation of caspase-3, preserved left ventricular function, and prolonged animal survival.64 Similarly, in rats with post-MI HF, treatment with GLP-1 (7–36) or exenatide significantly improved cardiac remodeling, cardiac function, and the survival of the model.65 In agreement with these results, infusion of GLP-1 (7–36) in dogs with dilated cardiomyopathy for 48hours increased myocardial glucose uptake, improving left ventricular function and decreasing systemic vascular resistance.66 These findings were confirmed one year later in the same experimental model, where GLP-1 (9–36) was also shown to have actions similar to GLP-1 (7–36) in terms of the stimulation of glucose uptake and the improvement of left ventricular function,67 thus supporting the cardioprotective role of GLP-1 (9–36) in experimental HF. Subsequent studies in experimental models of dilate cardiomyopathy showed that treatment with exenatide increased cardiac contractility and myocardial glucose uptake, decreased the production of brain natriuretic peptide (BNP), and prolonged survival. All these effects were associated with an increased activation of the RISK pathway, particularly AMP kinase and Akt-dependent intracellular pathways.68
Recent evidence has extended the cardioprotective action of GLP-1 to pathological conditions other than MI or HF. Specifically, the administration of liraglutide decreased cardiac hypertrophy and blood pressure in insulin-resistant obese mice.69 In this regard, the antihypertensive effect of GLP-1 had previously been reported in Dahl salt-sensitive rats.70 Linagliptin, a DPP-4 inhibitor, has also been shown to decrease the expression of proteins related to the presence of myocardial fibrosis and levels of cardiomyocyte stress markers in a rat model of uremic cardiomyopathy.71
Conclusion and perspectivesFindings reported to date suggest that therapies based on GLP-1 (7–36) may have beneficial actions in the heart beyond their metabolic effects by decreasing the susceptibility of cardiomyocytes to the activation of cell death processes and increasing their energy efficiency. Specifically, in vitro studies show a direct effect of GLP-1 on cardiomyocyte survival through activation of the RISK pathway in the presence of characteristic stimuli of ischemia-reperfusion injury and in the setting of HG (Table 1). In agreement with this, data from studies conducted in in vivo models of ischemia-reperfusion and HF show that GLP-1-mediated activation of the RISK pathway increases myocardial survival (Table 2). It should also be noted that in most experimental in vivo models, cytoprotection induced by GLP-1 is associated with an improved cardiac function (Table 2). To sum up, the direct effects of GLP-1 (7–36) in the myocardium, combined with other actions of the peptide on the cardiovascular system (reviewed in 21–24), may explain the beneficial effects of incretin-based therapies in cardiac structure and function, regardless of the presence or absence of T2DM (Fig. 1).
However, further research is required on the impact of therapies based on GLP-1 (7–36) on the different processes involved in myocardial remodeling, including evaluation of the pathways implicated in cardiomyocyte hypertrophy and death, as well as the mechanisms leading to excess collagen deposition and the occurrence of myocardial fibrosis. New experimental and clinical studies should also be proposed to assess the cardioprotective efficacy of incretin-based therapies, as compared to other drug strategies, in patients with or without T2DM in the presence or absence of MI or HF. In addition, new experimental studies should be designed to analyze the most adequate dosage and duration of treatments based on GLP-1 in each pathological condition to achieve a reasonable balance between side effects and the benefits seen in myocardial remodeling.
There is also a need to design additional experiments in the context of diabetes aimed at comparing the cardiovascular effects of incretin-based therapies to other glucose-lowering therapies and analyzing in depth the impact such therapies may have on different comorbid conditions associated with this disease, including obesity, hyperlipidemia, high blood pressure, renal failure, and so on.72 In this same conceptual framework, experiments to compare the cardioprotective efficacy of different therapies based on GLP-1 should be considered, particularly because GLP-1R and DPP-4 inhibitors have different actions depending on whether they are assessed in the metabolic or cardiovascular setting.14,72,73 In this regard, mention should be made of the multiple experimental observations attributing a cardioprotective activity to the GLP-1 (9–36) peptide, whose blood levels may be influenced by the type of incretin-based therapy administered. Such observations suggest that GLP-1R agonists may have a greater cardioprotective efficacy in terms of cardiomyocyte survival. In this regard, findings reported by Sauvé et al.33 show a clear trend to a greater activation of survival pathways in the myocardium of diabetic animals treated with liraglutide as compared to sitagliptin.
In conclusion, additional experimental studies should be conducted to investigate the feasibility and effectiveness of incretin-based therapies in the cardiovascular setting. Such an approach should include a combined analysis of the efficacy of therapies based on GLP-1 to preserve cardiomyocyte viability and function, and of their impact on other cell and tissue lesions characteristic of cardiac remodeling, in different experimental models in the presence and absence of diabetes.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Ravassa S, et al. Péptido similar al glucagón tipo 1 y supervivencia de la célula cardiaca. Endocrinol Nutr. 2012;59:561–9.